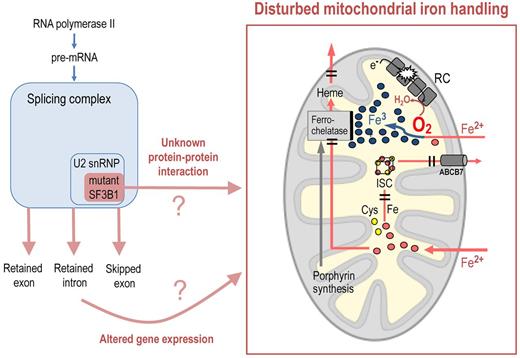
In this issue of Blood, Visconte and colleagues report on their investigations into the pathophysiologic effects of altered SF3B1 in patients with myelodysplastic syndromes (MDS).1
A crucial component of the spliceosomal U2snRNP complex, SF3B1 participates in normal RNA splicing. It was a surprise when whole genome sequencing studies showed that the most frequent recurrent mutations in MDS affect the spliceosome machinery. Collectively, spliceosome mutations are found in approximately 45% of all MDS patients. The strongest genotype-phenotype correlation exists between mutations of SF3B1 and the sideroblastic phenotype, which is caused by iron accumulation in erythroblast mitochondria. SF3B1 mutations are found in 60%-80% of patients with refractory anemia with ring sideroblasts (RARS) or RARS with thrombocytosis (RARS-T).2,3
Visconte et al found that patients with SF3B1 mutations have particularly coarse mitochondrial iron deposits.1 Knockdown experiments in K562 cells had an impact on intron-splicing, but did not produce RARS, probably because K562 cells are not sufficiently “erythroid.” However, meayamycin, a pharmacologic SF3B inhibitor, induced the formation of RARS in healthy bone marrow cells, corroborating the view that insufficiency of SF3B1 is important. Finally, the causative role of SF3B1 haploinsufficieny was confirmed in SF3B1 heterozygous mice showing RARS in the bone marrow.
RNA sequencing analysis of SF3B1 mutants yielded many exons differentially used in the SF3B1 mutant relative to normal groups, but none of them were obvious troublemakers for mitochondrial iron metabolism. In contrast to Papaemmanuil et al,3 Visconte et al did not find underexpression of key biologic pathways involved in mitochondrial function. It is also puzzling that they did not find any difference in exon-usage or gene expression of ABCB7, considering that Boultwood et al showed substantial down-regulation of ABCB7 expression in patients with RARS.4 A possible explanation is that Visconte and colleagues1 examined total bone marrow cells, while CD34+ cells were studied by the other investigators.3,4 Until these discrepancies are resolved, we cannot be sure that SF3B1 mutations interfere with mitochondrial iron metabolism through pathologic splicing and altered gene expression.
Other explanations should be considered. The absence of frameshift, nonsense, and splice-site mutations, the lack of key structural amino acid residues as sites for mutation, and the fact that the mutations are less deleterious than expected on the basis of chance all suggest that the mutated SF3B1 protein is likely to retain structural integrity, albeit with presumably altered function.3 Such altered function may either compromise a hitherto unknown physiologic role of SF3B1 in mitochondrial iron metabolism, or create a novel, unphysiologic effect on mitochondrial iron handling. SF3B1 does have functions unrelated to the splicing complex. For instance, its physical interaction with class II polycomb group proteins is needed for PcG-mediated repression of Hox genes, and skeletal abnormalities in SF3B1+/− mice appear to be independent of the alteration of general gene expression.5 Therefore, mutant SF3B1 may have pathophysiologic effects because of abnormal protein-protein interactions that have nothing to do with the splicing apparatus.
Which mitochondrial pathways might be targeted by an altered SF3B1 protein? As indicated in the figure, most of the iron that enters erythroblast mitochondria is inserted into protoporpyrin IX by ferrochelatase to make heme, and subsequently leaves the mitochondrion as heme iron. Therefore, a defect in heme export might lead to intra-mitochondrial heme degradation and iron accumulation, especially because feedback mechanisms would accelerate the import of iron into the deranged erythroblast mitochondria.6
The upstream components of the heme biosynthetic pathway are not impaired in patients with RARS, in contrast to congenital X-linked sideroblastic anemia, as shown by moderately elevated rather than reduced levels of protoporphyrin IX, the end product of porphyrin synthesis. Therefore, the incoming iron should find ample supply of its physiologic reactant. Ferrochelatase, the enzyme that catalyses the incorporation of ferrous iron into protoporphyrin IX, would be a plausible culprit, but ferrochelatase is neither mutated nor underexpressed in RARS.7 Nevertheless, diminished activity of ferrochelatase remains a possibility.
Iron also enters mitochondria for the biosynthesis of iron-sulfur clusters (ISCs). Thus, impaired assembly or export of ISCs may lead to mitochondrial iron accumulation. This is illustrated by mutations of the exporter molecule ABCB7 in patients with inherited X-linked sideroblastic anemia with ataxia (XLSA/A). Interestingly, impaired ISC assembly may impinge on ferrochelatase function because ferrochelatase is an iron-sulfur protein whose enzyme activity is strictly dependent on the presence of a functioning Fe/S cluster.
A defect in mitochondrial ISC assembly may also impair the mitochondrial respiratory chain (RC) because complexes I, II, and III of this electron transport chain are Fe/S proteins. RC dysfunction could explain why iron accumulates as ferric iron (Fe3+)8 after entering the mitochondria as ferrous iron (Fe2+; see figure). This may be attributable to a failure of the respiratory chain to effectively remove oxygen from the mitochondrial matrix. Oxygen consumption in erythropoietic cells appears to be stimulated by uncoupling protein-2, which is expressed during erythroid cell maturation.9 The resulting low oxygen concentration in the mitochondrial matrix would help to keep iron in its reduced form. An RC defect will diminish O2 consumption, thus increasing O2 in the mitochondrial matrix. If ferrous iron, after crossing the inner mitochondrial membrane, becomes oxidised (Fe2+ → Fe3+), it will be rejected by ferrochelase, which processes Fe2+ but cannot use Fe3+.10 An RC defect may thus lead to iron the wrong chemical form. The RC, because of its many components, can be disturbed by a variety of causes, perhaps including mutant SF3B1.
Mutations of SF3B1, a splicing complex component, may, via altered gene expression or abnormal protein-protein interaction, disturb mitochondrial iron handling in a way that causes mitochondrial iron accumulation in acquired sideroblastic anemia. Possible targets include (1) the final step of heme synthesis, that is, incorporation of Fe2+ into protoporphyrin IX by ferrochelatase; (2) the mitochondrial respiratory chain (RC), whose malfunction may lead to oxidation of Fe2+ (with Fe3+ being rejected by ferrochelatase); (3) iron sulfur cluster (ISC) assembly, whose malfunction can impair the RC as well as ferrochelatase; and (4) the export of heme or ISCs from the mitochondrial matrix.
Mutations of SF3B1, a splicing complex component, may, via altered gene expression or abnormal protein-protein interaction, disturb mitochondrial iron handling in a way that causes mitochondrial iron accumulation in acquired sideroblastic anemia. Possible targets include (1) the final step of heme synthesis, that is, incorporation of Fe2+ into protoporphyrin IX by ferrochelatase; (2) the mitochondrial respiratory chain (RC), whose malfunction may lead to oxidation of Fe2+ (with Fe3+ being rejected by ferrochelatase); (3) iron sulfur cluster (ISC) assembly, whose malfunction can impair the RC as well as ferrochelatase; and (4) the export of heme or ISCs from the mitochondrial matrix.
Therefore, we have at least 3 mitochondrial iron-related pathways that may be targeted by mutant SF3B1, either through abnormal splicing and disturbed gene expression or through abnormal protein-protein interaction. Visconte and colleagues have done a lot to consolidate the evidence that mutant SF3B1 is a basic cause of the sideroblastic phenotype in patients with MDS. However, the downstream events that are more immediate causes of ring sideroblast formation still have to be identified. The riddle of RARS remains to be solved.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■