Key Points
In HS, patients’ early referral for HCT, with the best available HLA-matched donor offers the best event-free survival.
In HS, patients’ HCT with a well-matched unrelated cord blood unit is particularly attractive because the unit is readily available.
We report transplantation outcomes of 258 children with Hurler syndrome (HS) after a myeloablative conditioning regimen from 1995 to 2007. Median age at transplant was 16.7 months and median follow-up was 57 months. The cumulative incidence of neutrophil recovery at day 60 was 91%, acute graft-versus-host disease (GVHD) (grade II-IV) at day 100 was 25%, and chronic GVHD and 5 years was 16%. Overall survival and event-free survival (EFS) at 5 years were 74% and 63%, respectively. EFS after HLA-matched sibling donor (MSD) and 6/6 matched unrelated cord blood (CB) donor were similar at 81%, 66% after 10/10 HLA-matched unrelated donor (UD), and 68% after 5/6 matched CB donor. EFS was lower after transplantation in 4/6 matched unrelated CB (UCB) (57%; P = .031) and HLA-mismatched UD (41%; P = .007). Full-donor chimerism (P = .039) and normal enzyme levels (P = .007) were higher after CB transplantation (92% and 98%, respectively) compared with the other grafts sources (69% and 59%, respectively). In conclusion, results of allogeneic transplantation for HS are encouraging, with similar EFS rates after MSD, 6/6 matched UCB, 5/6 UCB, and 10/10 matched UD. The use of mismatched UD and 4/6 matched UCB was associated with lower EFS.
Introduction
Hurler syndrome (HS) is the most severe phenotype in the spectrum of mucopolysaccharidosis type I (MPS I), a type of lysosomal storage disorder. Untreated HS develops into progressive and ultimately fatal multisystem deterioration, including psychomotor retardation, severe skeletal manifestations, and life-threatening cardiac and pulmonary complication caused by severe deficiency or complete absence of the lysosomal enzyme α-l-iduronidase.1 On the basis of in vitro studies documenting cross-correction of cells from MPS patients with normal cells,2 hematopoietic cell transplantation (HCT) was introduced as a treatment of severe phenotype MPS I in the early 1980s.3 After transplantation, engrafting donor cells serve as a permanent source of cellular enzyme replacement in the blood and in the central nervous system. Enzyme replacement therapy (ERT), available for MPS I patients since 2003, does not prevent central nervous system deterioration. Therefore, allogeneic HCT is currently considered the treatment of choice for patients with HS.4,5
Worldwide, more than 500 HCT procedures have been performed in HS patients, making HS the most frequently transplanted inborn error of metabolism (IEM). Successful outcomes have been reported for HS patients after HCT, provided transplantation is performed early in life.6 However, this treatment option historically was limited by donor availability, high rates of graft failure in some types of transplants, mixed chimerism, and transplantation-related morbidity and mortality.7 There have been several studies done recently to identify risk factors related to these limitations. In a European retrospective study, both T-cell depletion and the use of reduced-intensity conditioning regimen were found to be associated with graft failure, whereas busulfan pharmacokinetic targeting protected against graft failure.7 Interesting studies using unrelated cord blood (UCB) as a stem cell source for HCT in patients with IEM, including HS, showed high rates of full-donor chimerism associated with normal enzyme levels post engraftment.8,,-11 Because full-donor chimerism associated with normal enzyme levels are thought to be associated with a superior neurocognitive outcome after HCT, cord blood (CB) has been proposed as an alternative option or even preferential stem cell source for HS patients.6 However, a large series of patients comparing the different stem cell and donor sources is still needed to support this.
With this aim, we conducted a collaborative retrospective analysis of outcomes and risk factors after HCT for HS patients treated in multiple centers worldwide reported to the European Group for Blood and Marrow Transplantation (EBMT), Eurocord, and the Center for International Blood and Marrow Transplant Research (CIBMTR).
Patients and methods
Data collection
This retrospective analysis is based on data reported to the Promise database and/or Eurocord Registry from European and non-European centers through a standardized questionnaire that includes information about the patients, donor, diseases, and transplant outcomes. In addition, data were obtained from US transplant centers either directly (Duke University) or from the CIBMTR. CIBMTR data are also collected on standardized data reporting forms similar to those used by the Eurocord-EBMT. The collected information was reviewed by 2 physicians to ensure data quality. Overlaps between the databases were checked to eliminate duplicate reporting. All parents and or legal guardians of patients gave informed consent for hematopoietic stem cell transplantation (HSCT) according to the Declaration of Helsinki. In Europe, the study was approved by Eurocord and the Working Party for Inborn Errors of European Blood and Marrow transplant group (ie, EBMT). In the US, the institutional review boards of Duke University, the Medical College of Wisconsin, and the National Marrow Donor Program approved the study. One-hundred thirty-eight (53%) patients included herein were reported in 3 previous studies.7,10,12 For this study, data of the previously reported patients were updated. Thirty-eight patients who were reported to CIBMTR from 21 transplant centers were excluded from the analysis because we have planned a follow-up study of the current cohort that will systematically examine functioning and quality of life in surviving patients. Data collection for the planned study involved a member of the study team visiting transplant centers in Europe and the US. As such, it was not feasible to visit all US centers to collect additional data. In addition, important data for our analyses were collected, such as enzyme levels (plus the institutional reference ranges) on latest follow-up and the use of ERT before HCT (including the number of infusions). As well, missing data were completed with this on-site visit. The survival of these excluded patients was not statistically different from the patients reported in the analysis (data not shown).
Inclusion criteria
The following criteria were required: (1) Diagnosis of HS was confirmed by an increase in urinary glycosaminoglycan excretion, a deficiency or absence of α-l-iduronidase in peripheral blood leukocytes, and the clinical phenotype; (2) transplantation with either a HLA-matched sibling donor (MSD), a HLA-matched or mismatched unrelated donor (UD) (either T-cell–replete or T-cell–deplete [TCD-TUD]), or a nonexpanded, single, unmanipulated, UCB unit; (3) complete clinical data with at least 3 months of posttransplant follow-up; and (4) transplants using a myeloablative conditioning regimen performed between 1995 and 2007. Myeloablative conditioning in this nonmalignant disease was defined as a cumulative busulfan dose >14 mg/kg (either oral or intravenous: with or without therapeutic drug monitoring) or total body irradiation (TBI) >7 Gy delivered unfractioned or >10 Gy delivered in fractioned doses.
Definitions and endpoints
The primary endpoints were: (1) event-free survival (EFS), defined as survival from transplantation to last contact: autologous reconstitution (defined by documentation of <10% donor-derived engraftment), graft failure (defined as a lack of neutrophil recovery or transient engraftment of donor cells after transplantation and/or a requirement for a second transplant), or death were considered as events; (2) overall survival (OS) was defined as time from transplantation to death. All surviving patients were censored at date of last contact. Other endpoints reported include: (1) rate of neutrophil recovery, defined as the first day of achieving a neutrophil count of ≥0.5*10e9/L for 3 consecutive days, (2) incidence of acute and chronic graft-versus-host disease (GVHD), and (3) donor chimerism and enzyme level at most recent follow-up. Acute GVHD grade II-IV at day 100 was diagnosed and graded according to published criteria13 and chronic GVHD at 5 years was graded according to standard criteria14 and evaluated in patients who survived at least 100 days with sustained engraftment.
Donor-recipient HLA match
HLA matching in the UD recipients was scored using the classification of either low-resolution (LR)/intermediate-resolution (IR) or high-resolution (HR) typing. For CB, HLA matching was scored using LR or IR typing for HLA Class I (A and B) and HR typing for HLA Class II (DRB1). UD donors were grouped based on typing as follows: (1) Matched on HR (10/10), (2) mismatch on HR (7-9/10), and (3) any match grade on either LR or IR. UD recipients receiving an “ex vivo T-cell–depleted graft” (independent of the method used: not enough information available) were scored and grouped separately.
Donor-recipient chimerism
Chimerism was reported on the basis of data available at any time during the first 3 months after HCT and at the last assessment. Full-donor chimerism (on whole blood) was defined as ≥95% donor-derived hematopoietic cells, mixed-chimerism as 10% to 94% donor-derived hematopoietic cells were of donor origin and autologous recovery if <10% of hematopoietic cells and autologous recovery if <10% of hematopoietic cells were donor-derived. Data on the methodology of chimerism detection were not available. Enzymes were regarded as “normal” if they were within the range of normal according to the institutional reference range and “low” if the value was below the lower limit of normal but not within the range used to define MPS I.
Statistical analysis
The duration of follow-up was the time to the last assessment for surviving patients or death. To analyze risk factors for outcomes, we considered patients’ related factors (ie, median age at transplant, gender), the disease (ie, median interval time from diagnosis to transplant), use of “enzyme replacement therapy” before transplant (eg, at least 4 doses), the donor (ie, source, donor relationship, HLA-disparity, HR-typed or LR/IR-typed, T-cell depletion, and for CB median collected and infused total nucleated cell and CD34+cell doses), and the transplant (ie, year of transplant, conditioning regimen and the type of GVHD prophylaxis). Because of sample size, the median interval between diagnosis and HCT for CB median-collected and infused, nucleated cells and CD34+ was taken to dichotomize the group. Cumulative incidence estimates were calculated for neutrophil recovery and acute and chronic GVHD, with death as the competing event.15 Gray’s test was used for univariate comparisons.16 Probabilities of EFS and OS were calculated using the Kaplan-Meier estimate; the two-sided log rank test was used for univariate comparisons.
HLA (including type of donor) and CB cell dose were analyzed as prognostic factors for EFS and taken into consideration if a P value < .20 was observed. Factors associated with a P value < .10 by univariate analysis (described in the Results section) and other relevant factors, were included in multivariate analyses using Cox proportional hazards for EFS and OS, logistic regression for donor chimerism and enzyme levels (at latest follow-up), and the proportional subdistribution hazard regression model of Fine and Gray for neutrophil recovery.17 Then a stepwise regression was performed using a threshold of .05. All tests were two-sided. The type I error rate was fixed at .05 for determination of factors associated with time-to-event outcomes. Risk factor analysis for chronic GVHD was not performed because of the relative small number of events. Statistical analyses were performed with SPSS Inc. (IBM, Armonk, NY) and S-Plus (MathSoft, Inc., Seattle, WA) software packages.
Results
Patient, donor, and transplant characteristics
Two-hundred fifty-eight patients with HS receiving HCT between 1995 and 2007 were analyzed. The baseline patient, donor, and transplantation characteristics are shown in Table 1. The median age at transplant was 16.7 months. Nineteen percent (n = 48) of patients received intravenous ERT (at least 4 infusions before transplant). The most common graft source used was UCB (n = 116: mainly HLA-mismatched, 81%), followed by UD (n = 105) and MSD (n = 37). Approximately half of the patients receiving from a UD were “ex vivo” T-cell–depleted (n = 53). One-hundred thirty-eight patients in this report have also been in previously published studies (but updated for this analyses).7,10,12
The majority of children were conditioned with a busulfan-containing myeloablative chemotherapy (>97%), primarily busulfan/cyclophosphamide (83%; Table 1). Serotherapy, either anti-thymocyte globulin (ATG) (n = 207) or alemtuzumab (Campath-1H; n = 21), was also given to the majority of the patients (89%). All patients who received GVHD prophylaxis received a cyclosporine-containing regimen. Depending on cell source, patients received steroids (n = 76) or mycophenolate mofetil (MMF) (n = 16; in recipients of CB), or methotrexate (MTX) for UD or MSD (n = 74). The majority of the T-depleted recipients received no additional GVHD prophylaxis (n = 30). Given their young age, the patients receiving a CB graft underwent transplantation with relatively large doses of CB cells; the median total nucleated cell dose was 8.8 × 107 cells/kg, with a median of 3.0 × 105/kg CD34+ dose based on precryopreservation counts.
Neutrophil and platelets recovery and chimerism
Cumulative incidence of neutrophil recovery at day 60 was 91 ± 2% (Table 2), with 87 ± 2% engrafting at or before day 42. The median time to neutrophil recovery was 19 days (range, 9-60). In univariate analysis, the probability of neutrophil recovery was associated with stem cell source and then type of donor/donor type and patient age. The probability of neutrophil recovery at day 60 was 98% ± 4% for patients receiving a MSD- or HLA-matched UCB (6/6) transplant, and it was 94 ± 3% in recipients of a matched UD (MUD) graft, 91 ± 3% after a HLA-mismatched or T-cell–depleted UD transplant, 88 ± 4% after a 5/6 antigen-matched UCB transplant, and 79 ± 6% in those receiving a 4 of 6 antigen-matched UCB graft (P ≤ .001). Patient age was also associated with neutrophil recovery: recovery was 95 ± 3% for patients younger than 16.7 months compared with 88 ± 5% for older patients (P = .002). Multivariate analysis confirmed that both factors described were independently associated with neutrophil recovery. In UCB recipients, younger age (P = .01) and NC dose (> median; P = .038) were independent factors affecting engraftment. Cumulative incidence of platelet recovery at 180 days was 80 ± 3%.
Forty-nine patients had either autologous reconstitution (n = 18; 7%) or secondary graft failure (n = 31; 12%) during the first 3 months after HCT (Table 2). Chimerism data at last assessment were available for 157 patients of 164 “alive and engrafted” patients: 125 of them were full chimeras (79%) and 32 (21%) had mixed chimerism. The use of UCB as a stem cell source was associated with a higher rate of full-donor chimerism and normal enzyme levels at latest follow-up (median: 57 months). In fact, 98% of the “alive and engrafted” CB recipients had normal enzyme levels compared with 50% to 66% in the other 3 groups (P = .039; Table 3), reflecting higher rates of full engraftment in the CB cohort.
Acute and chronic GVHD
The cumulative incidence of acute GVHD II-IV at day 100 was 25 ± 3% (n = 65), with the majority of these patients having grade II GVHD (n = 46, 18%), grade III GVHD (n = 9, 3%), and grade IV (n = 10, 4%). In univariate analysis, cumulative incidence of grade II-IV GVHD was higher (P = .01) in patients receiving a UCB 5/6 (36 ± 3%) or 4/6 (33 ± 3%) compared with UCB 6/6 (19 ± 9%), MSD (19 ± 7%), HLA-MUD (15 ± 5%), and HLA-mismatched UD (22 ± 6%). The cumulative incidence of aGVHD was 17% for “ex vivo T-cell–depleted” matched and mismatched UD. No other factor analyzed was associated with an increased risk of acute GVHD II-IV. Concerning aGVHD grade III-IV, the overall CI was 8 ± 2%: 5% for MSD, 9% for CB recipients, and 6% for UD. The CI of chronic GVHD at 5 years was 16 ± 2% (n = 33: 17 patients extensive and 16 patients limited). By cell source, the incidence of cGVHD was not different among the cell source groups (P = .49): 10% for MUD (for TCD 7%, non-TCD 11%), 29% for CB, and 3% for sibling.
EFS and OS
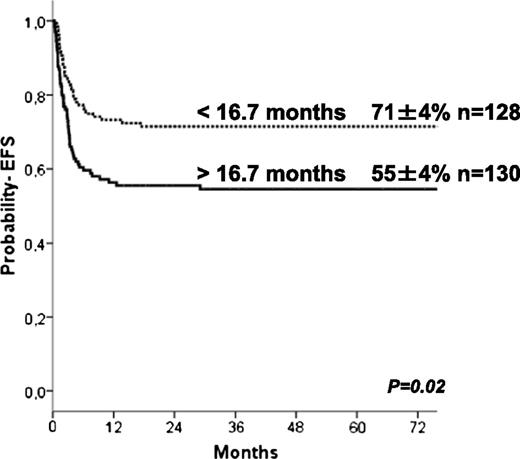
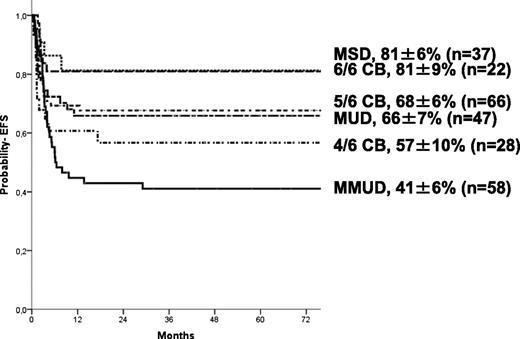
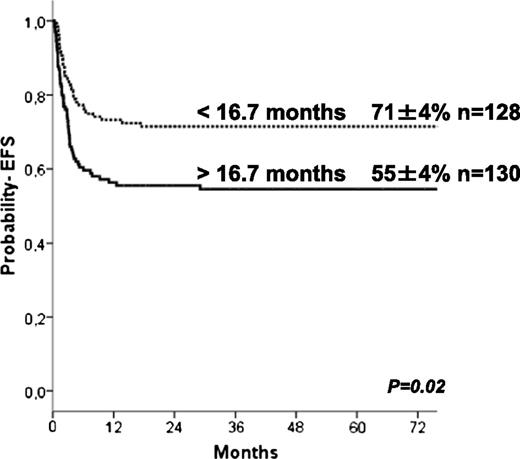
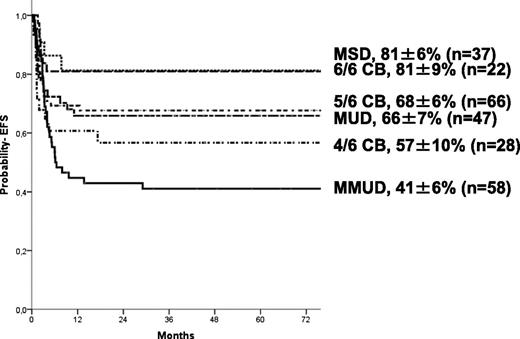
The 5-year estimate probabilities of EFS and OS after HCT in this series were 63 ± 3% and 74 ± 3%, respectively. In univariate analyses, the EFS rate was higher in younger patients (age at transplant less than the median of 16.7 months (P = .024; Figure 1), in those who underwent transplant more recently (after 2004: P = .01), and those who underwent transplant with a well-matched CB or related donor graft (MSD, 6/6 UCB, P = .009; Table 2). Patients with previous ERT showed a trend to significantly higher EFS (P = .07) in univariate analysis but not in multivariate analyses (Table 4). In regards to HLA typing and type of donor, transplantation using MSD, or fully-matched UCB (6/6) grafts, the 5-year estimated probability of EFS was 81%, whereas after 10/10 MUD and 5/6 matched UCB, EFS rates were 66% and 68%, respectively. Four of 6 matched UCB and mismatched HLA UD grafts were associated with lower 5-year probability of EFS (57% and 41%, respectively; Figure 2) compared with the index group (MSD/matched UCB). For patients receiving an ex-vivo T-cell–depleted UD transplant (43/53 were mismatched HLA UD), EFS was 36% compared with 69% in unmanipulated UD. Importantly, multivariate analysis (adjusted for year of transplantation and previous ERT) demonstrated that both the age at transplantation (younger than 16.7 months) and cell source affected EFS: 10/10 UD or 5/6 UCB showed a trend (P = .07 when grouped) to lower EFS compared with MSD and 6/6 UCB, whereas EFS was lower after 4/6 UCB (P = .031) and HLA-mismatched UD (P = .007; Table 4). ERT peri-HSCT did not influence the endpoint EFS (P = .775) in multivariate analyses. Also, year of HCT (< or >2004) was not significant in multivariate analyses (P = .157). Thirty-five patients underwent subsequent transplantation using either the same (n = 12) or a different donor (n = 19; 4 missing). The median interval to the second HCT was 207 (range, 36-630) days. A variety of conditioning regimens was used (eg, busulfan-based 16; fludarabine-based 7; all either ATG or Campath). Twenty-seven patients achieved donor engraftment in a median time of 16 days (range, 6-26), of whom 22 of 35 (63%) patients are “alive and engrafted” with donor cells with a median follow-up of 54 months.
Causes of death
Sixty-four patients (25%) died within 5 years post stem cell transplantation, 60 from a transplant-related cause (11 viral infection, 11 multi-organ failure, 10 GVHD, 6 hemorrhage, 4 idiopathic pneumonia syndrome/diffuse alveolar hemorrhage, 1 sepsis, 1 fungal, 13 other), and 3 from disease progression after graft failure. In 4 patients, the cause of death was unknown.
Discussion
We identified 2 factors associated with EFS in patients with HS undergoing HCT: age at transplantation and donor/graft source, which also considered donor-recipient HLA-match. EFS was significantly higher when transplantation was offered before 17 months of age. This supports the need for early referral for transplantation in infants diagnosed with HS, and for families with an index case, these data support perinatal screening. In addition, our data also support proceeding to transplantation with an alternative donor in the absence of an HLA-matched sibling. Such a donor should preferably be a 6/6 or 5/6 HLA-matched UCB or a 10/10 HLA-matched unrelated adult donor. Although we did not identify a NC dose threshold above which outcomes were superior, the cell content of the UCB unit is particularly important when considering UCB grafts. Others have shown that the minimal acceptable NC dose should be 5 × 107/kg when selecting mismatched UCB units for nonmalignant indications.18 In the current analysis, although the median TNC is 8.8 × 107/kg and only 13% of patients received NC dose <5 × 107/kg, this explains why we were unable to detect a NC dose threshold. With regard to hematopoietic recovery, however, younger age at transplantation as well as CB cell dose are important factors affecting engraftment after UCBT.
As with EFS, neutrophil recovery was also associated with patient age and donor/graft source: it was highest in MSD and 6/6 matched UCB (98%), whereas it was lowest in 4/6-matched UCB (79%) and higher among patients younger than 16 months (95% vs 88%). A limitation of the current analysis is a detailed and thorough examination of chimerism and its association with donor/graft source. The timing of chimerism and enzyme assay and comparative norms were transplant center–dependent and impossible to address when using registry data. Centralized measuring would obviously be superior. Nevertheless, our observation support that almost all “alive and engrafted” CB recipients achieved sustained full-donor chimerism and normal enzyme levels in circulating leukocytes (at most recent follow-up), which was in line with previous CB studies in patients with an IEM.8,,,-12 In the UD and MSD setting, mixed-chimerism was seen in 30% to 50% of the patients, leading to lower enzyme levels. Although information on the carriership (1 mutated gene) of the MSDs is lacking, it is expected that the majority will be carrier, which will lead to the observed lower enzyme levels in engrafted MSD patients, as has been shown by others.19 Others have also reported that higher enzyme levels are associated with better metabolic correction19 and improved long-term functional outcomes of children with HS.6,20,21 Although it is not the focus of the current analysis, an ongoing international study by our group is examining the association between enzyme levels and long-term functional outcome. The observed better engraftment after UCB transplants is difficult to explore in the current analysis. Nevertheless, this merits further examination in a population of patients receiving a homogeneous conditioning regimen and GVHD prophylaxis regimens with defined time-points for chimerism assay.
A European report (n = 146), mainly analyzing blood marrow (BM) grafts, showed that predictors for graft failure were “reduced intensity conditioning” and T-cell depletion.7 For myeloablative, T-replete BM transplantation, EFS was approximately 70%. Reports for CB transplantation suggest similar 3-year OS and EFS of 77% and 70%, respectively.12 Together, these data support alternative-donor HCT for HS when a HLA-matched sibling is not available. Although the current analysis supports superior EFS after 6/6 HLA-matched UCB, caution is necessary when interpreting data. In the unrelated setting, a 6/6 HLA-matched UCB unit containing a minimum NC dose of 5 × 107/kg may be preferred over a 10/10 HLA-matched adult UD.12,22 In the absence of a fully-matched donor or when the fully matched adult UD is not readily available, mismatched UCB units are a suitable alternative. However, it should be taken into account that the acute GVHD risks are higher after mismatched (CB) transplants. In the absence of a HLA-matched sibling or fully-matched UCB unit or adult UD, delaying HCT is unlikely to improve EFS, given the timing of HCT is an important determinant of success. Transplantation occurring beyond 17 months from birth had an adverse effect on EFS.10,12 Offering transplantation before organ involvement is an important determinant for a successful outcome because full-intensity conditioning regimens predict higher EFS.7,8,12 Others have suggested bridging for a short period with ERT to allow for some recovery so that these patients are able to tolerate full-intensity conditioning regimens. We showed, as others have in smaller series, that ERT was not found to be an influencing predictor for any of the endpoints (including engraftment; data not shown).23,24 In multivariate analyses, the year of transplantation (or time period) did not impact the survival rates. Furthermore, those who failed a first transplant showed satisfactory outcome after a second transplant (EFS 63%), indicating that this too should be considered in the event of graft failure.
Taken together, our findings extend and confirm those reported by others. Early referral for HCT, with the best available HLA-matched donor, offers the best EFS. UCB units are particularly attractive because they are readily available, but for mismatched-CB recipients, higher GVHD rates may be a concern. Increasing the CB inventory and better matching possibilities (including HR typing of the CBU) might improve these outcomes. Early referral implies relatively young patients and therefore identifying adequately dosed UCB units is an unlikely barrier. It is important to recognize that most MSDs are carriers and influence posttransplant enzyme levels. Completion of the ongoing study, an international collaborative effort to correlate enzyme levels with functional outcome, may further refine donor selection for clinical practice. In the absence of these data, the ideal donor is a MSD or a fully-matched UCB or an adult UD, and when an ideal donor is not available, mismatched transplantation in a timely manner is a suitable alternative.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the collaborators for sharing patient information: Teresa Kivisto (University of Minnesota, Minneapolis, MN), Mary Coussons (data manager, Manchester, UK), Anne Gahan (data manager, Dublin, UK), Isabelle Hirsch (data manager, Necker Hospital, Paris, France), Dr Stefania Varotto (Padua, Italy), Dr Vicky Borbon (Ghent, Belgium), Dr Petr Sedlacek (Prague, Czech Republic). and Dr José Sánchez de Toledo Codina (Barcelona, Spain).
Authorship
Contribution: J.J.B., M. Eapen, E.G., and V.R. designed and supervised the study; J.J.B. and M.A. wrote the manuscript; J.J.B., D.P., and A.R. analyzed and interpreted the data; M.A. visited the transplant centers for data collection; A.R. and V.R. performed statistical analysis; M. Eapen, M.C.-C., A.F., J.T., E.G., P.J.O., and J.K. contributed to the critical revision of the manuscript; M.A., T.D., R.W., E.W., M.C.-C., A.R., A.F., J.T. V.K.P., M. Escolar, A.O., P.J.O., P.V., and J.K. contributed to the acquisition of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Eurocord, Inborn Errors Working Party of European Blood and Marrow Transplant group, Duke University, and the Centre for International Blood and Marrow Research appears in “Appendix: study group members.”
Correspondence: Jaap Jan Boelens, Department of Pediatrics, Pediatric Blood and Marrow Transplantation Program, Room KC 03.063.0, University Medical Center Utrecht, Lundlaan 6, 3584 EA, Utrecht, The Netherlands; e-mail: j.j.boelens@umcutrecht.nl.
Appendix: study group members
The members of the Eurocord, Inborn Errors Working Party of European Blood and Marrow Transplant group, Duke University, and the Centre for International Blood and Marrow Research are: Members of study group CIBMTR: http://www.cibmtr.org/about/proceduresprogress/documents/2012_cibmtr_annual_r.pdf; Co-chairs working committee inborn errors: Paul Veys (London, UK); Harry Malech (NIH); Edwin Horwitz (Philadelphia); Scientific director: Mary Eapen (CIBMTR, Milwaukee); PhD Statistician: Jennifer Le-Rademacher (CIBMTR, Milwaukee); and MS Statistician: Wensheng He (CIBMTR, Milwaukee).
Members of study group EBMT: http://www.ebmt.org/Contents/Members-Sponsors/Members/MembershipList/Pages/Membership-List.aspx; Co-chairs of the working party inborn errors: Bobby Gaspar (London, UK) and Fulvio Porta (Brescia, Italy).
Members of study group Eurocord: Eliane Gluckman (Paris, France); Annalisa Ruggeri (Paris, France); and Vanderson Rocha (Paris, France; Oxford, UK).
Members of study group Duke University (Blood and Marrow Transplantation Program): Timothy A. Driscoll; Joanne Kurtzberg; Daniel B. Landi; Paul L. Martin; Kristin M. Page; Suhag H Parikh; and Vinod K. Prasad.