Abstract
Abstract 4100
Mobilized hematopoietic stem/progenitor cells (HSPC) have become the favored cell source for stem cell transplantation. The current gold standard mobilizing agent is G-CSF, where a 5-day mobilization regimen precedes stem cell harvest. More fast-acting and potent mobilizing agents would be desirable in the interest of donor and recipient safety and convenience. AMD3100, a currently available fast-acting mobilizing agent has proven weak for clinical mobilization as a single agent, with an efficiency of less than 1/5th of G-CSF in humans.
Binding properties (position, selectivity, affinity) of the novel PEM CXCR4 antagonist POL5551 to its target receptor were analyzed. In vivo mobilization efficiency was studied after injection into C57Bl/6 or DBA/2 mice. Different administration modes (Bolus vs. continous infusion) were tested as well as a combination with a standard regimen (9×100 μg/kg q12h) of G-CSF or Cyclophosphamide. Progenitor cell mobilization was monitored using clonogenic in vitro assays. Properties of mobilized cells were tested by flow cytometry, in vitro transwell migration assays and in vivo (homing, engraftment kinetics, stem cell contents, secondary engraftment) in lethally irradiated CD45.1 or CD45.1/2 recipient mice, alone or with CD45.1 competitor bone marrow cells.
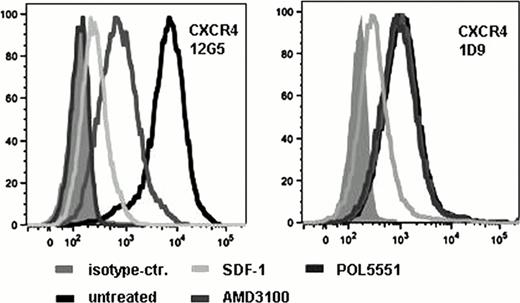
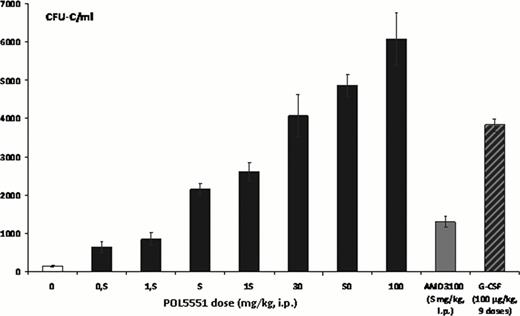
POL5551 showed selective binding to CXCR4 with an affinity exceeding that of its natural ligand SDF1, albeit occupying the extracellular receptor domains only (Fig.1). Mobilization peaked 4 hours after i.p. injection and a positive but non-linear dose-response relationship was documented for doses between 0.5 and 100 mg/kg (6000 CFU-C/ml, Fig. 2). A dose of 15 mg/kg mobilized more than twice the number of CFU-C as an equimolar dose of AMD3100, and a single dose of POL5551 at 30 mg/kg mobilized as strongly as a standard 5-day course of G-CSF treatment. POL5551 synergized with G-CSF in that injection of 5 mg/kg POL5551 after G-CSF treatment increased mobilization by 10-fold (3,000 to approx. 30,000 CFU-C/mL); this represents a 2.5 fold increase compared to a similar treatment regimen with AMD3100. Similarly, synergism with Cyclophosphamide was observed (9,900 to 50,000 CFU-C/mL).
Given as continous infusion, 5 mg/kg/day of POL5551 mobilized up to 8,000 CFU-C/ml, whereas at 30 mg/kg/day up to 40,000 CFU-C/ml were measured in circulation on day 3. Mobilized cells migrated efficiently in in vitro transwell assays and homed efficiently to the bone marrow of lethally irradiated recipients. Moreover POL5551 mobilized cells provided timely early engraftment and contained long-term engrafting stem cells with self-renewal capacity, including in serial transplantation. The immunophenotype of immature cells mobilized with POL5551 was characterized by low expression of several adhesion molecules.
POL5551 mobilizes murine stem and progenitor cells with rapid kinetics and unprecedented efficiency, markedly exceeding that of G-CSF and AMD3100. The combination of POL5551 with G-CSF mobilized more strongly than G-CSF with other CXCR4 antagonists. Similar to what we previously described for other mobilized stem cell specimen, POL5551-mobilized cells homed to marrow and engrafted efficiently. Immunophenotype was similar to that of AMD3100 mobilized cells. If the data can be corroborated in humans, POL5551 has the potential to substitute for G-CSF as a mobilizing agent.
Romagnoli:Polyphor Ltd.: Employment. Chevalier:Polyphor Ltd: Employment. Patel:Polyphor Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal