Abstract
The identification of the causative organism in invasive pulmonary aspergillosis (IPA) is recommended. We investigated whether a mycologic diagnostic strategy could be optimized based on patient characteristics. Fifty-five patients were enrolled in a prospective study. The presence of Aspergillus in respiratory samples occurred more frequently in non-acute leukemia (AL) patients than in AL patients (P = .0003), and in patients with leukocyte counts more than 100/mm3 (P = .002). In a logistic regression model, these 2 factors appeared to be independent, with an adjusted odds ratio of 7.14 (95% confidence interval, 1.40-36.5) for non-AL patients and an adjusted odds ratio of 6.97 (95% confidence interval, 1.33-36.5) for patients with leukocyte counts more than 100/mm3. A positive mycologic result was also more frequent among patients with lung CT scan signs of airway-invasive disease than among other patients (P = .043). Airway-invasive signs were more frequent among non-AL patients (P = .049), whereas angioinvasive disease was more frequent among both AL patients (P = .01) and patients with leukocyte counts less than 100/mm3 (P = .001). A concomitant pulmonary infection was identified more frequently among non-AL patients (P = .005 vs allogeneic hematopoietic stem cell transplant and P = .048 vs others). Our results suggest that different strategies for diagnosing IPA should be considered based on the underlying condition.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1956.
Disclosures
Anne Bergeron received advisory board fees from Schering-Plough and speaking fees from Schering-Plough and Pfizer. Emmanuel Raffoux received advisory board fees from Genzyme. Muriel Cornet received honoraria from Conidia. Benoit Brethon received funding from MSD-Chibret for manuscript preparation. Claire Lacroix received honoraria from Pfizer and Gilead and speaking fees from MSD. Patricia Ribaud received advisory board fees from Gilead, Merck Sharp & Dohme, Pfizer, and Schering-Plough and speaking fees from Gilead, Merck Sharp & Dhome, Pfizer, and Schering-Plough. The remaining authors, the Associate Editor Martin S. Tallman, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe factors predicting the presence of Aspergillus in respiratory samples as based on a prospective study of patients with invasive pulmonary aspergillosis.
Describe a recommended mycologic diagnostic stategy for patients with acute leukemia (AL) as based on this prospective study.
Describe a recommended mycologic diagnostic strategy for the specific diagnosis of aspergillosis in non-AL patients as based on this prospective study.
Release date: February 23, 2012; Expiration date: February 23, 2013
Introduction
Invasive aspergillosis, which mainly affects the lungs, is an important cause of mortality and morbidity in patients with hematologic malignancies, especially those with prolonged neutropenia, recipients of allogeneic hematopoietic stem cell transplants (HSCTs), and those with prolonged use of corticosteroids and/or T-cell immunosuppressants.1,2 The incidence of invasive aspergillosis is however different depending on the underlying condition: up to 24% for patients with acute leukemia, up to 10% in patients with allogeneic HSCT, and up to 7% in patients with lymphoid malignancies.3,4
The diagnosis of invasive pulmonary aspergillosis (IPA) is typically difficult to make. Except for when a tissue specimen is available, this diagnosis relies on the association of a host factor, characteristic lung CT findings, and microbiologic findings that may be detected via either direct tests (such as direct microscopy or culture) or indirect tests (such as galactomannan antigen [GM] or β-D glucan).1
Over the last decade, the CT scanning has become a cornerstone of the diagnosis of IPA. The presence of a nodule with a halo sign has been shown to be highly suggestive of IPA in neutropenic patients.5 Although little is known about CT scan features in non-neutropenic patients, this test has been applied to hematologic patients with any underlying host factor, even leading to the inclusion of high-risk hematologic patients in a therapeutic clinical trial only on the basis of the presence of a nodule with a halo sign.6 However, up to 40% of patients with IPA do not present with a nodule with a halo sign.7 Some recent data have shown that other CT scan findings may be the only signs present in non-neutropenic patients with IPA, demonstrating the necessity of considering these less characteristic CT scan features for the diagnosis of IPA.8,9 In this context, some authors have previously identified 2 different patterns of IPA, relying on correlations between histology and CT scan patterns: angioinvasive pulmonary aspergillosis is characterized by vascular invasion by Aspergillus and a nodule with a halo sign, whereas airway-invasive aspergillosis is characterized by the destruction of the bronchiolar wall by Aspergillus and centrilobular micronodules and tree-in-bud opacities.9-13
In the last few years, breakthrough infections with new Aspergillus species and other fungi, whose sensitivity to the various antifungal drugs is variable, have been reported.14-17 As clinical presentation does not allow for the differentiation of the causative organisms of invasive fungal infections, direct mycologic tests are now recommended for both epidemiologic reasons and for the optimal management of the patients.18
However, the best strategy to isolate the fungus in suspected IPA is not well established. Although bronchoscopy remains the most frequently used tool to explore the lungs of immunocompromised patients, its reported diagnostic yield does not exceed 40% to 60%.19-22 In the context of hematologic patients who are admitted to the intensive care unit for various respiratory complications, the overall diagnostic yield of bronchoscopy was previously shown to be associated with the underlying disease and with neutropenia.23 These results raise the question of whether the mycologic diagnostic yield of bronchoscopy would differ according to the underlying hematologic condition in the specific context of IPA. This hypothesis is supported by the fact that hematologic patients constitute a heterogeneous population of patients who differ with respect to the specific defects in host defense mechanisms leading to the risk of developing IPA.24,25 In this context, it has been shown that host immunosuppression may influence the radiologic presentation26 and the prognosis, which seems better for patients with neutropenia than for those who have undergone allogeneic HSCT.27
With the intention of optimizing the mycologic diagnostic yield of respiratory samples, we wondered whether mycologic diagnostic strategies for IPA could be adapted based on patient characteristics. To answer this question, we analyzed baseline characteristics of patients with invasive aspergillosis who were included in a prospective study that evaluated the performance of microbiologic tools to predict the outcome of invasive aspergillosis (A.B., R.P., J. Menotti, J.L.P., K.C., A.V., M.C., F.I., E.R., B.B., C.L., S. Touratier, J. P. Latgé, C.B., A.T., F.D., P.R., and A.S., manuscript in revision, November 2011).
Methods
Patients
The clinical database used for this analysis included the baseline clinical, mycologic, and lung high-resolution CT (HRCT) scan characteristics from 55 patients with invasive aspergillosis who were enrolled in a prospective study that was designed to evaluate the ability of several microbiologic tools to predict the day 45 outcome (A.B., R.P., J. Menotti, J.L.P., K.C., A.V., M.C., F.I., E.R., B.B., C.L., S. Touratier, J. P. Latgé, C.B., A.T., F.D., P.R., and A.S., manuscript in revision, November 2011). The protocol was approved by the ethics committee of the Saint Louis Teaching Hospital, Paris, France. All patients gave informed consent in accordance with the Declaration of Helsinki. All consecutive patients who were diagnosed with proven or probable invasive aspergillosis according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC) and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (MSG) definitions between May 2005 and March 2007 were included.1 In a proper clinical setting, patients with abnormalities on their lung CT, other than those retained for the diagnosis of invasive aspergillosis based on the revised definition, who had a microbiologic criteria (ie, positive antigen and/or mycology) and for whom an alternative diagnosis was ruled out were considered as probable invasive aspergillosis cases (in accordance with the 2002 definition28 ). Patients with possible invasive aspergillosis were also included and retained for the study if an alternative diagnosis was ruled out.29 A Data Review Committee assessed the diagnosis of invasive aspergillosis for each case (P.R., A.B., A.S., and K.C.).
Both the clinical features and the mycologic diagnostic yield were analyzed for 3 groups of patients: those who had acute leukemia and no HSCT (AL), those who underwent an allogeneic HSCT, and those who had another underlying hematologic disorder and no allogeneic HSCT (others). For further analysis, because of similar characteristics, the 2 latter groups of patients were pooled as the non-AL patient group to simplify hypothesis testing. Profound neutropenia was defined as leukopenia < 100/mm3.
Lung CT scan and fiberoptic bronchoscopy
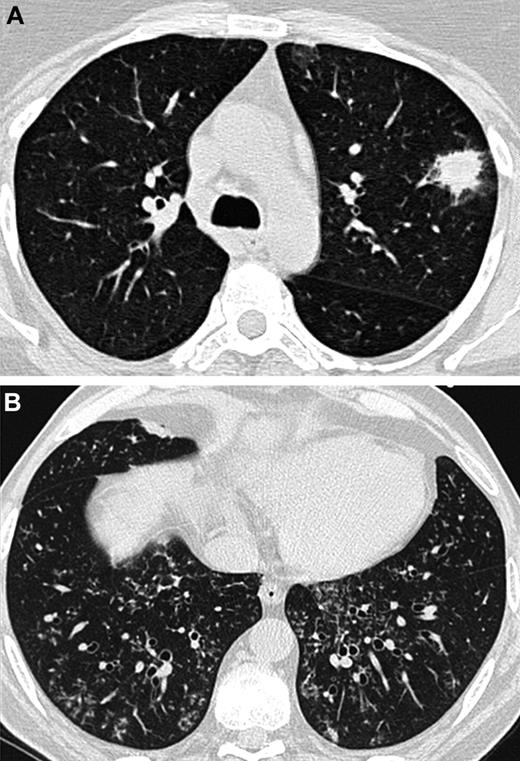
At baseline, lung HRCT scans were performed on each patient using multidetector CT scanners. A Data Review Committee reviewed and analyzed the lung CT scans (A.B., C.d.B., and K.C.). The following features were noted: nodules with or without halo sign or cavitation (in particular air crescent sign), alveolar consolidation with or without halo sign or cavitation, centrilobular micronodules and tree-in-bud opacities, ground glass opacities, septal thickening, and pleural effusion. Particular attention was paid to differentiate features that are known to be attributed to angioinvasive aspergillosis and those indicative of airway-invasive aspergillosis: in accordance with what was previously demonstrated in patho-radiologic correlations, the presence of a nodule with a halo sign without any airway-invasive features was defined as an angioinvasive disease (Figure 1A). In contrast, the presence of centrilobular micronodules and/or tree-in-bud without any nodule with a halo sign was defined as an airway-invasive disease (Figure 1B).7,9,11-13 As consolidations may occur with both aspergillosis-induced hemorrhagic infarctions (ie, angioinvasive disease) and Aspergillus bronchopneumonia (ie, airway-invasive disease), consolidations were not considered when differentiating between the 2 entities.10,12,13
Lung CT scans from 2 different patients. (A) Nodule with a halo sign revealing angioinvasive aspergillosis in an AL patient. (B) Centrilobular nodules and tree-in-bud opacities showing airway-invasive aspergillosis in a patient who underwent an allogeneic HSCT.
Lung CT scans from 2 different patients. (A) Nodule with a halo sign revealing angioinvasive aspergillosis in an AL patient. (B) Centrilobular nodules and tree-in-bud opacities showing airway-invasive aspergillosis in a patient who underwent an allogeneic HSCT.
Fiberoptic bronchoscopy was performed within 24 hours of the baseline HRCT. Only results from bronchoscopies performed within 2 days of inclusion were analyzed. The site of bronchoalveolar lavage (BAL) was guided by the lung HRCT, and BAL was performed using four 50-mL aliquots of sterile saline solution. An extensive analysis for pathogens, including bacteria, viruses, and parasites, was performed on BAL fluid. Direct examination and culture for fungi were also systematically performed on BAL fluid, bronchial aspirate recovered from all along the bronchoscopy, bronchial biopsy, and sputum when available.
Antigen detection technique
GM antigen was detected using a sandwich immunocapture ELISA (Platelia Aspergillus, Bio-Rad) according to the manufacturer's recommendations. Positive and negative controls were included in each assay. Results were expressed as an index of positivity and were considered positive if ≥ .5, both in the serum and in the BAL.22
Statistical analysis
The clinical characteristics of patients were compared across the 3 patient groups using the χ2, Fisher exact, or Kruskal-Wallis tests. In case of a significant overall difference, posthoc 2-by-2 group comparisons were carried out using Fisher exact test or the Wilcoxon rank-sum test, with P values corrected by the Holm method to account for multiplicity. In addition, a priori defined specific hypotheses were tested with a P value correction to control the false discovery rate (ie, the expected proportion of false rejection among the rejected hypotheses, at a 5% rate).30 In these cases, no pair-wise group comparisons were performed. Paired comparisons of sensitivities of mycologic examinations were performed using the McNemar or Liddell exact tests.
All tests were 2-sided, and P values ≤ .05 were considered statistically significant. Analyses were carried out using R Version 2.6.2 statistical software.31
Results
Among the 55 patients included in the study, 23 had undergone allogeneic HSCT (median time between transplantation and invasive aspergillosis, 132 days [Q1-Q3: 72-189]; 17 had GVHD, acute ≥ grade 2, n = 9; chronic, n = 8), 22 were treated for AL (acute myeloid leukemia, n = 18; acute lymphoid leukemia, n = 4), and 10 had other hematologic disorders (malignant lymphoma, n = 4; chronic lymphocytic leukemia, n = 3; multiple myeloma, n = 1; myelodysplastic syndrome, n = 1; hairy cell leukemia, n = 1). The characteristics of invasive aspergillosis among the different groups of patients are reported in Table 1.
Patients' clinical features of IPA according to the underlying condition and leukocyte status
Patients' clinical presentations at diagnosis of IPA are reported in Table 1. We noted the following significant differences between the 3 groups of patients: (1) a higher proportion of allogeneic HSCT recipients were receiving steroids at diagnosis compared with AL patients (P = .0002); (2) patients with AL had lower leukocyte counts than non-AL patients (P = .005 vs allogeneic HSCT, P = .005 vs others); (3) whereas almost all patients who had not undergone allogeneic HSCT had a fever at the diagnosis of invasive aspergillosis, fever was present in fewer patients who had undergone allogeneic HSCT (P = .0007 vs AL, P = .14 vs others); and (4) AL patients had a less frequent concomitant pulmonary infection (P = .005 vs allogeneic HSCT and P = .048 vs other).
The characteristics of the lung CT scans at diagnosis in the 3 groups of patients are summarized in Table 2. We analyzed the CT scan features with respect to the patient characteristics and found a strong association between CT findings and both the underlying condition and the leukocyte status. We found that angioinvasive aspergillosis was significantly more frequent in AL patients (P = .01). Conversely, airway-invasive signs were significantly more frequent in non-AL patients (P = .049). Seven patients (3 with allogeneic HSCT, 2 with AL and 2 others) had both angioinvasive and airway-invasive signs of IPA.
Furthermore, when we compared the lung CT scan characteristics of IPA with the leukocyte counts, we found strong associations between angioinvasive disease and leukocyte counts < 100/mm3 and between airway-invasive disease and leukocyte counts > 100/mm3 (Table 3).
Mycologic diagnostic yield differs according to the underlying condition and leukocyte status
The overall mycologic diagnostic yield for bronchoscopy was 58%. The performance of the identification of fungus in respiratory samples was significantly different between the 3 groups of patients. Isolation of Aspergillus was rare in patients with AL compared with those who had undergone allogeneic HSCT or others (P = .013 for each comparison). In non-AL patients, mycologic examination of bronchial aspirate was apparently more sensitive than examination of BAL, although this difference was not statistically significant (P = .31, Liddell test; Table 4).
Results of GM detection both in serum and BAL were not significantly different between the 3 groups of patients with respect to both the sensitivity of the test and its index value (Table 4). Previous anti–mold therapy had no significant influence on either serum or BAL GM testing: 47% of patients with anti–mold treatment at diagnosis of invasive aspergillosis and 62% of patients who did not receive anti–mold treatment had a positive serum GM (P = .28); 71% of patients with anti–mold treatment at diagnosis of invasive aspergillosis and 57% of patients with no anti–mold treatment had a positive BAL GM (P = .69). Notably, Aspergillus was present in respiratory samples from 5 of the 6 patients who had undergone allogeneic HSCT and who were negative for serum GM antigen. A BAL GM index was available for 28 patients, among whom 10 had a negative serum GM index; only 4 of the latter had a positive BAL GM index (1 allogeneic HSCT patient, 2 AL patients, and 1 other patient). Aspergillus was identified in respiratory samples from 2 of these 4 patients.
Beyond the underlying condition, we analyzed other factors that may have influenced the mycologic examination yield. Steroid therapy (yes/no and > or < 1 mg/kg), as well as previous anti–mold therapy, had no significant influence on the results (P = .17, P = .074, and P = .62, respectively). In contrast, the identification of Aspergillus in respiratory samples was significantly less frequent in patients with leukocyte counts < 100/mm3 than in others (P = .002). In a logistic regression model, underlying condition and leukocyte status were 2 independent factors (odds ratio = 7.14; 95% confidence interval [CI], 1.40-36.5 for non-AL patients and odds ratio = 6.97; 95% CI, 1.33-36.5 for leukocyte counts > 100/mm3). Aspergillus was identified in the respiratory samples of 95% of the non-AL patients with leukocyte counts > 100/mm3 versus 23% of samples from AL patients with leukocyte counts < 100/mm3 (P = .0004).
Association between CT scan features and the performance of mycologic examination of respiratory samples
We further postulated that the performance of isolation of Aspergillus in respiratory samples might be different with respect to lung CT scan features. We found a significant association between the presence of airway-invasive signs on the lung HRCT scan and the identification of Aspergillus in respiratory samples. Among the 18 patients with at least one airway-invasive sign and for whom mycologic examination of respiratory samples was available, Aspergillus was found in the respiratory samples of 15 patients (83%), whereas only 12 of 26 patients (46%) who had no signs of airway-invasive disease had a positive mycologic examination (P = .043). When we analyzed the 11 patients who had angioinvasive disease, 9 had no Aspergillus in their respiratory samples; only 2 of the 11 patients were positive for Aspergillus. These data show a strong association between poor performance of the isolation of Aspergillus from respiratory samples and angioinvasive disease (P = .004).
Of note, CT scan findings were not found to be an independent factor to predict the performance of isolation of Aspergillus from respiratory samples (odds ratio = 1.59; 95% CI, 0.14-17.9 for airway-invasive disease and odds ratio = 0.28; 95% CI, 0.03-2.29 for angioinvasive disease after adjusting for underlying condition and leukocyte status).
No association was found between the lung HRCT pattern and serum or BAL GM. In particular, 7 of 14 (50%) patients with angioinvasive disease and 7 of 15 patients (47%) with airway-invasive disease were positive for serum GM. When analyzing data from patients for whom a BAL GM was available, 3 of 7 (43%) patients with angioinvasive disease and 5 of 7 (71%) patients with airway-invasive disease were positive for BAL GM (P = .63).
Discussion
In our prospective study, we found that the performance of isolation of Aspergillus from respiratory samples from hematologic patients with IPA strongly depended on both the underlying condition and the leukocyte count. Furthermore, mycologic examination was more frequently positive when the CT scan showed signs of small airway-invasive disease. These airway-invasive signs were associated with non-AL patients and leukocyte counts > 100/mm3. Finally, a concomitant pulmonary infection was frequently associated with IPA in non-AL patients.
Overall, we found a mycologic diagnostic yield of bronchoscopy in hematologic patients with IPA that was similar to previously reported results.19-22 However, for the first time, we identified a significant discrepancy with respect to the underlying condition of the patients, with especially poor results in neutropenic patients with AL compared with the other patients.
Most previous studies concerning the clinical presentation of IPA have focused on neutropenic AL patients,5,32 whereas fewer data are available on IPA occurring in allogeneic HSCT recipients9,33 or in patients with another hematologic disorder. In addition, to our knowledge, no previous studies have compared the characteristics of IPA in these different patients. However, strong data have previously shown specificities in the histopathology of IPA according to the underlying condition, which is consistent with our results. Thus, as shown by autopsy, the histopathologic pattern of IPA in HSCT recipients with GVHD consists of severe lung inflammation; in contrast, in patients with neutropenia, the histopathology is characterized by scant inflammation, hyphal angioinvasion, and extensive coagulative tissue necrosis.34 These data are supported by data from animal models, which further detailed pulmonary histology of IPA in these different settings.24,25 Indeed, the lungs of corticosteroid-treated mice showed large foci of pneumonia and exudative bronchiolitis with the destruction of bronchi and alveoli. In contrast, in chemotherapy-treated mice, few bronchiolar lesions were observed other than diffuse pneumonia with edema and congestion within alveoli.25
Lung CT scanning is a major diagnostic tool for IPA, and different CT scan patterns have been correlated to histopathology.10,11,13 Most of the studies about IPA in immunocompromised patients have focused on angioinvasive aspergillosis, although airway-invasive aspergillosis has previously been recognized by radiologists and accounts for 14% to 34% of cases of IPA.9,10,13,35
Histology of a nodule with a halo sign demonstrates occlusion of arteries by plugs of hyphae and the subsequent development of infected pulmonary infarcts, whereas histologic examination of airway-invasive aspergillosis shows colonies of Aspergillus invading through bronchiolar walls together with peribronchiolar inflammation.10,11,13 Therefore, the fact that we found a high performance of mycologic examination of respiratory samples from patients with small-airway lesions on CT scan is consistent with the presence of Aspergillus in the wall of the bronchioles. In contrast, the poor mycologic diagnostic yield of respiratory sample examination from neutropenic AL patients who had nodules with a halo sign on their CT scan may be explained by the observation that no Aspergillus was found in the bronchioles in this context.
Although airway-invasive lesions have received little attention in past studies on aspergillosis, we think that they should be taken into consideration, not only because of our data showing that these lesions are related with the performance of isolation of Aspergillus but also as a diagnostic criterion of IPA. Indeed, in our study, we found that up to 38% of patients with IPA had small-airway lesions on their CT scan, whereas lesions other than “dense, well-circumscribed lesions, air crescent sign and cavity” are not part of the diagnostic criteria in the revised EORTC/MSG consensus.1
Another striking insight from our results is that, in contrast to AL patients, a high proportion of non-AL patients had a concomitant pulmonary infection together with IPA. This finding has a significant impact on the diagnostic approach to confirming suspected IPA in hematologic patients. Indeed, (1) this result should be taken into account for CT scan interpretation, and (2) this finding strengthens the indication of bronchoscopy in non-AL patients. Conversely, for AL patients, in particular for those with profound neutropenia and those without any signs of airway disease on CT scan, bronchoscopy may not be indicated for the identification of a fungus because of the poor mycologic performance of respiratory sample examination, as well as the fact that other pathogens were only occasionally identified. In this setting, however, we could not evaluate the contribution of the BAL GM index to the diagnosis of IPA because of an insufficient number of samples. In AL patients, CT-guided lung biopsy may be an interesting alternative tool to isolate and identify the fungus.36
In conclusion, our results strongly suggest applying a different mycologic diagnostic strategy for AL patients with leukocyte counts < 100/mm3 compared with non-AL patients. Thus, we strongly recommend bronchoscopy for the specific diagnosis of aspergillosis in non-AL patients, in particular in allogeneic HSCT recipients. In AL patients with severe neutropenia, another diagnostic strategy might be considered to isolate the fungus.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by La Ligue Contre le Cancer and Merck Sharp & Dohme (MSD) laboratories.
Authorship
Contribution: A.B., A.S., and P.R. designed research; A.B., A.S., K.C., E.R., A.V., M.C., F.I., B.B., C.L., J.L.P., C.B.M., and P.R. performed research and collected data; A.B., R.P., A.S., C.d.B., K.C., F.D., A.T., and P.R. analyzed and interpreted data; R.P. performed statistical analysis; and A.B., R.P., A.S., F.D., A.T., and P.R. wrote the manuscript.
Conflict-of-interest disclosure: A.B. received advisory board fees from Schering-Plough and speaking fees from Schering-Plough and Pfizer. E.R. received advisory board fees from Genzyme. M.C. received honoraria from Conidia. B.B. received funding from MSD-Chibret for manuscript preparation. C.L. received honoraria from Pfizer and Gilead and speaking fees from MSD. P.R. received advisory board fees from Gilead, Merck Sharp & Dohme, Pfizer, and Schering-Plough and speaking fees from Gilead, Merck Sharp & Dhome, Pfizer, and Schering-Plough. The remaining authors declare no competing financial interests.
Correspondence: Anne Bergeron, Service de Pneumologie, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75475, Paris cedex 10, France; e-mail: anne.bergeron-lafaurie@sls.aphp.fr.