Class II MHC molecules present antigenic peptides derived from exogenous and endogenous proteins to CD4+ T cells. In this issue of Blood, Kremer et al demonstrate that the nonclassic class II MHC molecule HLA-DO significantly expands the peptide repertoire of MHC II.1
CD4+ T lymphocytes recognize short peptides, generally less than 20 residues long, presented on the cell surface by the “classic” class II MHC molecules HLA-DR, -DQ, and -DP. Although the complex repertoire of peptides presented on class II+ cells by their complement of class II molecules has not yet been comprehensively defined, it is clear that this repertoire comprises peptides derived from the degradation of both exogenous and endogenous proteins.2 HLA-DR, -DQ, and -DP molecules are loaded with their peptide cargo in a late endosomal compartment called the MHC class II compartment (MIIC) in a process that is catalyzed by the nonclassic class II molecule HLA-DM, and involves displacement of CLIP, a peptide derived from proteolysis of invariant chain. Although HLA-DM does not itself have peptide-binding activity, it does bind to classic class II molecules such as HLA-DR and in so doing modifies their affinity for specific peptides, thus functioning as a peptide editor. Before exiting the MIIC and moving to the cell surface, HLA-DR, -DQ, and -DP molecules can undergo repetitive cycles of binding to HLA-DM with repeated peptide exchange, leading to a finely honed repertoire of peptides presented on the surface.
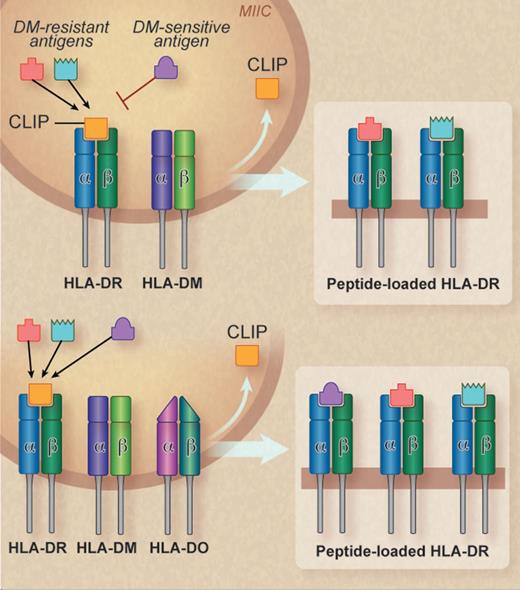
The peptide editing activity of HLA-DM in the MIIC can be modified by HLA-DO, another nonclassic class II MHC molecule that likewise has no peptide-binding activity of its own. Although the consensus view of HLA-DO function holds that it primarily inhibits the peptide editing activity of HLA-DM, relatively few published studies have evaluated the effect of HLA-DO expression on the presentation by class II+ cells of specific antigenic peptides, particularly those derived from endogenous proteins. The study from Kremer et al now argues quite convincingly that this traditional view of HLA-DO function is due for thorough re-evaluation.1 Through precise dissection of the effect of HLA-DM and HLA-DO expression on the presentation of 6 antigenic peptides, all derived from endogenous proteins, which elicited CD4+ T cell responses in vivo, the authors demonstrate that the net effect of HLA-DO expression is to expand the repertoire of peptides presented on the surface by class II MHC. This expanded peptide repertoire, however, is only presented by the limited range of cell types in which HLA-DO is expressed, namely, B lymphocytes, dendritic cells, and thymic epithelial cells (see figure).
HLA-DO expands the repertoire of peptides presented by class II MHC molecules such as HLA-DR. (Top) In the MIIC, HLA-DM facilitates the exchange of CLIP for specific DM-resistant peptides into the binding groove of class II molecules, which are then transported to the cell surface. The loading of DM-sensitive antigens is inhibited. (Bottom) In the presence of HLA-DO, both DM-resistant and DM-sensitive antigens are loaded, leading to a more diverse repertoire of peptide/class II MHC molecules displayed on the cell surface. Professional illustration by Debra T. Dartez.
HLA-DO expands the repertoire of peptides presented by class II MHC molecules such as HLA-DR. (Top) In the MIIC, HLA-DM facilitates the exchange of CLIP for specific DM-resistant peptides into the binding groove of class II molecules, which are then transported to the cell surface. The loading of DM-sensitive antigens is inhibited. (Bottom) In the presence of HLA-DO, both DM-resistant and DM-sensitive antigens are loaded, leading to a more diverse repertoire of peptide/class II MHC molecules displayed on the cell surface. Professional illustration by Debra T. Dartez.
Kremer and colleagues had previously isolated 6 alloreactive CD4+ T-cell clones from patients who had undergone allogeneic hematopoietic cell transplantation (HCT) from MHC-matched donors. They subsequently established that these clones recognized peptides derived from endogenous recipient proteins encoded by polymorphic genes, that is, that they recognized minor histocompatibility (H) antigens, and identified the specific class II MHC alleles that presented the 6 peptides for recognition by CD4+ T cells.3–5 Using the T-cell clones as sensitive reagents to detect the presence of their cognate antigens on the surface of target cells expressing the appropriate class MHC II restriction molecules, they could evaluate the effect of HLA-DM, HLA-DO, or both, on presentation of the antigenic peptides.
Kremer et al found that expression of the peptide editor HLA-DM in the absence of HLA-DO allowed presentation of 3 of the 6 minor H antigens, which they labeled DM-resistant antigens, but did not permit presentation of the other 3, which they accordingly termed DM-sensitive antigens. Coexpression of HLA-DO with HLA-DM, however, allowed presentation of the 3 DM-sensitive antigens, with negligible effect on the presentation of the 3 DM-resistant antigens. Epitope-exchange experiments assessing the recognition of target cells transduced with genes encoding recombinant proteins in which a DM-resistant peptide had been replaced by a DM-sensitive peptide, as well as the reciprocal manipulation, demonstrated that DM-sensitivity was an intrinsic property of the antigenic peptide and did not depend on the identity of the protein from which it was derived. Mutation of specific residues within the HLA-DR α chain that have been shown to be involved in the physical interaction of HLA-DR αβ heterodimers with HLA-DM had no effect on the presentation of 2 DM-resistant antigens but enabled the presentation of 2 DM-sensitive antigens, demonstrating that the property of DM-sensitivity was dependent on these DM-DRα contact residues.
To determine whether the property of DM-sensitivity was specific to the 3 antigenic peptides the authors had previously characterized, or a more general phenomenon, Kremer et al tested the effect of HLA-DM expression on target cell recognition by random CD4+ T-cell libraries established from peripheral blood of healthy donors. The DM-sensitive phenotype was common among the pools from each of the libraries, suggesting that peptide editing by HLA-DM has an extensive influence on the repertoire of peptides presented by class II MHC.
The results of these studies have potentially broad implications that extend from self-tolerance and autoimmunity to tumor immunology and immunotherapy. The narrow range of cells in which HLA-DO is expressed, and the fact that its expression is poorly, if at all, induced in other cell types by inflammatory stimuli such as interferon-γ, suggest that DM-sensitive antigens will likewise show expression that is limited to a very narrow range of cell types. Kremer et al point out that DM-sensitive antigens expressed in malignant cells that express HLA-DO represent attractive targets for immunotherapy, even under inflammatory conditions where class II MHC expression is likely to be induced in many different tissues. Could the exquisitely tissue-specific expression of HLA-DO be exploited, for example, to enhance graft-versus-leukemia effects after allogeneic HCT without inducing graft-versus-host disease? The results of Kremer et al provide us with a promising path to explore.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal