The study by Topp et al in this issue of Blood confirms that blinatumomab can significantly reduce the relapse risk and improve survival in patients with minimal residual disease (MRD)+ B-precursor acute lymphoblastic leukemia (ALL).1
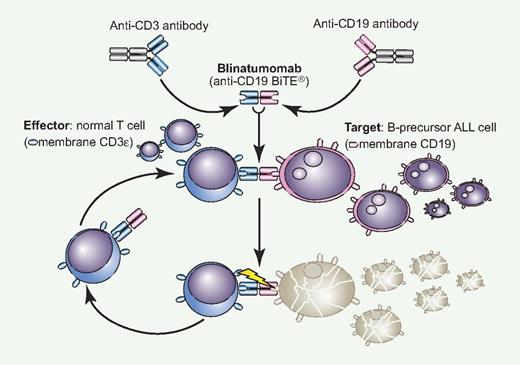
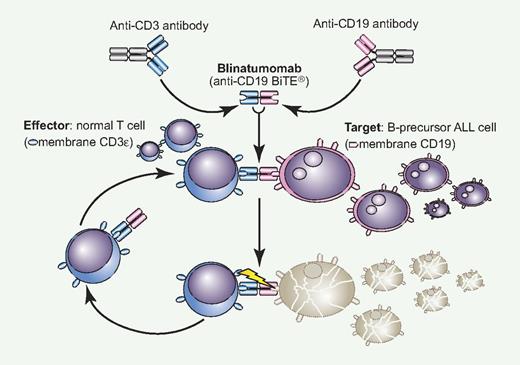
Blinatumomab is the result of an original translational research project leading to the production of BiTE (Bispecific T-cell Engager) antibodies. A BiTE monoclonal antibody (MoAb) is a novel construct with 2 variable regions, one specific to CD3 for T-cell recruitment and activation and the other targeting a different leukemic or neoplastic membrane antigen. With blinatumomab, CD19+ blast cells of B-precursor ALL are linked to CD3+ T cells and subject to perforin-mediated cytotoxicity (see figure).2 Conceptually, this is immunotherapy at its best because autologous effectors cells are brought into direct contact with the target and nothing else. This is a key distinction with the immune effects provided by an allogeneic stem cell transplantation, where unrestricted T-cell activation can lead to the serious clinical consequences of graft-versus-host disease. Blinatumomab, literally B-lineage antitumoral MoAb, is active at a very low concentration, easily achievable in the bone marrow microenvironment, and can induce serial killing with an optimal E/T ratio of 1:90. These are desirable goals in this disease. For example, in in vitro experiments against MEC-1 target cells, incubation with blinatumomab 101 pg/mL induced a 50% cell lysis, compared with 20% using rituximab 103 pg/mL.
Blinatumomab is a novel bispecific construct that reacts simultaneously to normal CD3+ T cells and CD19+ ALL cells, creating a tight intercellular connection followed by T cell–mediated cytotoxicity exerted on CD19+ blast cells (BiTE mechanism).2 The drug is active at very low concentration and once cell lysis is completed, the effector-blinatumomab complex is released to start over again. With single-agent blinatumomab, a complete and durable molecular remission was observed in approximaely 70% of adult patients with MRD+ ALL (see article by Topp et al1 on page 5185), and a similar activity is also being reported in relapsed ALL. Professional illustration by Paulette Dennis.
Blinatumomab is a novel bispecific construct that reacts simultaneously to normal CD3+ T cells and CD19+ ALL cells, creating a tight intercellular connection followed by T cell–mediated cytotoxicity exerted on CD19+ blast cells (BiTE mechanism).2 The drug is active at very low concentration and once cell lysis is completed, the effector-blinatumomab complex is released to start over again. With single-agent blinatumomab, a complete and durable molecular remission was observed in approximaely 70% of adult patients with MRD+ ALL (see article by Topp et al1 on page 5185), and a similar activity is also being reported in relapsed ALL. Professional illustration by Paulette Dennis.
The long-term results of the first single-agent blinatumomab trial carried out by the German Multicenter Study Group for Adult ALL in MRD+ ALL are presented here. The prognostic role of MRD in adult ALL is widely recognized3 as the most sensitive individual indicator of the relapse risk, whatever the treatment choice (chemotherapy or transplantation) and clinical risk class. MRD+ patients relapse within a few weeks to months, especially when the MRD amount measured molecularly is equal to or greater than 10−4 at treatment weeks 10-22.4,5 In the study by Topp et al,1 16 of 20 MRD+ patients responded completely to blinatumomab. Their response was durable, with or without consolidation by stem cell transplantation, and the majority of them remained disease free for more than 24 months. The data presented here support that blinatumomab strikes hard at MRD, should be applicable to virtually all cases of B-precursor ALL, is noncross-resistant with chemotherapy and transplantation, is possibly synergistic to both, and is effective in all disease subsets, including Philadelphia chromosome-positive ALL carrying the T315I mutation and older patients, in whom neither intensive chemotherapy or transplantation are an optimal choice because of lack of efficacy and/or toxicity. The study was numerically limited and requires confirmation in a larger patient cohort (as already planned/performed). It also demonstrated potential drawbacks, like the occurrence of neurologic toxicity in a fraction of patients (which appears transient and may be preventable) and the occasional progression of ALL at extramedullary sites (meninges, testes) due to insufficient drug penetration, or selection of CD19-negative ALL subclones.
While these problems need to be resolved, the results should prompt evaluation of blinatumomab in relapsed/refractory disease and, if promising, in frontline treatment. Preliminary data in patients with overt disease confirmed high response rates (complete clinical and molecular eradication of ALL observed in 17 of 25 patients, 68%), with a 4-log and greater reduction of the ALL tumor load after a single treatment cycle.6
If one considers that in untreated adult ALL complete remission (CR) rates to single agents are low (36% with corticosteroids, 40% with vincristine or cytarabine, 14% with methotrexate, 8% to 9% with thiopurines or cyclophosphamide),7 the 68% CR rate obtained in relapsed/refractory patients with MRD clearing may qualify blinatumomab as the most active single agent for B-precursor ALL. Blinatumomab entered the clinical trial arena much after other cytotoxic MoAbs that revolutionized treatment for B-cell neoplasms (ie, rituximab). Few clinical studies with MoAbs have been performed in adult ALL, and therapeutic success has only recently been demonstrated in some series (rituximab, inotuzumab ozogamicin).8,9
If the safety and efficacy of blinatumomab is shown in MRD+ and relapsed disease, blinatumomab will have to prove its value as a first-line drug, through well-designed clinical studies to define its exact place in induction and postinduction therapy, in different risk and patient subsets, in relation to transplants and, hopefully, in substitution for some of the more toxic traditional treatment elements. With no more than 50% of adult patients cured of their illness under the best of circumstances and with a high burden of cumulative toxicity,10 it seems likely that blinatumomab may contribute in several possible ways to the cure of B-precursor ALL.
Conflict-of-interest disclosure: The author has participated on an advisory board and received honoraria from Amgen. ■