Key Points
IL1RAP is overexpressed on candidate AML stem cells and is a promising target for antibody-based therapy.
Abstract
IL1RAP, a co-receptor for interleukin (IL)-1 and IL-33 receptors, was previously found to be highly upregulated on candidate chronic myeloid leukemia stem cells, allowing for leukemia-selective killing using IL1RAP-targeting antibodies. We analyzed IL1RAP expression in a consecutive series of 29 patients with acute myeloid leukemia (AML) and, based on the level of expression in mononuclear cells (MNCs), we divided the samples into 3 groups: IL1RAP low (n = 6), IL1RAP intermediate (n = 11), and IL1RAP high (n = 12). Within the CD34+CD38− population, the intermediate and high groups expressed higher levels of IL1RAP than did corresponding normal cells. With the aim to target AML stem cells, an anti-IL1RAP monoclonal antibody was generated followed by isotype switching for improved antibody-dependent, cell-mediated cytotoxicity activity. Using this antibody, we achieved selective killing of AML MNC, CD34+CD38+, and CD34+CD38− cells. Our findings demonstrate that IL1RAP is a promising new therapeutic target in AML.
Introduction
The interleukin-1 receptor accessory protein (IL1RAP) is a co-receptor of the IL1 and IL33 receptors and has so far mainly been studied in the context of inflammatory responses.1,2 In a recent study, we demonstrated that IL1RAP is upregulated on the cell surface of candidate chronic myeloid leukemia (CML) stem cells, allowing for the first time a prospective isolation of such cells using fluorescence-activated cell sorting (FACS).3 By generating polyclonal antibodies against IL1RAP, we further showed that candidate CML stem cells expressing this receptor could be killed by antibody-dependent, cell-mediated cytotoxicity (ADCC), while sparing corresponding normal hematopoietic stem cells.
Despite the promising role of IL1RAP as a target in CML, it was until recently unknown whether IL1RAP is overexpressed also on stem cells of other hematological malignancies. However, a recent study focusing on acute myeloid leukemia (AML) displaying monosomy 7 identified IL1RAP as upregulated on candidate AML stem cells.4 Interestingly, IL1RAP upregulation at the transcriptional level in AML and myelodysplastic syndrome was associated with poor prognosis.4 The functional role of IL1RAP in AML is currently unknown, but previous studies have demonstrated that IL-1 signaling supports proliferation of AML cells and that this effect can be blocked by an IL-1 receptor antagonist.5-7
Herein, we investigated IL1RAP cell surface expression in a consecutive series of AML samples of various genetic subtypes. Of these patients, a large fraction (79%) expressed IL1RAP and, importantly, both leukemic (mononuclear) bulk cells and leukemic stem cell and progenitor-enriched cell fractions could be killed ex vivo by ADCC using a newly generated monoclonal antibody against IL1RAP.
Study design
Samples
At the time of diagnosis, bone marrow (BM) or peripheral blood (PB) from a consecutive series of AML patients and healthy donors was collected after informed consent according to a protocol approved by the regional ethics committee. This study was conducted in accordance with the Declaration of Helsinki. The samples were separated on Lymphoprep (Axis-Shield PoC AS) and mononuclear cells (MNC) were viably frozen. For details on the flow cytometric analysis, see supplemental Methods, Flow cytometry, on the Blood Web site.
ADCC, in vitro culture, and in vivo repopulation analysis
The ADCC assay was performed as previously described,3 using monoclonal antibody (mAb) 81.2 (see supplemental Methods for details of antibody production) or human IgG1 isotype control (Eureka Therapeutics). A total of 5000 AML cells was used; either unsorted MNCs or stained with anti-CD34-APC and anti-CD38-PECy7 (BD) mAbs and sorted with a FACSAria (BD). The AML cells were cultured overnight together with mAbs and 100 000 freshly isolated natural killer (NK) cells from healthy donors. The next day, cells were analyzed on a FACSCanto to measure the frequency of dead cells (7AAD+). KU812 cells were used in parallel as a positive control. Because of interexperimental variance because of different NK cell donors, all values were normalized to the rate of cell death obtained using KU812 cells. Details regarding the in vitro culture and in vivo repopulation experiments are outlined in supplemental Methods.
Results and discussion
AML is a genetically and clinically heterogeneous malignancy with poor overall survival; thus, there is a demand for new therapeutic strategies. Herein, we investigated whether IL1RAP is upregulated on various populations of AML cells in a consecutive series of 29 non-acute promyelocytic leukemia AML patients (Table 1) and if such cells could be selectively killed ex vivo by ADCC.
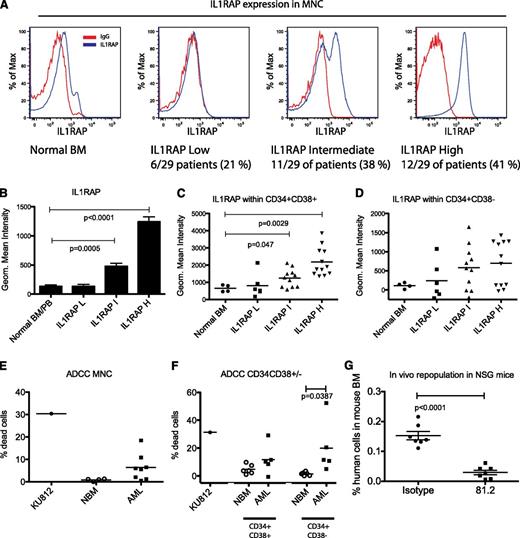
By analyzing IL1RAP expression on MNCs, we could distinguish three subgroups among the AML patients defined as: low (IL1RAP-L), intermediate (IL1RAP-I), and high (IL1RAP-H) (Figure 1A-B). BM and PB from healthy donors all exhibited IL1RAP levels similar to the IL1RAP-L group. These results show that in a majority of the AML patients (23 of 29, 79%), IL1RAP is expressed in the bulk leukemic population. Although additional studies involving more patients are needed to determine a possible correlation between IL1RAP expression and molecular subtype of AML, it is of interest to note that NPM1 mutations, the most common genetic abnormality in AML, was associated with the IL1RAP-H group (Table 1).
IL1RAP is overexpressed in AML and can be used as a target for selective antibody-mediated killing of AML cells. (A) AML patients were subdivided into 3 subgroups based on IL1RAP expression levels in mononuclear cells (MNC): IL1RAP low (IL1RAP-L), IL1RAP intermediate (IL1RAP-I), and IL1RAP high (IL1RAP-H). (B) Geometric mean fluorescence intensity (MFI) of IL1RAP expression within the IL1RAP-L, IL1RAP-I, and IL1RAP-H groups compared with normal BM and PB samples. Mean and standard error of the mean is presented. (C) Geometric MFI of IL1RAP expression within CD34+CD38+ cells from normal BM or AML patients. (D) Geometric MFI of IL1RAP expression within CD34+CD38− cells from normal BM or AML patients. (E) Frequency of dead cells (measured as % 7AAD+ cells after subtracting % 7AAD+ cell in the corresponding isotype control group) in an ADCC assay of normal BM MNC and MNC from AML patients (AML 17, 19-23, 27, and 29). The KU812 cell line with a high IL1RAP expression was used as a positive control. (F) Frequency of dead cells in an ADCC assay of sorted CD34+CD38− and CD34+CD38+ cells from normal BM or AML MNCs. Based on availability of cells, we selected 3 patients from the IL1RAP-I group (AML 7, 9, and 10) and 1 patient from the IL1RAP-L group (AML 2). (G) Engraftment of human AML cells (CD45+) in BM of immunodeficient mice, following in vitro ADCC using mAb 81.2 or an isotype control. Flow cytometry was performed at time of sacrifice 3 weeks posttransplantation.
IL1RAP is overexpressed in AML and can be used as a target for selective antibody-mediated killing of AML cells. (A) AML patients were subdivided into 3 subgroups based on IL1RAP expression levels in mononuclear cells (MNC): IL1RAP low (IL1RAP-L), IL1RAP intermediate (IL1RAP-I), and IL1RAP high (IL1RAP-H). (B) Geometric mean fluorescence intensity (MFI) of IL1RAP expression within the IL1RAP-L, IL1RAP-I, and IL1RAP-H groups compared with normal BM and PB samples. Mean and standard error of the mean is presented. (C) Geometric MFI of IL1RAP expression within CD34+CD38+ cells from normal BM or AML patients. (D) Geometric MFI of IL1RAP expression within CD34+CD38− cells from normal BM or AML patients. (E) Frequency of dead cells (measured as % 7AAD+ cells after subtracting % 7AAD+ cell in the corresponding isotype control group) in an ADCC assay of normal BM MNC and MNC from AML patients (AML 17, 19-23, 27, and 29). The KU812 cell line with a high IL1RAP expression was used as a positive control. (F) Frequency of dead cells in an ADCC assay of sorted CD34+CD38− and CD34+CD38+ cells from normal BM or AML MNCs. Based on availability of cells, we selected 3 patients from the IL1RAP-I group (AML 7, 9, and 10) and 1 patient from the IL1RAP-L group (AML 2). (G) Engraftment of human AML cells (CD45+) in BM of immunodeficient mice, following in vitro ADCC using mAb 81.2 or an isotype control. Flow cytometry was performed at time of sacrifice 3 weeks posttransplantation.
Because it previously has been shown that leukemic stem cells (LSCs) in AML most often reside among the CD34+CD38− and CD34+CD38+ cells,8-11 both populations were analyzed for IL1RAP expression. Within the CD34+CD38+ population, cells from IL1RAP-L patients exhibited IL1RAP expression comparable to normal BM. However, a significant increase in IL1RAP expression was seen in the IL1RAP-I and IL1RAP-H groups (Figure 1C). For the most primitive cells, enriched in the CD34+CD38− population, there was a clear tendency of increased IL1RAP levels in both the IL1RAP-I and IL1RAP-H groups (Figure 1D). Because the most primitive cell compartment often contains substantial numbers of normal cells, we speculate that low IL1RAP expression within the CD34+CD38− cells in certain patients from the IL1RAP-I and IL1RAP-H groups reflect larger numbers of normal cells, but this remains to be addressed. Altogether, these results indicate that IL1RAP is upregulated on both immature and mature AML cells.
For the patients in the IL1RAP-H group, the majority of the MNCs (mean 68%, data not shown) expressed IL1RAP. This prompted us to investigate whether we could target the AML cells using an immunotherapy-based approach with a newly generated chimeric anti-IL1RAP mAb (81.2) of human IgG1 subtype, allowing for improved ADCC activity. BM MNCs from healthy donors expressed low levels of IL1RAP (Figure 1A) and, as expected, the frequency of antibody-induced cell death in the ADCC assay was almost undetectable (Figure 1E). In contrast, when MNCs from 8 patients within the IL1RAP-H and IL1RAP-I groups were analyzed, a distinct ADCC effect was observed within the majority of the samples (Figure 1E). To investigate whether it is possible to also target candidate LSCs, ADCC was performed on sorted CD34+CD38+ and CD34+CD38− cells. Whereas low ADCC was observed in normal BM CD34+CD38+ and CD34+CD38− cells, a substantially higher ADCC was observed in corresponding AML cells (Figure 1F).
To further evaluate the cellular consequences of targeting IL1RAP on AML cells, we performed ADCC on primary CD34+ AML cells (AML 7) followed by colony assay, liquid culture, and transplantation of the cells into immunodeficient mice. Although a modest ADCC effect of around 5% was detected 24 hours after adding of the NK cells and mAb 81.2, the treated AML cells did not engraft immunodeficient mice, demonstrating an almost complete eradication of AML cells (Figure 1G; supplemental Figure 1A). Similarly, liquid culture and colony-forming assays confirmed the strong depletion of AML cells after ADCC (supplemental Figure 1B-C).
Because mAb 81.2 does not inhibit IL-1 signaling as measured in a routine IL-1 reporter cell line assay (data not shown), we speculate that the mechanism of cell killing is likely mediated by NK cells. Given the demonstrated antiproliferative effects following inhibition of IL-1 signaling in AML,5-7 we are currently focusing on generating new anti-IL1RAP mAbs that both block IL-1 signaling and potently induce ADCC.
In conclusion, our results show that a clear majority of the AML patients in our series (23 of 29, 79%) express IL1RAP on the cell surface of leukemic cells. Importantly, antibody-based targeting of IL1RAP could be used to selectively kill IL1RAP-expressing leukemic blasts and LSC-enriched populations, while sparing corresponding normal hematopoietic stem and progenitor cells. These findings indicate that IL1RAP is a promising new therapeutic target in AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kjell Sjöström (Innovagen AB, Lund, Sweden) for producing mAbs, Agneta Svedberg (Cantargia AB, Lund, Sweden) for providing the 81.2 mAb, and Ingbritt Åstrand-Grundström (Stem Cell Center, Lund University, Lund, Sweden) for assistance in the collection of patient material.
This work is supported by the Swedish Cancer Society, the Swedish Children’s Cancer Foundation, the Inga-Britt and Arne Lundberg Foundation, the Gunnar Nilsson Cancer Foundation, the Medical Faculty of Lund University, and Swedish Research Council Hemato-Linné and BioCARE strategic research program grants and a project grant (T.F.).
Authorship
Contribution: M.A., H.Å., N.H., A.A., G.J., J.R., M.J., and T.F. designed the study; M.A., H.Å., N.H., S.G., and M.R. performed experiments; A.A., G.J., and J.R. collected patient material and provided clinical data; M.A., H.Å., N.H., A.A., G.J., J.R., M.J., and T.F. analyzed results; M.A., H.Å., N.H., and T.F. wrote the paper; and S.G., A.A., M.R., G.J., J.R., and M.J. suggested improvements to the manuscript.
Conflict-of-interest disclosure: M.J. and T.F. are cofounders and have equity ownership in Cantargia AB (Ideon Medical Village, Lund, Sweden) formed with Lund University Bioscience AB. Cantargia AB is the owner of the intellectual property rights for agents targeting IL1RAP for use in the treatment and diagnosis of neoplastic hematologic disorders. J.R. has stock options in Cantargia. The remaining authors declare no competing financial interests.
Correspondence: Thoas Fioretos, Department of Clinical Genetics, Lund University, BMC B13, Klinikgatan 26, SE-221 84 Lund, Sweden; e-mail: thoas.fioretos@med.lu.se.