Human γδ T cells potently kill a variety of tumor cells, suggesting their usefulness in cancer therapy. In this issue of Blood, Gründer and colleagues report on in vitro–manipulated γδ–T cell receptors (TCRs) with substantially improved tumor cell recognition and killing characteristics.1
Recent clinical trials involving the adoptive transfer of autologous, ex vivo–expanded γδ T cells into cancer patients was shown to be safe, even at very large numbers exceeding 109 cells/infusion.2 Unfortunately, the clinical benefits do not match current expectations associated with this novel type of cellular vaccine. Why? Well, Gründer and colleagues argue that in vitro–expanded blood γδ T cells are polyclonal and, consequently, differ in their tumor cell killing activities. Ideal cellular vaccines would feature γδ T cells with high avidity γδ-TCRs and specificity for a broad range of tumor cells. In the present study, the authors focused on Vγ9Vδ2 T cells for several reasons, including availability (relative abundance in peripheral blood) and ease of in vitro manipulation. In fact, human blood Vγ9Vδ2 T cells have a unique responsiveness toward “phosphoantigens,”3 such as the mevalonate pathway metabolite isopentenyl pyrophosphate (IPP), which is believed to accumulate in cancer cells4 and other “stressed” cells. Of note, aminobisphosphonates, such as pamidronate or zoledronate, that are frequently used to treat patients with osteoporosis or bone metastasis, are known to inhibit IPP-metabolizing enzymes thereby causing the accumulation of IPP in treated cells. Simple addition of phosphoantigens or aminobisphosphonates together with growth factors to peripheral blood mononuclear cells results in the selective outgrowth of Vγ9Vδ2 T cells, which is useful for clinical application. The current view holds that adult Vγ9Vδ2 T cells represent a spin-off product of their engagement in antimicrobial defenses in infants exposed to the common microbial phosphoantigen, hydroxymethylbutenyl pyrophosphate (HMBPP).5,6 How Vγ9Vδ2 T cells are recognizing these unusual stress- or microbe-associated antigens is not clear and represents the single most important question that needs to be addressed to fully understand the significance of Vγ9Vδ2 T cells in antimicrobial and antitumor immunity. Encouraging new findings have just been published in Blood.7
Gründer and colleagues report that human blood–derived Vγ9Vδ2 T-cell clones differed substantially in their target cell selectivity and avidity to kill tumor cells, and further studies revealed differences in the sequences of their respective TCRs, notably in complementary determining region (CDR) 3. This region was shown to be crucially involved in the recognition of the T10/T22 antigens by murine γδ T cells.8 In comparison with a prototype Vγ9Vδ2-TCR (clone G115), individual Vγ9Vδ2-TCRs were grouped into highly active and poor performers. Of note, and in agreement with previous studies,9 treatment of tumor cells with aminobisphosphonates (pamidronate) increased their recognition and killing by Vγ9Vδ2 T-cell clones, most notably by those with high-avidity TCRs. Finally, extensive single alanine mutations in the CDR3 regions in combination with what the authors have termed combinatorial-γδ-TCR chain-exchange methodology, involving the co-expression of individual recombinant Vγ9 and Vδ2 chains in carrier αβ T cells, led to the following conclusions: (1) length and amino acid composition in CDR3 regions are important for tumor cell recognition and killing, and (2) combination of individual (engineered) Vγ9 and Vδ2 chains creates novel TCRs with superior performance both in vitro and in vivo.
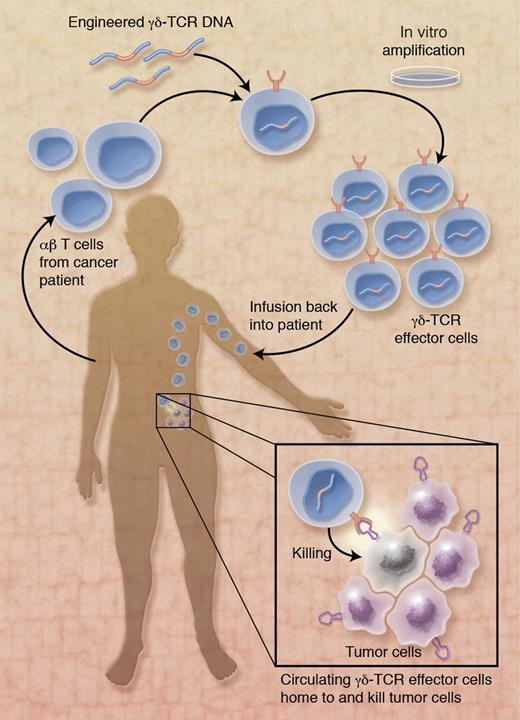
Collectively, Gründer and colleagues provide compelling new evidence in favor of translating engineered human γδ-TCRs into the clinic (see figure). Conceptually, novel γδ-TCRs that target primary tumor cells (and tumor stem cells) are expressed in carrier T cells derived from cancer patients. After in vitro expansion and transfer back into cancer patients, γδ-TCR–expressing effector cells interact with and kill tumor cells, thereby fixing the endogenous, ailing tumor defenses. Of course, numerous technical and physiologic issues remain to be addressed, including potential problems with γδ-TCR signaling in CD4- and CD8-expressing carrier cells,10 inadequate tumor homing potential, and reduced lifespan of engineered effector cells. Perhaps this novel vaccine approach may benefit from the recent finding of γδ T-APCs (human Vγ9Vδ2 T cells with professional antigen-presentation functions)11 in that their tumor cell killing activity may synergize with their ability to mobilize endogenous, tumor-specific αβ T-cell responses. Nonetheless, the concept of treating cancer patients with engineered γδ-TCR–expressing effector cells bears numerous advantages over traditional cellular vaccines. Above all, the HLA-independent recognition and killing of a broad range of tumor cells portrays Vγ9Vδ2 T cell–based effector cells as a promising new tool in the armament of antitumor therapies.
Application of γδ-TCR–expressing effector cells in immunotherapy of cancer patients. αβ T cells from a cancer patient are genetically modified to stably express optimized, high-avidity γδ-TCRs with broad range tumor cell killing activity. After expansion during in vitro culture and infusion into the cancer patient, circulating γδ-TCR effector cells home to the tumor and kill tumor cells. The ligand on tumor cells that is recognized by engineered γδ-TCRs is not known. Professional illustration by Alice Y. Chen.
Application of γδ-TCR–expressing effector cells in immunotherapy of cancer patients. αβ T cells from a cancer patient are genetically modified to stably express optimized, high-avidity γδ-TCRs with broad range tumor cell killing activity. After expansion during in vitro culture and infusion into the cancer patient, circulating γδ-TCR effector cells home to the tumor and kill tumor cells. The ligand on tumor cells that is recognized by engineered γδ-TCRs is not known. Professional illustration by Alice Y. Chen.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal