Abstract
Abstract 4101
Despite the curative promise of hematopoietic cell transplantation (HCT), many patients with hematologic malignancies relapse and others may not proceed to HCT due to the unavailability of a matched donor. Toxicities remain high with HCT, due in part to the administration of non-specific therapies such as total body irradiation (TBI) as part of preparative regimens. We aim to overcome these limitations by replacing TBI with anti-CD45 radioimmunotherapy (RIT) for haploidentical HCT to deliver radiation directly to leukemic cells while sparing normal organs and minimizing non-specific toxicities.
We established an initial TBI HCT regimen in B6SJLF1/J mice (H-2Db haplotype) conditioned with fludarabine (FLU, days -6 to -2), followed by TBI (250, 500, 750 cGy; day -1). The mice then received 15 million donor (CB6F1/J, H-2Dd) BM cells (day 0), followed by cyclophosphamide (CY) for graft-versus-host disease (GvHD) prophylaxis (day +2). Subsequent RIT HCT studies involved B6SJLF1/J mice conditioned with and without fludarabine (FLU) and escalating doses (200–400 μCi) of 90Y-anti-CD45 Ab (30F11) RIT without TBI, followed by infusion of haploidentical BM cells from CB6F1/J mice and a single dose of cyclophosphamide (CY) 2 days after HCT. Chimerism studies were performed using flow cytometric analysis to assay for engraftment of donor CD8+ cells. Therapeutic studies were performed in B6SJLF1/J mice given 105 syngeneic leukemia cells via tail vein (day -5), followed by 200 or 400 μCi 90Y-30F11 (day -3), and 1.5 × 107 BM donor cells (day 0) and two doses of CY (days -2 and +2) without FLU.
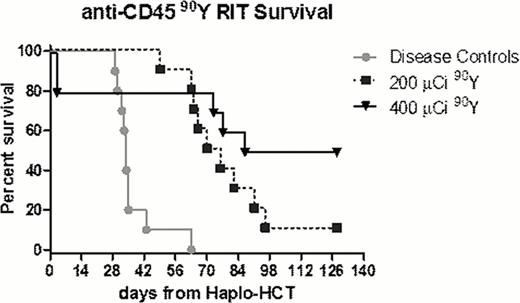
Using this model we have demonstrated that mixed chimerism was established in mice transplanted with TBI or escalating doses (200–400 μCi) of 90Y-30F11 RIT followed by injection of haploidentical BM donor rescue cells. TBI-based HCT showed that chimerism as determined by flow cytometric analysis for donor CD8+ cells was TBI dose-dependent; mice receiving ≥500 cGy were fully chimeric 4 weeks post-HCT, and persisted ≥12 months. RIT-based HCT also revealed mice with mixed chimerism, with up to 89% of donor CD8+ cells 1 month after HCT. Elimination of FLU from the conditioning regimen did not significantly decrease chimerism, as mice transplanted without FLU showed up to 70% donor CD8+ cells 1 month after HCT. Subsequent RIT experiments in B6SJLF1/J mice harboring AML were treated with escalating doses of 90Y-30F11 prior to HCT without FLU. Mice treated with anti-CD45 RIT using 200 μCi and 400 μCi of 90Y-30F11 had a median overall survival (OS) of 73 (p<0.001) and 107.5 (p=0.0015) days, respectively, compared to untreated leukemic control mice which had a median OS of 34 days after HCT (p values by Log rank (Mantel-Cox) testing)(Figure). Two mice in the 400 μCi-90Y-30F11 group were euthanized on day 3 for excessive weight loss, without gross histology abnormality in kidneys or liver.
These studies suggest that anti-CD45 RIT in the absence of TBI and FLU prior to haploidentical HCT can lead to establishment of mixed chimerism. Moreover, this anti-CD45 RIT in combination with haploidentical HCT can lead to improvement in survival for mice with AML. These results suggest that clinical studies with anti-CD45 RIT in lieu of TBI and FLU in a haploidentical HCT regimen should be considered for further investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.