Abstract
The calcium-binding protein calreticulin (CRT) regulates protein folding in the endoplasmic reticulum (ER) and is induced in acute myeloid leukemia (AML) cells with activation of the unfolded protein response. Intracellular CRT translocation to the cell surface induces immunogenic cell death, suggesting a role in tumor suppression. In this study, we investigated CRT regulation in the serum of patients with AML. We found that CRT is not only exposed by exocytosis on the outer cell membrane after treatment with anthracyclin but also ultimately released to the serum in vitro and in AML patients during induction therapy. Leukemic cells of 113 AML patients showed increased levels of cell-surface CRT (P < .0001) and N-terminus serum CRT (P < .0001) compared with normal myeloid cells. Neutrophil elastase was identified to cleave an N-terminus CRT peptide, which was characterized as vasostatin and blocked ATRA-triggered differentiation. Levels of serum vasostatin in patients with AML inversely correlated with bone marrow vascularization, suggesting a role in antiangiogenesis. Finally, patients with increased vasostatin levels had longer relapse-free survival (P = .04) and specifically benefited from autologous transplantation (P = .006). Our data indicate that vasostatin is released from cell-surface CRT and impairs differentiation of myeloid cells and vascularization of the bone marrow microenvironment.
Introduction
Acute myeloid leukemia (AML) can be characterized by deregulated proliferation and the blocking of normal myeloid differentiation, ultimately leading to the accumulation of immature myeloid cells within bone marrow and peripheral blood. Lineage-specific transcription factors such as CCAAT/enhancer binding protein-α (CEBPA) are crucial for normal maturation of neutrophils, and they are obvious targets for disruption in AML.1-3 Indeed, CEBPA function can be deregulated in patients with AML by genomic mutations and altered mRNA expression but also by blocked translation mediated by the mRNA-binding protein calreticulin (CRT).3,4
Accumulation of misfolded proteins in the endoplasmic reticulum (ER) triggers the unfolded protein response (UPR) with 3 major consequences: (1) reduced translation of misfolded proteins; (2) enhanced translation of ER chaperones such as glucose-regulated protein, 78 kDa (GRP78), protein disulfide isomerase (PDI), and CRT; and (3) ER-associated degradation of misfolded proteins.5 The UPR can be initiated by the 3 ER transmembrane receptors: PERK (double-stranded, RNA-dependent protein kinase–like ER kinase), IRE1 (inositol-requiring enzyme 1), and ATF6 (activating transcription factor 6). Activation of IRE1 causes the cleavage of the X-box binding protein-1 (XBP1), thereby generating a potent transcriptional activator of UPR target genes. If ER homeostasis cannot be restored, prolonged UPR ultimately induces apoptosis.5,6 In previous reports, we found that the UPR is activated in up to 25% of patients with AML at diagnosis, which correlates with increased expression of mediators of the UPR such as (among others) CRT.4
CRT is a multifunctional protein involved in the regulation of intracellular calcium homeostasis, protein synthesis, cell adhesion, and chaperoning. First described as a resident of the ER, numerous studies also indicated CRT localization in the cytoplasm and at the outer surface of the cell membrane.6,7 In some solid-tumor types, treatment with anthracyclin was reported to trigger CRT translocation to the cell membrane surface.8,9
The various mechanisms contributing to intracellular CRT delocalization and the effects of CRT presentation on the cell surface for induction of immunogenic cell death and apoptosis recently have been studied intensely.8-10 In contrast, the extracellular expression and regulation of CRT are poorly understood. In this report, we investigated the regulation and function of CRT in the serum of patients with AML. We found that a stable N-terminus CRT peptide is cleaved in a neutrophil elastase (NE)–dependent manner, triggered by treatment with anthracyclin. This peptide was identified as vasostatin, and it blocked all-trans retinoic acid (ATRA) triggered differentiation of myeloid cells. In patients with AML, the levels of serum vasostatin inversely correlated with bone marrow vascularization, indicating a role in antiangiogenesis. Our data suggest that vasostatin is cleaved from cell-surface CRT in AML cells and that it impairs myeloid differentiation and vascularization of the bone marrow microenvironment.
Methods
Patients and healthy volunteers
Peripheral blood samples from 113 patients with AML at diagnosis that included all French-American-British classification subtypes and from 75 healthy volunteers were studied. Purification of mononuclear cells and isolation of RNA were described previously.11 Informed consent from patients and volunteers was obtained according to the Declaration of Helsinki. Patients fit for intensive treatment underwent uniform, consecutive HOVON/SAKK protocols for the first-line treatment of AML. Studies were approved by the local ethics committee of Bern, Switzerland. Patient characteristics and treatment are summarized in Table 1, and clinical outcome in Table 2.
CRT enzyme-linked immunoassay
MaxiSorb 96-well plates (Milian SA) were incubated overnight at 4°C with polyclonal goat anti–human against N-terminus CRT or polyclonal rabbit anti–human against C-terminus CRT (Santa Cruz Biotechnology) at 1:300. Samples diluted 1:50 and a standard dilution from 1000 ng/mL to 7.6 ng/mL of recombinant human CRT (Stressgen) were incubated for 2 hours at room temperature. Monoclonal mouse anti–human CRT (Abnova) at 1:150 was used to detect bound CRT. Horseradish peroxidase–conjugated sheep anti–mouse antibody (GE Healthcare Bio-Sciences) at 1:1000 was used as secondary antibody. The peroxidase system (Seramun Diagnostica) was applied with 100 μL of 3,3′,5,5′-tetramethylbenzidine, and the reaction was stopped by adding 50 μL of 2M H2SO4. Optical density at 450 nm (with reference wavelength at 620 nm) was assessed using an Infinite 200 Fluoro-/Luminometer (Tecan). Dilutions were performed in phosphate-buffered saline (PBS), and 0.05% Tween20 (Grogg) was added for washing steps. The enzyme-linked immunoassay (ELISA) to detect human NE in the serum of patients with AML was performed according to the manufacturer's instructions (Hycult Biotech).
Immunoprecipitation and immunoblotting
Dynabeads protein A (Invitrogen) diluted in 0.02% PBS were incubated with 10 μg of goat anti–human CRT (N-19; sc-6468) for 30 minutes at room temperature. Serum of AML patients was diluted 1:10 in PBS, and the mixture was incubated at 4°C for 30 minutes. Washing was performed 3 times with 0.02% PBS, and immunoprecipitates were eluted with 25 μL of SDS-loading dye at 70°C for 10 minutes. Samples were run on a SDS-page gel and detected by mouse anti–human CRT (Abnova).
The following antibodies were used: for N-terminal CRT: sc-6468, and for C-terminal CRT: sc-11398, all from Santa Cruz; for eIF2-alpha: L57A5, and for p-eIF2-α Ser51: 119A11, all from Cell Signaling Technology; for ATF6: IMG-273, from IGENEX; for β-actin: MAB1501, from Chemicon/Milipore; and secondary antibodies (all at 1:10′000) were against goat (926-68024), mouse (926-68020), or rabbit (926-32211), all from LICOR Biosciences. The LI-COR Odyssey Infrared Imaging System and Odyssey Version 3.0 software were used (LI-COR Biosciences).
Limited CRT digestion
A total of 15 μg of recombinant CRT was incubated with 0.02 μg and 0.005 μg of NE (activity 10.8 U/mg) in 45 μL of 200mM Tris-HCL at pH 6.0 and 7.4 for 2 hours at 37°C, as previously described.12 The addition of an equal volume of SDS-page sample buffer was used to stop the reaction.
Quantitative RT-PCR and detection of XBP1 spliced variant
CRT on cell membrane surface
CRT translocation was induced with doxorubicin (Adriblastin solution). CRT on cell membrane surface (cs-CRT) was detected by mouse monoclonal FITC-conjugated antibodies against human CRT (Mabtech AB). In addition, leukocytes of 500 μL of fresh whole blood were lysed and stained with mouse monoclonal PE-conjugated antibodies, human CD33, and Pacific-blue/eFlour450–conjugated antibodies against human CD45 (eBioscience). Blood needed to be processed within 5 hours after blood taking. Because of this limitation, our sample cohort measured by flow cytometry is smaller than that assessed by ELISA. For compensation, the anti–mouse IgG/Negative Control (FBS) Compensation Particles Set was used (BD Biosciences).
Cell culture conditions and in vitro differentiation of leukemic cells
The human leukemic cell lines HL-60 and Kasumi-1 (DSMZ) were cultured in RPMI-1640 medium with 10% and 20% fetal calf serum, respectively (Sigma-Aldrich). For differentiation, HL-60 cells were seeded at a density of 0.2 × 106/mL and incubated with 1μM ATRA (Sigma-Aldrich) or with 2 μg of recombinant vasostatin. Myeloid differentiation was assessed by CD11b surface antigen expression (Dako) and morphologic assessment.
Cell viability assay and cell-cycle analysis
The Almar Blue Cell Viability Assay (Invitrogen) was used according to the manufacturer's instructions.
Cloning, expression, and purification of recombinant vasostatin
The cDNA encoding vasostatin corresponding to amino acids 1-180 of CRT was amplified using the forward primer 5′-GCGGGATCCCTGCTATCCGTGCCGTTG-3′ and the reverse primer 5′-GGCGGCAGAACCGCGTGGCACCAGGTTGTCTGGCCGCACAATCAGTGTGTAC-3′), ligated into the pBAD TOPO TA plasmid, and expressed in TOP10 Escherichia coli (Invitrogen). Bacteria were grown at 37°C until optical density at 450 nm of 0.5-0.8, and the protein was induced with 0.02% L-arabinose for 4 hours. Cells were centrifuged at 4°C at 5000g for 12 minutes and resuspended in buffer A at pH 8.0 containing 10mM imidazole, 300mM NaCl, 20mM phosphate buffer, 2 μg/mL DnaseI, and 25× Protease Inhibitor complete (Roche). Cells were opsonized using 3 times French press at 4000 psi and incubated for 1 hour with Ni-NTA beads at 4°C. After washing with 20 column volumes of buffer B (20mM imidazole, 3000mM NaCl, 20mM phosphate buffer; at pH 8.0), the protein was eluted with 10 column volumes of buffer C (250mM imidazole, 300mM NaCl, 20mM phosphate buffer; at pH 8.0). The eluted protein solution was desalted using G-25 Sephadex columns (GE Healthcare). The recombinant protein was cleared of endotoxin using Detoxi-G Gel (Pierce) and concentrated using Vivaspin6 (Sartorius).
Immunhistochemistry
A total of 2- to 3-μm formalin-fixed, paraffin-embedded sections were dewaxed, rehydrated, and digested with 1 mg/mL trypsin at a dilution of 1:250 (Difco). Endogenous peroxidase activity was blocked by incubation for 5 minutes with 3% hydrogen peroxide (H2O2) with 0.1% sodium acid. Sections were washed in TBS and incubated for 60 minutes at room temperature with a polyclonal rabbit anti-VWF antibody (Dako), diluted 1:600 in TBS with 0.5% casein and 5% normal goat serum. A polymer-based visualization system (secondary anti–rabbit antibody bound to a common dextran backbone) with horseradish peroxidase (Envision+; Dako) was applied for 30 minutes. Finally, sections were developed in 0.02% of 3,3′-diaminobenzidine (Sigma-Aldrich) with 0.01% H2O2 and counterstained with hematoxylin.
The entire biopsy (or 10 high-power fields, HPF, if the biopsy specimen was larger) was examined. Staining intensity and vessel density were scored with an arbitrary semiquantitative, 3-tiered system (1 indicates low staining intensity, low blood vessel density; 2, medium staining intensity, medium blood vessel density; and 3, high staining intensity, high blood vessel density), and absolute vessel numbers were counted in 10 HPF. We defined low, medium, and high by using 3 biopsies as “reference” biopsies for visual scoring.
Statistical analysis
Overall survival (OS) was defined as the time from diagnosis until death. Relapse-free survival (RFS) was assessed as the time from achievement of first complete remission to relapse or death, whichever occurred first. Survival curves were generated via use of the Kaplan-Meier method and analyzed with Log-rank (Mantel-Cox test). Analyses were performed using Graph Prism 5.0 software (2007) and statistical significance was set at P < .05.
Results
Anthracyclin treatment induces cs-CRT in leukemic cells
In some solid tumor types, anthracyclin treatment induces ER stress and initiates CRT translocation to the cell membrane surface.9 Our group showed previously that induction of ER stress by compounds such as thapsigargin, tunicamycin, or calcimycin induces CRT mRNA and protein levels in leukemic cells.5,13 To investigate whether ER stress is activated by anthracyclins in leukemic cells, we treated myeloid leukemic HL-60 (Figure 1A) and Kasumi-1 cells (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) with increasing concentrations of doxorubicin for 20 hours. With the exception of CHOP, no changes in mRNA expression of UPR effectors, including CRT, were observed, and no splicing of XBP1 was detectable (data not shown).
Anthracyclin treatment induces CRT expression on the cell surface and in the serum.CRT, GRP78, and CHOP mRNAs were determined by RT-PCR in HL-60 cells (A) stimulated for 20 hours with different concentrations of doxorubicin and (B) treated with 1μM of doxorubicin in a time course. (C) HL-60 cells were stimulated with 1μM of doxorubicin, and protein levels in whole cell lysates were determined by Western blot analysis; β-actin served as a loading control. (D) HL-60 cells were treated with 1μM of doxorubicin, and cs-CRT was determined by flow cytometry. (E) Again, HL-60 cells were treated with 1μM doxorubicin, and cs-CRT was determined by flow cytometry (black bars). White bars depict levels (ng/mL) of serum CRT detected by an N-terminus–specific CRT ELISA. Untreated control cells (0h) served as controls (1-fold). (F) HL-60 cells were treated with 1μM doxorubicin (dox; light gray bars) or with 0.5μM thapsigargin to induce ER stress (th; dark gray bars). Both stimulations were performed with (scattered bars) or without 1 hour before treatment of 10μM brefeldin A (BFA) to block anterograde traffic. (G) Cells from panel F were assessed for CRT protein levels and for XBP1 mRNA. u indicates unspliced; and s, spliced variant. β-actin and GAPDH served as loading controls, respectively. Because of limited slot number, Western blot and gel electrophoresis images were modified to arrange desired order. Vertical lines have been inserted to indicate a repositioned gel lane.
Anthracyclin treatment induces CRT expression on the cell surface and in the serum.CRT, GRP78, and CHOP mRNAs were determined by RT-PCR in HL-60 cells (A) stimulated for 20 hours with different concentrations of doxorubicin and (B) treated with 1μM of doxorubicin in a time course. (C) HL-60 cells were stimulated with 1μM of doxorubicin, and protein levels in whole cell lysates were determined by Western blot analysis; β-actin served as a loading control. (D) HL-60 cells were treated with 1μM of doxorubicin, and cs-CRT was determined by flow cytometry. (E) Again, HL-60 cells were treated with 1μM doxorubicin, and cs-CRT was determined by flow cytometry (black bars). White bars depict levels (ng/mL) of serum CRT detected by an N-terminus–specific CRT ELISA. Untreated control cells (0h) served as controls (1-fold). (F) HL-60 cells were treated with 1μM doxorubicin (dox; light gray bars) or with 0.5μM thapsigargin to induce ER stress (th; dark gray bars). Both stimulations were performed with (scattered bars) or without 1 hour before treatment of 10μM brefeldin A (BFA) to block anterograde traffic. (G) Cells from panel F were assessed for CRT protein levels and for XBP1 mRNA. u indicates unspliced; and s, spliced variant. β-actin and GAPDH served as loading controls, respectively. Because of limited slot number, Western blot and gel electrophoresis images were modified to arrange desired order. Vertical lines have been inserted to indicate a repositioned gel lane.
Because 1μM doxorubicin closely mimicked the therapeutic situation in patients, HL-60 (Figure 1B) and Kasumi-1 (supplemental Figure 1B) cells were treated for shorter time periods with this concentration. We observed an early and transient increase of CRT mRNA levels (P < .05), but this modest (maximum 2-fold) mRNA increase remained undetectable on the protein level when we used both N- and C-terminus calreticulin antibodies (Figure 1C). In conclusion, we found no evidence of significant CRT de novo protein synthesis in leukemic cells after treatment with anthracyclin.
Using FACS analysis, we found that stimulation of HL-60 cells with 1μM doxorubicin initiated a time-dependent increase of CRT exposure on the outer cell membrane (Figure 1D). To investigate CRT proteins in the supernatant of leukemic cells, a CRT-specific ELISA was established. In HL-60 (Figure 1E) and Kasumi-1 (supplemental Figure 1D) cells, the increase of cs-CRT correlated with an increase of CRT in the supernatant of stimulated cells. In contrast, induction of ER stress with 0.5μM thapsigargin failed to induce CRT exposure (Figure 1F). Blocking the anterograde traffic by treatment with 10μM Brefeldin A14,15 impaired the translocation of CRT on doxorubicin treatment (Figure 1F), whereas no changes in de novo CRT synthesis were observed by Western blot analysis (Figure 1G). These observations support previous reports that the exocytosis pathway is involved in CRT delocalization after treatment with anthracyclin.7,9,16 Because no significant de novo CRT protein synthesis was observed, our data suggest that calreticulin is actively translocated to the cell membrane surface and released to the supernatant in response to treatment with anthracyclin.
Leukemic cells from patients with AML at diagnosis have increased extracellular CRT levels
On the basis of our in vitro data mentioned previously, we determined expression of cs-CRT and serum CRT protein in leukemic cells from patients with AML at diagnosis. We detected by flow cytometry that blasts from patients with AML showed increased (22-fold, P < .0001) mean levels of cs-CRT compared with mature neutrophils or monocytes from healthy donors (Figure 2A), which hardly expressed cs-CRT. Similarly, 5 samples of normal CD34+ selected autologous stem cells, obtained from patients with lymphoma in second remission before autologous transplantation, also showed very low cs-CRT expression (range, 0%-3%; data not shown). When we analyzed immunoprecipitates of serum from patients with AML by using an N-terminus CRT antibody (Figure 2B), we confirmed the presence of the 55-kDa wild-type CRT protein; consistently, a smaller 27-kDa peptide also was observed that was not detectable when we used a C-terminus CRT antibody (data not shown).
Calreticulin surface expression (cs-CRT) and extracellular serum CRT levels in patients with AML. (A) Leukemic cells from patients with AML at diagnosis and myeloid cells from 75 healthy volunteers were CD45+ gated and CD33+/CRT+ cells were determined. (B) Immunoprecipitation of serum from 2 patients with AML (#5 and #6) and 1 healthy volunteer (H1) demonstrated the 55-kDa wild-type CRT and a smaller 27-kDa peptide. (C) CRT concentration in serum from 113 patients with AML at diagnosis and from 86 healthy volunteers was investigated by C-terminus and N-terminus specific CRT ELISA. (D) cs-CRT and N-terminus CRT serum concentrations of AML patients at diagnosis were studied for correlation. (E) CRT on the cell surface by flow cytometry and (F) N-terminus CRT in the serum by ELISA were assessed in 3 de novo AML patients (#1 and #4 had FAB type -M1, and #2 had -M4Eo) during the first 30 hours of chemotherapy in induction cycle 1. Anthracyclin (anth; idarubicin 12 mg/m2) was given over 3 hours and cytarabine (cyt; 200 mg/m2) over the following 21 hours.
Calreticulin surface expression (cs-CRT) and extracellular serum CRT levels in patients with AML. (A) Leukemic cells from patients with AML at diagnosis and myeloid cells from 75 healthy volunteers were CD45+ gated and CD33+/CRT+ cells were determined. (B) Immunoprecipitation of serum from 2 patients with AML (#5 and #6) and 1 healthy volunteer (H1) demonstrated the 55-kDa wild-type CRT and a smaller 27-kDa peptide. (C) CRT concentration in serum from 113 patients with AML at diagnosis and from 86 healthy volunteers was investigated by C-terminus and N-terminus specific CRT ELISA. (D) cs-CRT and N-terminus CRT serum concentrations of AML patients at diagnosis were studied for correlation. (E) CRT on the cell surface by flow cytometry and (F) N-terminus CRT in the serum by ELISA were assessed in 3 de novo AML patients (#1 and #4 had FAB type -M1, and #2 had -M4Eo) during the first 30 hours of chemotherapy in induction cycle 1. Anthracyclin (anth; idarubicin 12 mg/m2) was given over 3 hours and cytarabine (cyt; 200 mg/m2) over the following 21 hours.
We further assessed CRT protein serum levels in 113 patients with AML and 86 healthy controls by ELISA (Figure 2C). We observed a significant median increase of N-terminus CRT levels in AML patients at diagnosis (2.1-fold, P < .0001) compared with N- and C-terminus CRT levels in healthy controls. In contrast, C-terminus CRT levels in patients with AML were not increased. N-terminus CRT levels were significantly (4.3-fold; P < .01) increased compared with C-terminus CRT levels in patients with AML, whereas N- and C-terminus CRT levels did not differ among healthy controls.
CRT levels on the cell surface of blasts correlated with N-terminus CRT serum levels of AML patients at diagnosis (Figure 2D; Pearson correlation coefficient: r = .003; P = n.s.). Because we observed in vitro CRT translocation and release to the serum after treatment with anthracyclin, we investigated whether induction therapy in patients with AML yielded similar effects. Analyzing 3 patients with AML during the first 30 hours of chemotherapy of the first induction cycle, we found no correlation between cs-CRT and serum-CRT: chemotherapy, including anthracyclin treatment, decreased the expression of CRT on the surface of the blasts (Figure 2E), whereas N-terminus serum CRT was induced (Figure 2F). These data suggest that extracellular CRT is regulated by cellular translocation and secretion during induction therapy in patients with AML.
NE regulates N-terminus CRT levels
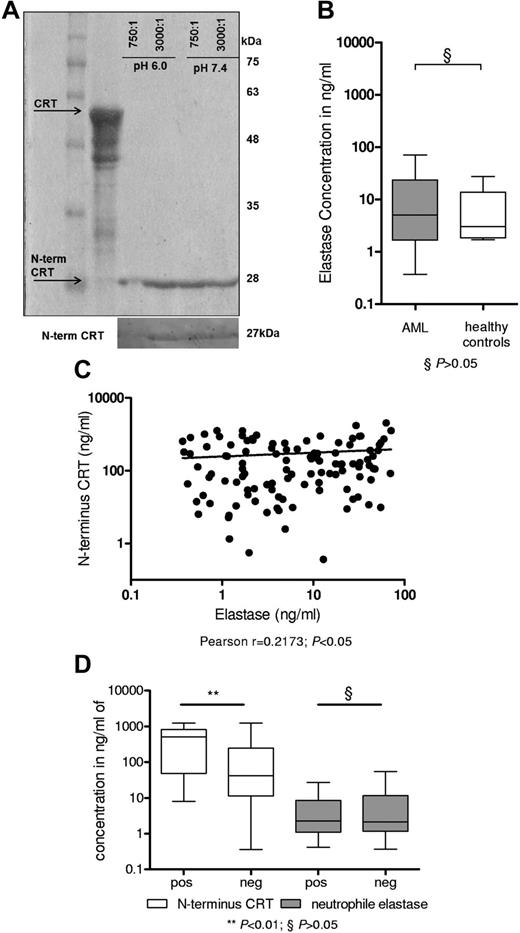
We observed divergent N- and C-terminus serum CRT levels in patients with AML, and we identified a smaller 27-kDa peptide using an N-terminus CRT antibody. This finding suggests preferential formation of an N-terminus CRT peptide in patients with AML. Assuming a sequence of roughly 180 amino acids and based on the MEROPS database,17-19 NE, a proteolytic enzyme released on activation and degranulation,17 was identified as a prominent candidate protease. In fact, proteolytic digest of recombinant CRT by NE produced a 27-kDa peptide that could be detected by the N-terminus CRT antibody (Figure 3A) but not the C-terminus CRT antibody. This 27-kDa peptide corresponds to the N-terminus 180aa of CRT and was previously characterized as vasostatin.20 Previous reports suggested a positive correlation of increased levels of NE with AML blast count.21 Consequently, we determined NE protein levels by ELISA in the serum of our cohort of patients with AML (n = 113), and healthy donors (n = 7) served as controls (Figure 3B). We detected increased NE levels in the serum of AML patients; however, this difference was not significant compared with healthy controls. NE serum levels modestly tended to correlate with N-terminus CRT levels in patients with AML at diagnosis (Figure 3C; Pearson correlation coefficient: r = .2173, P < .05). We found no evidence that NE levels were increased in patients with AML activation of the UPR (characterized by the presence of the spliced variant of XBP1). However, AML patients with activated UPR, observed in 30% of our cohort, displayed increased N-terminus CRT serum levels (P < .01; Figure 3D). In summary, these data indicate proteolytic digestion of CRT by NE and formation of a stable N-terminus 27-kDa peptide, also called vasostatin, in patients with AML.
NE releases N-terminus CRT in patients with AML. (A) Recombinant full-length CRT protein was digested by NE at pH 6.0 and pH 7.4 for 2 hours. Coomassie staining depicted a band at 27 kDa. A Western blot analysis using an N-terminus CRT antibody is depicted below. (B) ELISA to detect human NE in 113 patients with AML at diagnosis and 7 healthy volunteers was performed. (C) The N-terminus CRT levels were correlated to NE levels (Pearson correlation coefficient: r = .2173; P < .05). (D) A total of 63 patients with AML could be investigated for the presence of the XBP1-spliced variant (positive: activated UPR; 19 patients vs negative: 44 patients). Patients with activated UPR had greater levels of N-terminal CRT (P < .001) but did not differ in serum neutrophil elastase levels (P = n.s.).
NE releases N-terminus CRT in patients with AML. (A) Recombinant full-length CRT protein was digested by NE at pH 6.0 and pH 7.4 for 2 hours. Coomassie staining depicted a band at 27 kDa. A Western blot analysis using an N-terminus CRT antibody is depicted below. (B) ELISA to detect human NE in 113 patients with AML at diagnosis and 7 healthy volunteers was performed. (C) The N-terminus CRT levels were correlated to NE levels (Pearson correlation coefficient: r = .2173; P < .05). (D) A total of 63 patients with AML could be investigated for the presence of the XBP1-spliced variant (positive: activated UPR; 19 patients vs negative: 44 patients). Patients with activated UPR had greater levels of N-terminal CRT (P < .001) but did not differ in serum neutrophil elastase levels (P = n.s.).
Vasostatin impairs ATRA-triggered differentiation
In some solid tumors, vasostatin was reported to have tumor suppressor and antiangiogenic function in vitro22 and in vivo20,23,24 ; however, its significance for hematologic malignancies is so far unknown. Therefore, recombinant vasostatin protein was purified as described previously and depicted in Figure 4A. Leukemic HL-60 cells were treated with 1μM ATRA (positive control) for up to 6 days to induce differentiation, as assessed by increased CD11b expression and morphology. Sole treatment with 2μM recombinant vasostatin had no effect on CD11b expression (Figure 4B). Simultaneous stimulation with ATRA and vasostatin showed blocked differentiation after 6 days compared with ATRA alone (Figure 4B), and no morphologic changes were observed (data not shown).
Vasostatin impairs differentiation. (A) Coomassie staining of steps during purification of recombinant Vasostatin; lane 1: whole protein lysates fraction; 2: unbound protein fraction; 3: washing step fraction; and 4: purified and concentrated protein (5 μg protein/lane). Bottom panel indicates Western blot with N-terminus CRT antibody. (B) 0.2 × 106/mL HL-60 cells were treated with 1μM ATRA and/or 2μM vasostatin. CD11b expression was determined by flow cytometry. (C) The proportion of metabolizing cells was assessed via use of the Alamar Blue Assay, and we identified a decrease in metabolizing cells in ATRA-treated HL-60 cells. (D) Cell-cycle analysis (propidium iodide staining) of HL-60 cells treated with ATRA and/or vasostatin.
Vasostatin impairs differentiation. (A) Coomassie staining of steps during purification of recombinant Vasostatin; lane 1: whole protein lysates fraction; 2: unbound protein fraction; 3: washing step fraction; and 4: purified and concentrated protein (5 μg protein/lane). Bottom panel indicates Western blot with N-terminus CRT antibody. (B) 0.2 × 106/mL HL-60 cells were treated with 1μM ATRA and/or 2μM vasostatin. CD11b expression was determined by flow cytometry. (C) The proportion of metabolizing cells was assessed via use of the Alamar Blue Assay, and we identified a decrease in metabolizing cells in ATRA-treated HL-60 cells. (D) Cell-cycle analysis (propidium iodide staining) of HL-60 cells treated with ATRA and/or vasostatin.
To estimate whether proliferation of cells is affected by treatment with vasostatin, we verified the amount of metabolizing cells in comparison with nontreated cells by using the Alamar Blue Assay. Whereas the proportion of metabolizing cells decreased as expected in HL-60 cells treated with ATRA alone, we did not observe changes in cells treated with vasostatin (Figure 4C). Again, vasostatin blocked the effects of ATRA treatment. These results were confirmed by cell-cycle analysis depicting a G0/G1 arrest of ATRA-treated cells (Figure 4D). In contrast, vasostatin-treated cells showed a similar cell-cycle distribution as untreated cells, and vasostatin treatment abolished the effects of ATRA. These experiments suggest that vasostatin impairs ATRA-triggered differentiation.
Vasostatin levels inversely correlate with microvessel density in the bone marrow of patients with AML
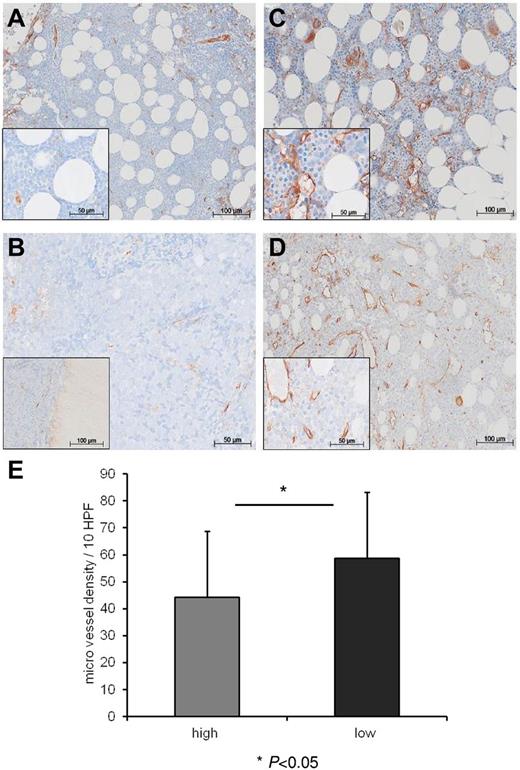
Whereas the role of angiogenic factors is intensely studied in solid and hematologic tumors, the impact of antiangiogenic factors in leukemias hardly has been investigated. We hypothesized that levels of antiangiogenic vasostatin might be associated with differing microvessel density (MVD) at diagnosis in the bone marrow of patients with AML. We stratified our patient cohort according to a threshold of 200 ng/mL vasostatin in the serum in groups of low versus high vasostatin levels. This threshold was selected because 98% of our healthy controls scored below this value. The clinical characteristics of the 2 groups are summarized in Table 1. We compared bone marrow biopsies of AML patients with low (n = 20) versus high (n = 20) vasostatin levels for MVD, and we applied VWF staining for the detection of endothelial cells. We found that patients with high vasostatin levels showed lower MVD (Figure 5B and D) than patients with low vasostatin serum levels (Figure 5A and C). There was a significantly lower mean microvessel number (enumerated in 10 HPF) in the group with high compared with the group with low vasostatin serum levels (P = .0495; mean 44 ± 25 vs 59 ± 24/10 HPF, Figure 5E). These results suggest that vasostatin might suppress bone marrow vascularization in patients with AML.
Vasostatin levels inversely correlate with MVD in patients with AML. Immunohistochemical staining of bone marrow biopsies with von VWF identifying endothelial vessel structures (stained brown). Panels A and B represent 2 patients with high vasostatin levels and low MVD, whereas panels C and D depict 2 patients with low CRT serum levels and high MVD. Inlays reveal greater magnification of the individual overview images. Panel E depicts the number of microvessels (enumerated in the VWF staining) per 10 HPF in the individual bone marrow biopsies (P = .0495; mean ± SD; high vasostatin levels: 44 ± 25/10 HPF vs low vasostatin levels: 59 ± 24/10 HPF).
Vasostatin levels inversely correlate with MVD in patients with AML. Immunohistochemical staining of bone marrow biopsies with von VWF identifying endothelial vessel structures (stained brown). Panels A and B represent 2 patients with high vasostatin levels and low MVD, whereas panels C and D depict 2 patients with low CRT serum levels and high MVD. Inlays reveal greater magnification of the individual overview images. Panel E depicts the number of microvessels (enumerated in the VWF staining) per 10 HPF in the individual bone marrow biopsies (P = .0495; mean ± SD; high vasostatin levels: 44 ± 25/10 HPF vs low vasostatin levels: 59 ± 24/10 HPF).
Patients with high vasostatin levels have a more favorable course of their disease
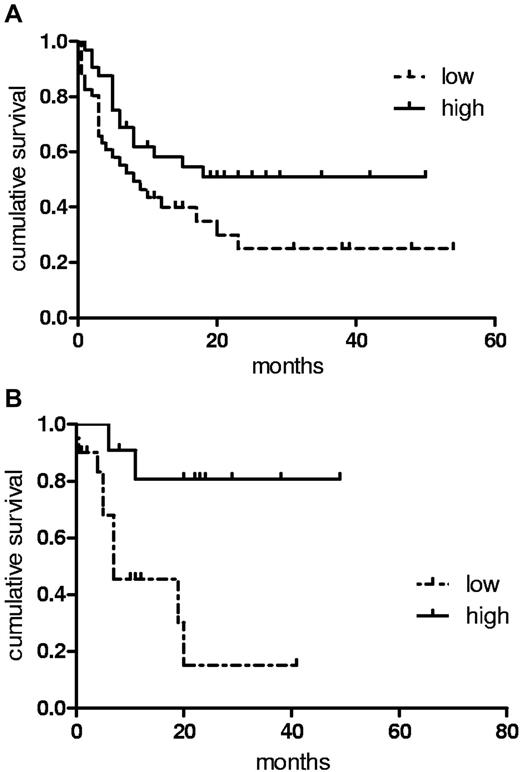
We assessed the impact of differing vasostatin serum levels in our cohort of patients with AML at diagnosis with regards to response to chemotherapy and survival. We observed no differences in the rate of achieving a first complete remission or in the relapse rate between patients expressing high or low levels of vasostatin. However, patients with high levels of vasostatin (Figure 6A) enjoyed longer RFS compared with the group with low vasostatin levels (P = .0479; median 11.5 vs 7.2 months; hazard ratio [HR] 0.66; 95% confidence interval [95% CI] 0.50-0.95). OS also tended to be longer in the high vasostatin group (16.1 vs 10.3 months; P = .1160; HR 0.76; 95% CI 0.53-1.12). In patients undergoing consolidation treatment, relapse rate and OS after allogeneic transplantation were not different between the 2 vasostatin groups (summarized in Table 2). However, we found that patients with elevated vasostatin serum levels receiving high-dose chemotherapy and autologous SCT had significantly (P = .0057; 23.3 vs 7.5 months; HR 0.62; 95% CI 0.45-0.88) longer OS (Figure 6B), suggesting increased sensitivity to chemotherapy of AML patients with high vasostatin levels. These data propose that high levels of serum vasostatin in patients with AML at diagnosis are associated with a more favorable course of the disease and that such patients might preferentially benefit from autologous transplantation for consolidation.
Patients with increased vasostatin levels have a favorable outcome. (A) A threshold of 200 ng/mL separated groups with high vs low serum N-terminus CRT-expressing patients with AML. RFS applying Kaplan-Meier analysis was determined for the 2 groups (P = .0479; median 11.5 vs 7.2 months). (B) OS is depicted for patients with AML consolidated in first complete remission with autologous stem cell transplantation (P = .0057; 23.3 vs 7.5 months).
Patients with increased vasostatin levels have a favorable outcome. (A) A threshold of 200 ng/mL separated groups with high vs low serum N-terminus CRT-expressing patients with AML. RFS applying Kaplan-Meier analysis was determined for the 2 groups (P = .0479; median 11.5 vs 7.2 months). (B) OS is depicted for patients with AML consolidated in first complete remission with autologous stem cell transplantation (P = .0057; 23.3 vs 7.5 months).
Discussion
In this study, we investigated the regulation and function of the chaperone calreticulin (CRT) in the serum of patients with AML. We found that a stable N-terminus CRT peptide is released to the serum in a NE-dependent manner triggered by treatment with anthracyclin or activation of the UPR. This peptide was characterized as vasostatin and blocked ATRA-triggered in vitro differentiation of AML cells. In patients with AML, levels of serum vasostatin inversely correlated with bone marrow vascularization, indicating a role in antiangiogenesis. Our data suggest that vasostatin is cleaved from cell surface CRT in AML cells and that it impairs ATRA-induced differentiation of leukemic cells and vascularization of the bone marrow microenvironment.
Besides its role for intracellular calcium homeostasis and protein folding control, CRT has gained increased attention for mediating immunogenic cell death by its presentation on the outer cell membrane.6-9,25 Thereby, CRT acts as an “eat-me” signal on tumor cells for dendritic cells and turns nonimmunogenic apoptosis into cell death that elicits an immune response.8,9,26 Translocation of CRT from the endoplasmic reticulum via the Golgi apparatus to the surface of the cell membrane is a highly orchestrated process.27,28 The UPR mediator CRT is induced by ER stress or anthracyclin treatment. CRT requires the protein disulfide isomerase family member ERp57 for translocation or interaction with phosphatidylserine.27-29 Treatment with anthracyclin triggers the PERK pathway of the UPR involving phosphorylation of eIF2a and induction of CHOP.30 Phosphatidylserine-associated externalization of CRT follows increased nitric oxide levels, thereby activating ATF6 and IRE1 pathways.29-31 Treatment with anthracyclin induces nitric oxide levels and translocation of CRT to the cell membrane as demonstrated in the human colon cancer cell line HT29.32
Our group previously reported in leukemic cells that induction of ER stress by compounds such as thapsigargin or tunicamycin induces potent activation of calreticulin transcription mediated by 2 tandem ER stress responsive elements in the CRT promoter.12 In this study, we found the treatment with anthracyclin induced exposure of cs-CRT in leukemic cells. However, the elevated cs-CRT occurred without increasing CRT transcription or CRT protein levels. This finding suggests that cellular CRT translocation, but not de novo synthesis, is the main mechanism involved in CRT presentation on the cell membrane, allowing rapid distribution of intracellular CRT protein. Similar conclusions were reported for doxorubicin-induced CRT exposure in HT29 colon cancer cells,32 and it is supported by our observation that blocking the anterograde traffic by brefeldine A impairs exposure of cs-CRT.
In our cohort of patients with AML, we found that roughly 30% of all patients with AML display activation of the UPR as assessed by the presence of the spliced variant of XBP1. Patients with activated UPR had greater levels of cs-CRT. Moreover, in general blasts from patients with AML expressed greater median cs-CRT levels than granulocytes or monocytes from healthy controls, suggesting that additional mechanisms are triggering cs-CRT induction in AML cells besides UPR activation. This is exemplified by our observation that treatment with anthracyclin can induce cs-CRT exposure without inducing mRNA and protein expression of UPR associated effector genes such as, among others, CRT.
CRT lacks a specific transmembrane domain.7 Thus, CRT translocation to the cell membrane requires chaperoning of other proteins such as ERp57.28,29 Interaction with such proteins might mask the retrieval signal of CRT and allow temporary adherence of CRT to the cell membrane surface.
In this study and in accordance with previous reports, we showed that CRT is targeted by NE for proteolysis. NE is a membrane-associated protein originally purified from leukemic cells from patients with AML.33 It is a myeloid-specific protease and is transcriptionally regulated during early myeloid differentiation.34 The protease-resistant core of CRT is an N-terminus 27-kDa peptide, which was found to be identical to the antiangiogenic protein vasostatin.19,20,35 In the serum of patients with AML, we consistently observed, besides the wild-type 55kDa CRT protein, a 27-kDa peptide detectable only with N-terminus CRT antibodies. This N-terminus CRT peptide correlated with NE levels in the serum of patients with AML. These findings suggested processing of CRT by NE.
A variety of reports described increased bone marrow vascularization in leukemias.36,37 However, the role of antiangiogenic factors per se remains poorly understood in hematologic malignancies. In some solid tumors, vasostatin inhibits angiogenesis20 and proliferation of endothelial cells,37 but so far, it had not been investigated whether vasostatin is involved in the differentiation block of myeloid leukemic cells. We found that the protease-resistant core of CRT, vasostatin, inhibited ATRA-induced differentiation of leukemic cells, but it did not affect proliferation. These observations confirm a report in P19 embryonic carcinoma cells in which overexpression of wild-type CRT impaired ATRA-induced differentiation.38 Our results suggest that extracellular vasostatin may also affect differentiation.
To further elucidate the role of vasostatin for antiangiogenesis in AML, we analyzed bone marrow biopsies of patients with AML in our cohort. Commonly used endothelial markers such as CD31, CD34, and VEGF have to be disregarded because of staining interference of leukemic cells,39,40 whereas immunohistochemical staining against VWF was able to overcome these limitations. We found that AML patients with high vasostatin serum levels had lower MVD in the bone marrow compared with patients with low vasostatin levels. Our data suggest a role of vasostatin in the regulation of endothelial growth and vessel expansion in the bone marrow of patients with AML. This growth inhibitory effect seemed to be limited to endothelial cells because vasostatin blocked differentiation in leukemic cells in vitro but did not affect proliferation.
We also investigated the prognostic relevance of divergent vasostatin serum levels at diagnosis of AML. We observed that AML patients with high vasostatin serum levels (and low bone marrow microvascular density) at diagnosis had significantly longer RFS and tended to have longer overall survival. Strikingly, patients with high vasostatin levels specifically benefited from consolidation with high-dose chemotherapy and autologous transplantation. However, this latter observation awaits confirmation in a prospective study. Apart from this observation, AML patients with low microvascular density might be more susceptible to high-dose chemotherapy (with myleran and cyclophosphamide) as a conditioning regimen before autologous transplantation, and high vasostatin levels could serve as a marker predicting response to this therapy in AML patients.
In summary, we found that an elastase-inert N-terminus CRT peptide is released into the serum triggered by treatment with anthracyclin. This CRT peptide was identified as vasostatin and was shown to block ATRA-triggered differentiation of leukemic cells. In patients with AML, levels of serum vasostatin inversely correlated with bone marrow vascularization, indicating an important role in antiangiogenesis. Together with reports on the growth inhibitory function of vasostatin in solid tumors, our study points toward vasostatin as a promising therapeutic candidate.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Deborah Shan for help with preparing patient samples and Stefano Fontana for assisting in collecting samples from healthy volunteers; Marc Solioz, Helge Abicht, Kerstin Schaubitzer, and Thomas Heitkamp for support in protein purification; and the team of Andreas Kappeler for help with immunohistochemistry.
This study was supported by grants to T.P. from the Swiss National Science Foundation (SNF) no. 310030-127509 and from the Swiss Cancer League (KLS) no. 02520-20-2010.
Authorship
Contribution: S.M. and T.P. conceived and designed experiments; S.M., B.U.M., and T.P. performed and analyzed data; Y.B. performed and analyzed immunohistochemistry; and all authors were involved in drafting or critically revising the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Pabst, MD, Associate Professor, Department of Medical Oncology, University Hospital, 3010 Bern, Switzerland; e-mail: thomas.pabst@insel.ch.