Abstract
Dysfunction of T cells and natural killer (NK) cells has been proposed to determine the course of disease in acute myeloid leukemia (AML), but only limited information is available on the mechanisms of lymphocyte inhibition. We aimed to evaluate to what extent human malignant AML cells use NADPH oxidase-derived reactive oxygen species (ROS) as an immune evasion strategy. We report that a subset of malignant myelomonocytic and monocytic AML cells (French-American-British [FAB] classes M4 and M5, respectively), recovered from blood or BM of untreated AML patients at diagnosis, expressed the NADPH oxidase component gp91phox. Highly purified FAB M4/M5 AML cells produced large amounts of ROS on activation and triggered poly-[ADP-ribose] polymerase-1−dependent apoptosis in adjacent NK cells, CD4+ T cells, and CD8+ T cells. In contrast, immature (FAB class M1) and myeloblastic (FAB class M2) AML cells rarely expressed gp91phox, did not produce ROS, and did not trigger NK or T-cell apoptosis. Microarray data from 207 AML patients confirmed a greater expression of gp91phox mRNA by FAB-M4/M5 AML cells than FAB-M1 cells (P < 10−11) or FAB-M2 cells (P < 10−9). Our data are suggestive of a novel mechanism by which monocytic AML cells evade cell-mediated immunity.
Introduction
Acute myeloid leukemia (AML) is characterized by a deficiency of hematopoietic progenitor and stem cell development with a resulting accumulation of immature myeloid cells in BM.1-3 The current treatment in AML comprises an initial phase of intensive chemotherapy, induction, and consolidation that aims to achieve and maintain complete remission (CR).4,5 Younger patients may subsequently undergo allogeneic stem cell transplantation,6 whereas few therapeutic options are available in the postconsolidation phase for other patients.7 The occurrence of relapse after CR along with the poor postrelapse survival significantly explains the dismal long-term survival of patients with AML.4,8
In several studies investigators point to a role for lymphocytes, such as cytotoxic T cells and natural killer (NK) cells, in the surveillance of the malignant clone in AML and in determining prognosis.9 T cells are considered to mediate the graft-versus-leukemia reaction that significantly accounts for the reduced rate of leukemic relapse after allogeneic stem cell transplantation,10,11 and several tumor-associated antigens of relevance to T-cell reactivity are expressed by AML cells.12 A role for NK cells in surveillance of AML cells was demonstrated, as exemplified by the favorable outcome when authors used transplants with donor/recipient class I disparities, which facilitates NK cell–mediated destruction of residual leukemic cells.13 In addition, multiple deficiencies of T- and NK-cell functions, with ensuing relapse risk and poor prognosis, have been observed in patients with AML who did not undergo transplantation.14-18
In earlier studies, investigators demonstrated that nonmalignant phagocytic cells down-modulate lymphocyte functions by producing and releasing NADPH oxidase-derived reactive oxygen species (ROS).19-23 These findings have formed the basis for the use of a NADPH oxidase inhibitor in conjunction with IL-2 as a relapse-preventive strategy in patients with AML.24,25 In this study, we monitored the surface expression of gp91phox, a component of the ROS-generating NADPH oxidase,26 on leukemic cells recovered from BM and blood of newly diagnosed patients with AML and explored whether ROS produced by leukemic cells compromise T- and NK-cell function. These analyses were performed with cells recovered from patients with defined morphologic subtypes of AML cells on the basis of French-American-British (FAB) classification.27 We report that AML cells from patients with monocytic forms of AML (FAB classes M4/M5), but not cells from patients with myeloblastic AML (FAB class M2) or immature AML (FAB class M1), express the NADPH oxidase, produce ROS, and trigger extensive apoptosis in adjacent T and NK cells. Our results are suggestive of a novel mechanism of immune evasion in myelomonocytic and monocytic AML.
Methods
Sampling of BM and peripheral blood
Peripheral blood or BM from 26 untreated patients with newly diagnosed AML (10 fresh blood samples and 16 frozen BM) was obtained from Sahlgrenska University Hospital or Lund University Hospital. Patient characteristics are described in detail in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Identification of chromosomal or subchromosomal aberrations and FAB classification were performed at participating centers by the use of standard procedures for BM morphology, cytochemistry, and flow cytometry, along with cytogenetics via the use of FISH and/or RT-PCR. Informed written consent was obtained from all patients and blood donors included in this study. The study was approved by the Ethical Committee at the University of Gothenburg.
Cell preparation
Frozen BM samples were thawed quickly, washed in warm medium, and resuspended in Krebs Ringer glucose (KRG) buffer for ROS production measurement and phenotyping. Peripheral blood from patients was diluted 1:1 in sterile PBS, carefully layered on top of Ficoll-Paque (Lymphoprep; Nyegaard) and centrifuged (850g, 20 minutes, room temperature). Mononuclear cells were recovered from the interphase, and pure myeloid populations were obtained by FACS sorting. Purity and phenotype of myeloid cell populations were determined by flow cytometry via the use of fluorochrome-conjugated antibodies against CD14, CD15, CD33, CD34, and gp91phox. In some cases, phenotyping of PBMC was performed after cryopreservation of fresh samples. No difference in the density of gp91phox expression (median fluorescence intensity; MFI) was observed between frozen and fresh samples, nor was there any difference between PBMC or BM from fresh samples. The gp91phox MFI values were normalized against an internal negative lymphocyte control.
PBMCs from healthy donors, obtained by Ficoll-Paque density centrifugation, were further separated into lymphocytes and mononuclear myeloid cells (monocytes) by the use of counter-current centrifugal elutriation technique as described elsewhere.21 Lymphocyte fractions were pooled and subjected to further separation with MACS NK-cell, CD4+ T-cell, or CD8+ T-cell isolation kits (Miltenyi Biotec) according to the instructions provided by the manufacturer.
Flow cytometry
Samples were collected via a 3-laser BD FACSAria (405, 488, and 633 nm) or a 4-laser BD LSRFortessa (405, 488, 532, and 640 nm; BD Biosciences). Sorting was performed via the BD FACSAria flow cytometer. Data were analyzed via the FACSDiva Version 6 software (BD Biosciences) or FlowJo Version 8.4 software (TreeStar).
Confocal microscopy
To obtain confocal photomicrographs, PBMC from patients with AML were stained with anti-CD33 and gp91phox (FITC) and FACS sorted directly onto poly-L-lysine–coated slides (Sigma-Aldrich). Cells were mounted in Prolong Gold Antifade with DAPI (Invitrogen). Photomicrographs were acquired with a Zeiss LSM700 confocal microscope with a 63×/1.40 Oil DIC objective and analyzed with ZEN software.
Detection of ROS
Extracellular superoxide anion production by malignant myeloid cells was determined by the use of isoluminol-enhanced chemiluminescence as described.28 In brief, myeloid cells were suspended at a concentration of 106/mL in KRG buffer supplemented with isoluminol (10 μg/mL) and HRP (4 U/mL). After incubation (5 minutes) at 37°C, phorbol myristate acetate (PMA; 5 × 10−8M) or formyl-methionyl-leucyl phenylalanine (fMLF; 10−7M) was added as NADPH oxidase stimuli. Light emission was recorded continuously in a 6-channel Berthold Biolumat LB 9505 (Berthold Technologies Co).
Assessment of apoptosis in NK and T cells
NK cells and T cells were obtained from healthy donors by use of the MACS technique and cocultured overnight with malignant myeloid cells in 96-well round-bottomed plates. The purity of CD56+ NK and CD4+ T cells was consistently > 95%, and the purity of CD8+ T cells was approximately 90%. The experiments were performed in the presence or absence of the poly[ADP-ribose] polymerase-1 (PARP-1) inhibitor PJ34 (0.5μM), catalase (200 U/mL), the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI; 3μM), or the nitric oxide synthase (NOS) inhibitor nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; 100μM). Lymphocyte apoptosis was determined after 18 hours by use of the violet amine-reactive Dead Cell Stain Kit (Invitrogen).
FISH analysis
FISH analysis was performed on interphase cells via the use of LSI probes from Vysis (Abbott Scandinavia AB) for the detection of CBFB (Dual Color, Break Apart Rearrangement Probe) according to the manufacturer's instructions. Typically, 200 nuclei were scored by 2 independent reviewers.
Quantification of CBFB-MYH11 transcripts (quantitative RT-PCR)
RNA was extracted by the use of an automated poly-A RNA purification method, and cDNA was generated by reverse transcription with random primers and the Superscript II enzyme (Invitrogen) according to the manufacturer's instructions. The CBFB-MYH11 transcripts were detected and quantified by real-time PCR with a RotorGene 3000 instrument (QIAGEN) with GUS as the endogenous control gene.
Mutational analysis of CEBPA, NPM1, and FLT3-ITD
Western blot detection of PAR
Lymphocytes were exposed to hydrogen peroxide in the presence or absence of PARP-1 inhibitor PJ34 (0.5μM) for 20 minutes. Whole-cell lysate was obtained by lysis in RIPA buffer and PAR detected, after electrophoresis and transfer to nitrocellulose membrane, with an anti-poly(ADP)–ribose monoclonal antibody (BD Biosciences), followed by a HRP-conjugated secondary antibody (Dako).
H2O2 consumption assay
H2O2 consumption was assayed via a p-hydroxyphenylacetic acid–based system described elsewhere.31 In brief, DPI, PJ34, and catalase were incubated with 50μM of H2O2 for 15 minutes (RT) and remaining H2O2 measured as fluorescent p-hydroxyphenylacetic acid dimers.
Antibodies and reagents
The following anti–human monoclonal antibodies were purchased from BD Biosciences: anti-CD33 (clone P67.6, PE-Cy7), anti-CD34 (clone 8G12, PE), anti-CD56 (clone NCAM16, PE-Cy7, APC), anti-CD14 (clone MϕP9, APC-Cy7), anti-CD15 (clone HI98, APC), anti-CD4 (clone SK3, FITC), and anti-CD8 (clone SK1, FITC). Antiflavocytochrome b558 (gp91phox; clone 7D5, FITC) was from MBL International. CD14 (clone TüK4, Pacific blue) was from Invitrogen. The following compounds were used: isoluminol, PMA, fMLF, DPI, L-NAME, and catalase (Sigma-Aldrich); dextran (Kabi Pharmacia); Ficoll-Hypaque, Lymphoprep (Nycomed); Live/Dead fixable violet dead cell stain (Invitrogen); and HRP (Boehringer-Mannheim).
Statistical analysis
For multiple comparisons within a dataset, 1-way ANOVAs with the Bonferroni posttest were performed. Student t test was used for single comparisons. Correlations were calculated by use of the Spearman rank correlation. All indicated P values are 2-sided. P < .05, P < .01, and P < .001.
Results
BM cells from patients with FAB-M4 and FAB-M5 AML produce ROS and express the NADPH oxidase subunit gp91phox
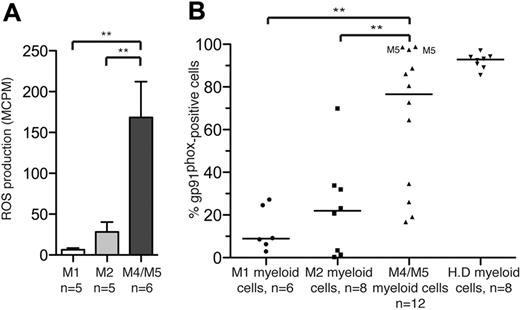
The FAB classification divides AML into 8 subtypes, M0 to M7, on the basis of the morphology of the dominating malignant clone and its maturation stage.27 In a first series of experiments, we aimed to determine to what extent nonerythroid cells in BM of newly diagnosed AML patients produced ROS. Using a sensitive chemiluminescence method,28 we assessed extracellular ROS production on stimulation with the NADPH oxidase activator PMA32 in BM samples from untreated patients diagnosed with FAB-M1, -M2, -M4, or -M5 AML. It was found that the production of ROS in BM was almost exclusively confined to monocytic forms of AML, that is, FAB classes M4 and M5. BM cells of these FAB classes produced significantly greater levels of ROS than BM cells from FAB-M1 (P < .01) or FAB-M2 (P < .01) AML (Figure 1A).
FAB-M4 and -M5 AML cells produce ROS and express the NADPH oxidase subunit gp91phox. (A) Nonerythroid BM cells recovered at diagnosis from untreated patients with FAB-M1 AML, FAB-M2 AML, or FAB-M4/M5-AML (patients 11-26) were stimulated with PMA (5 × 10−8M) and assayed for ROS production. Bars represent extracellular ROS ± SEM. (B) Myeloid cells in BM or peripheral blood cells from newly diagnosed, untreated AML patients were analyzed by FACS for expression of the NADPH oxidase subunit gp91phox. The data points represent the percentage of CD33+ and/or CD34+ cells expressing gp91phox, with medians indicated. Among FAB-M4/M5 cells, M5 indicates FAB-M5 cells. H.D represents gp91phox expression by peripheral blood monocytes from healthy donors.
FAB-M4 and -M5 AML cells produce ROS and express the NADPH oxidase subunit gp91phox. (A) Nonerythroid BM cells recovered at diagnosis from untreated patients with FAB-M1 AML, FAB-M2 AML, or FAB-M4/M5-AML (patients 11-26) were stimulated with PMA (5 × 10−8M) and assayed for ROS production. Bars represent extracellular ROS ± SEM. (B) Myeloid cells in BM or peripheral blood cells from newly diagnosed, untreated AML patients were analyzed by FACS for expression of the NADPH oxidase subunit gp91phox. The data points represent the percentage of CD33+ and/or CD34+ cells expressing gp91phox, with medians indicated. Among FAB-M4/M5 cells, M5 indicates FAB-M5 cells. H.D represents gp91phox expression by peripheral blood monocytes from healthy donors.
We next analyzed the proportion of cells expressing of gp91phox, a subunit of the ROS-generating NADPH oxidase,26 on myeloid cells in BM and blood from patients with FAB class M1 (n = 6), M2 (n = 8), M4 (n = 10), and M5 (n = 2) AML. It was found that gp91phox was significantly more frequently expressed by cells from FAB-M4/M5 AML than by FAB-M1 or FAB-M2 cells (P < .001 and P < .01, respectively; Figure 1B). A strong correlation was observed between the proportion of gp91phox/NADPH oxidase-expressing myeloid cells and ROS production in unsorted BM from patients with AML (P < .0001; supplemental Figure 1). In addition, gp91phox mRNA expression levels were investigated in microarray data deposited by Valk et al.33 The data discussed in this publication have been deposited in NCBI's Gene Ex-pression Omnibus and are accessible through GEO Series accession number GSE1159. This analysis showed that the expression of gp91phox mRNA was significantly greater in AML cells, recovered at diagnosis, from FAB-M4/M5 patients compared with FAB-M1 (P < 10−11) and FAB-M2 (P < 10−9) patients (supplemental Figure 2).
Expression of functional gp91phox/NADPH oxidase by mature myeloid cells of the FAB-M4/M5 subclasses
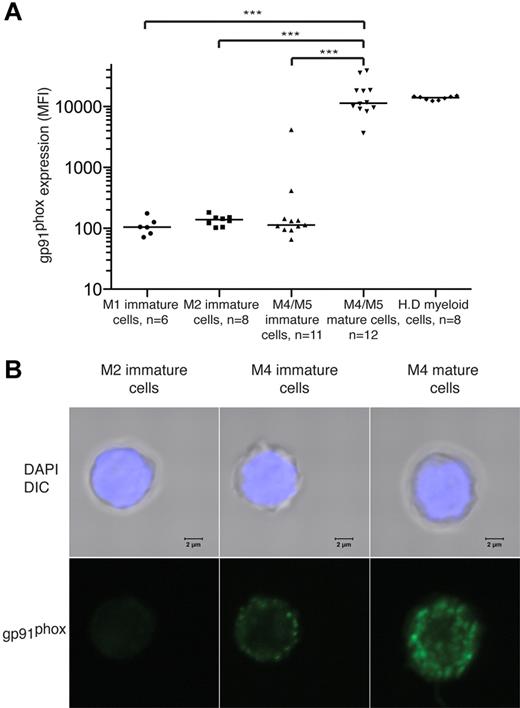
To further define the gp91phox+ cell population, we compared the intensity of gp91phox expression by mature (CD14+) FAB-M4/M5 cells and immature (CD14− or CD15−) FAB-M1, -M2, and -M4/M5 cells. As shown in Figure 2A, gp91phox was almost invariably expressed by CD14+ cells from patients with FAB-M4/M5 AML. Figure 2B visualizes by confocal microscopy the presence of membrane-bound gp91phox in FACS-sorted mature cells from a patient with FAB-M4 AML. One hundred percent of the sorted cells carried the t(16;16) chromosomal rearrangement.
gp91phox is expressed by mature malignant FAB-M4 and FAB-M5 AML cells. BM or peripheral blood cells from patients with FAB-M1, FAB-M2, or FAB-M4/M5 AML were stained for gp91phox. Results show intensity of gp91phox expression (MFI) of cells with CD33+ and/or CD34+ phenotype with medians indicated. Immature cells were defined by absence of CD14 and CD15. Mature FAB-M4/M5 cells were defined as cells expressing CD14. H.D represents gp91phox expression by peripheral blood monocytes from healthy donors. (B) Confocal photomicrographs of FACS-sorted immature M2 cells (left), immature M4 cells (center), and mature M4 cells (right) showing gp91phox expression. The sorted FAB-M2 (patient 2) cells were NPM1+ and FLT3/ITD+. Immature and mature cells from the patient with FAB-M4 AML (patient 6) carried t(16;16). The nucleus was made visible by DAPI staining (blue) and gp91 was visualized using a FITC-conjugated primary antibody. Bars represent 2 μm.
gp91phox is expressed by mature malignant FAB-M4 and FAB-M5 AML cells. BM or peripheral blood cells from patients with FAB-M1, FAB-M2, or FAB-M4/M5 AML were stained for gp91phox. Results show intensity of gp91phox expression (MFI) of cells with CD33+ and/or CD34+ phenotype with medians indicated. Immature cells were defined by absence of CD14 and CD15. Mature FAB-M4/M5 cells were defined as cells expressing CD14. H.D represents gp91phox expression by peripheral blood monocytes from healthy donors. (B) Confocal photomicrographs of FACS-sorted immature M2 cells (left), immature M4 cells (center), and mature M4 cells (right) showing gp91phox expression. The sorted FAB-M2 (patient 2) cells were NPM1+ and FLT3/ITD+. Immature and mature cells from the patient with FAB-M4 AML (patient 6) carried t(16;16). The nucleus was made visible by DAPI staining (blue) and gp91 was visualized using a FITC-conjugated primary antibody. Bars represent 2 μm.
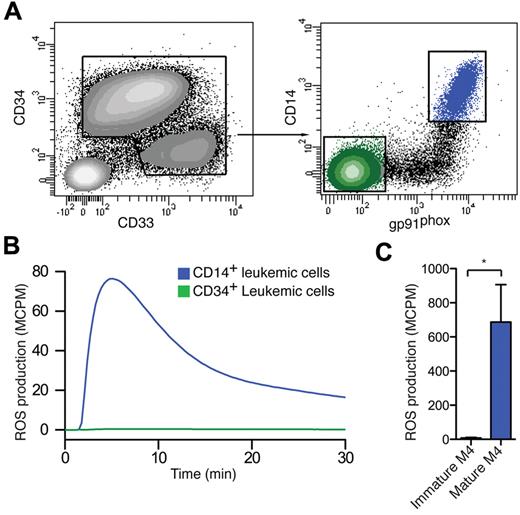
To clarify whether the gp91phox expression by AML cells reflected a functional NADPH oxidase, mature and immature myeloid cells from patients with FAB-M4 AML were sorted with FACS by the use of the gating strategies shown in Figure 3A and stimulated with the NADPH oxidase activator PMA for assay of ROS production. The sorted mature FAB-M4 cells carrying gp91phox produced ROS in response to NADPH oxidase activation, whereas immature M4 cells did not (Figure 3B). Figure 3C shows a comparison of ROS production between sorted mature and immature FAB-M4 cells. Immature as well as mature cells used in these experiments belonged to the malignant clone, as verified by FISH, quantitative RT-PCR, or mutational PCR analysis
Sorted CD14+ gp91phox + mature malignant from FAB M4/M5 AML produce ROS. (A) A representative set of FACS plots of a FAB-M4 AML patient (patient 6) with 2 distinct myeloid populations, one immature blast population CD34+CD33−CD14− (green) and one monocyte-like CD34−CD33+CD14+ (blue) population, is shown. (B,C) FACS-sorted mature CD14+ leukemic cells (blue) and immature CD34+ leukemic cells (green) were stimulated with PMA and assayed for ROS production. Panel B shows the kinetics of a representative experiment, and panel C shows total ROS production (n = 3, patients 5-7).
Sorted CD14+ gp91phox + mature malignant from FAB M4/M5 AML produce ROS. (A) A representative set of FACS plots of a FAB-M4 AML patient (patient 6) with 2 distinct myeloid populations, one immature blast population CD34+CD33−CD14− (green) and one monocyte-like CD34−CD33+CD14+ (blue) population, is shown. (B,C) FACS-sorted mature CD14+ leukemic cells (blue) and immature CD34+ leukemic cells (green) were stimulated with PMA and assayed for ROS production. Panel B shows the kinetics of a representative experiment, and panel C shows total ROS production (n = 3, patients 5-7).
We next aimed to define the size of the gp91phox+ mature population of FAB-M4/M5 AML. As expected, a greater proportion of myeloid cells from BM or blood of patients with FAB-M4 and FAB-M5 AML expressed CD14 and/or CD15, which were used as markers of maturation, (range 25.2%-98.8%) compared with FAB-M1 AML (3.6%-42.7%, P < .001) or FAB-M2 AML (0.3%-69.2%, P < .01; supplemental Figure 3). In addition, the median fluorescence intensity of gp91phox/NADPH expression was significantly greater (median, 5468) in mature FAB-M4/M5 AML cells than in similarly gated FAB-M2 cells (median, 782; P < .05; supplemental Figure 3). To determine whether the percentage of circulating monocytes differs between subclasses of AML in CR, we used blood counts from 109 patients24 and found no differences between the M1, M2, and M4/M5 FAB classes (supplemental Figure 4A). Phenotyping of PBMC from 15 patients in first CR revealed similar levels of gp91phox expression on circulating monocytes between FAB classes (supplemental Figure 4B).
Mature M4/M5 AML cells trigger ROS-dependent cell death in lymphocytes
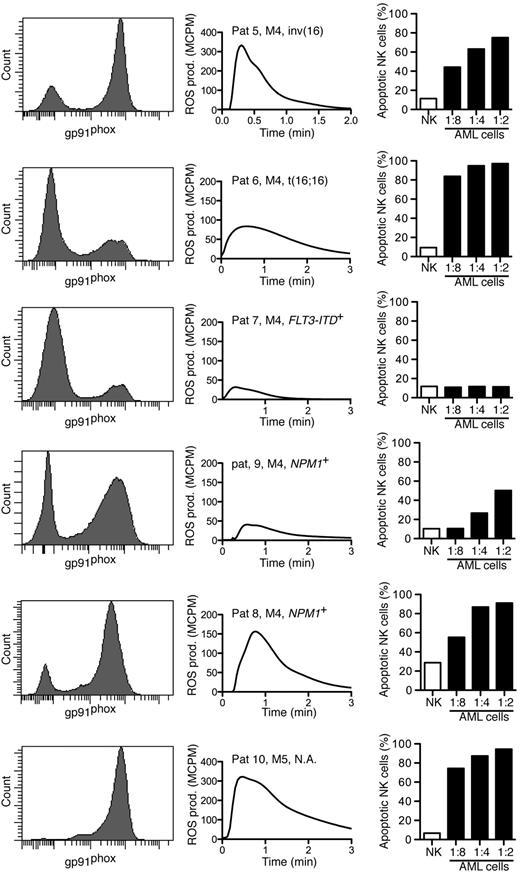
To elucidate whether NADPH oxidase-derived ROS produced by FAB-M4/M5 AML cells triggered apoptosis in cytotoxic lymphocytes, NK cells from healthy donors were exposed to FACS-sorted mature malignant or immature malignant cells by use of the gating strategies outlined previously. In accordance with the results shown in Figure 3, all patients carried a population of gp91phox+ cells that produced ROS on NADPH oxidase activation (Figure 4 center). In 5 of 6 patients, the sorted mature myeloid cells triggered apoptosis in a significant fraction of NK cells, albeit at different NK to AML cell ratios (Figure 4 right). Immature leukemic AML cells did not trigger lymphocyte apoptosis regardless of FAB class origin (data not shown). In these experiments, it was confirmed that the ROS-producing cell populations belonged to the malignant clone, as verified by use of FISH or PCR, in 5 of 6 patients; the sixth patient had AML with normal karyotype with no known subchromosomal aberrations.
Sorted mature FAB-M4/M5 AML cells trigger apoptosis in NK cells. Each row shows data obtained from individual patients with FAB-M4 or FAB-M5 AML as indicated. Histograms show the expression of gp91phox in myeloid cells. Center panels show fMLF-induced ROS production by the corresponding sorted gp91phox-positive population. Right panels show NK-cell apoptosis after overnight incubation with mature leukemic cells at indicated ratios of AML cells to NK cells.
Sorted mature FAB-M4/M5 AML cells trigger apoptosis in NK cells. Each row shows data obtained from individual patients with FAB-M4 or FAB-M5 AML as indicated. Histograms show the expression of gp91phox in myeloid cells. Center panels show fMLF-induced ROS production by the corresponding sorted gp91phox-positive population. Right panels show NK-cell apoptosis after overnight incubation with mature leukemic cells at indicated ratios of AML cells to NK cells.
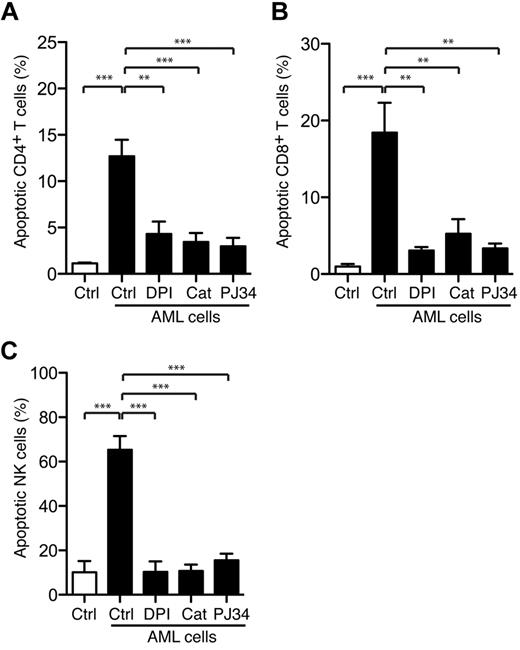
The leukemia-induced apoptosis was not confined to NK cells because mature FAB-M4/M5 AML cells also induced significant apoptosis in CD4+ and CD8+ T cells (Figure 5A-C; P < .001 for all comparisons). To clarify whether the leukemia-induced apoptosis was mediated by NADPH oxidase-derived ROS, we pretreated mature FAB-M4 or FAB-M5 AML cells with the combined NADPH-oxidase and NOS inhibitor DPI (3μM), which rescued NK cells as well as CD4+ and CD8+ T cells from apoptosis (Figure 5A-C; P < .001, P < .01, and P < .01, respectively), whereas the specific NOS inhibitor L-NAME (100μM) did not. In contrast, catalase (200 U/mL), which degrades hydrogen peroxide into water and molecular oxygen,34 significantly prevented AML cell-induced apoptosis (Figure 5). Taken together, these results imply that lymphocyte apoptosis induced by FAB-M4/M5 AML cells was specifically mediated by ROS produced by NADPH oxidase/gp91phox-expressing cells.
Leukemic cells trigger PARP-1–dependent T-cell and NK-cells apoptosis. Bars show the percentage of apoptosis ± SEM. (A) CD4+ T cells (n = 3), (B) CD8+ T cells (n = 3), and (C) NK cells (n = 5) after overnight incubation with FACS-sorted mature malignant FAB-M4/M5 AML cells in the presence or absence of the NADPH oxidase inhibitor DPI (3μM), catalase (200 U/mL), or the PARP-1 inhibitor PJ34 (0.5μM). For T cells, the AML to T-cell ratio was 1:1, whereas the lowest AML to NK-cell ratio to reach 50% NK-cell apoptosis is presented (range, 1:8-1:2).
Leukemic cells trigger PARP-1–dependent T-cell and NK-cells apoptosis. Bars show the percentage of apoptosis ± SEM. (A) CD4+ T cells (n = 3), (B) CD8+ T cells (n = 3), and (C) NK cells (n = 5) after overnight incubation with FACS-sorted mature malignant FAB-M4/M5 AML cells in the presence or absence of the NADPH oxidase inhibitor DPI (3μM), catalase (200 U/mL), or the PARP-1 inhibitor PJ34 (0.5μM). For T cells, the AML to T-cell ratio was 1:1, whereas the lowest AML to NK-cell ratio to reach 50% NK-cell apoptosis is presented (range, 1:8-1:2).
Role of poly[ADP-ribose] polymerase activity in leukemia-induced apoptosis
Nonmalignant phagocytic cells produce ROS that trigger activation of PARP-1, which results in the formation of polymers of ADP-ribose (PAR) that translocate to mitochondria to release apoptosis-inducing factor with ensuing DNA fragmentation and cell death.20,35 To determine whether AML cell-induced lymphocyte apoptosis was triggered via the PARP-1/apoptosis-inducing factor pathway, we used PJ34, a PARP-1 inhibitor.36 To ensure the specificity of PJ34, we verified that PAR formation in lymphocytes after exposure to exogenous ROS was completely prevented by PJ34 and that PJ34 was devoid of nonspecific scavenging activity for hydrogen peroxide (supplemental Figure 5). The addition of PJ34, at a concentration sufficient to inhibit PAR formation, efficiently blocked both the NK-cell (P < .001) and T-cell apoptosis (CD4+ T cells, P < .001; CD8+ T cells, P < .01) induced by FAB-M4 and -M5 AML cells (Figure 5).
Discussion
A main finding in this study was that primary mature AML cells of monocytic origin (FAB classes M4 and M5) generate extracellular ROS via the NADPH oxidase complex and that ROS produced by FAB-M4/M5 AML cells kill T cells and NK cells by triggering PARP-1–dependent apoptosis. Although similar ROS-mediated immunosuppression has been reported to be exerted by nonmalignant myeloid cells,20,21,23,37 this is the first observation to suggest that malignant AML cells use ROS to evade antileukemic effector lymphocytes. The capacity of leukemic cells to induce apoptosis correlated with the presence of a functional NADPH oxidase, as reflected by the expression of the oxidase component gp91phox and ROS production by the leukemic cells.
Our results imply that the leukemia-induced lymphocyte apoptosis was specifically or selectively mediated by mature leukemic cells. Thus, among FAB-M4/M5 cells, only mature cells carried a functional NADPH oxidase and triggered T- and NK-cell apoptosis. FAB-M0, FAB-M1, and FAB-M2 AML are dominated by immature cells: FAB-M0 and FAB-M1 leukemic cells are almost invariably immature, whereas the malignant clone in FAB-M2 AML typically comprises a high proportion of immature granulocyte progenitor cells (myeloblasts).27 Accordingly, myeloid cells with immunosuppressive properties (eg, high gp91phox expression) were rarely detected in these morphologic subtypes of AML, and the capacity of BM cells to generate ROS was almost exclusively confined to FAB-M4/M5 leukemias. The source of ROS in FAB-M4/M5 BM was not formally established, but there was a strong correlation between ROS production and the percentage of BM cells expressing the gp91phox/NADPH oxidase, implying that ROS in BM was contributed mainly by mature NADPH oxidase-expressing myeloid cells. ROS production and the capacity to trigger apoptosis in antileukemic lymphocytes were exerted by AML cells with defined chromosomal and/or subchromosomal aberrations, thus establishing immunosuppressive properties of cells of the leukemic clone. We observed similar gp91phox expression by circulating monocytes recovered from monocytic and nonmonocytic AML in CR, thus implying that the observed FAB class-related differences regarding ROS production and immunosuppression are not applicable to nonmalignant cells.
Although the clinical course in monocytic forms of AML is not distinctly different from that of AML of other FAB classes,38,39 we speculate that this novel mechanism of leukemia-related immunosuppression may impact on immune-mediated clearance of leukemic cells, and thus influence prognosis. In this regard, it is of note that FAB-M4 or -M5 AML has been identified as an independent risk factor for leukemic relapse after allogeneic stem cell transplantation,40-43 and the possibility that this phenomenon may be related to inactivation of the graft-versus-leukemia reaction by M4/M5 cell–derived ROS should be further studied. In addition, posthoc analyses within a phase 3 trial in which investigators used the NADPH oxidase inhibitor histamine dihydrochloride in conjunction with the T- and NK-cell–activating cytokine IL-2 for relapse prevention in AML24,25,44 showed that immunotherapy with this combination significantly improved the leukemia-free survival of patients with FAB-M4/M5 AML but did not improve outcome in those with FAB-M2 AML.44
In summary, our data are suggestive of novel mechanism of immune escape in AML: mature malignant cells in monocytic forms of leukemia have the capacity to produce ROS via the NADPH oxidase and thus trigger PARP-1–dependent cell death in adjacent antileukemic lymphocytes. These findings may have implications for the development of improved immunotherapeutic regimens in AML of the FAB-M4/M5 subclasses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Ulla Gingsjö and Jenny Hendel for expert technical assistance.
This study was supported by the Swedish Cancer Society (Cancerfonden), the Swedish Research Council, Torsten och Ragnar Söderberg's Foundation, the Inga-Britt and Arne Lundberg Research Foundation, the Gunvor and Ivan Svensson Foundation, the Assar Gabrielsson Foundation, Sahlgrenska University Hospital, and the Sahlgrenska Academy at University of Gothenburg.
Authorship
Contribution: J.A., F.B.T., A.A., L.P., and A.M. performed experiments and drafted the figures; all authors contributed in collecting and interpreting data; M.B., M.H., and L.P. provided clinical samples; and J.A., F.B.T., K.H., and A.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: K.H. holds issued and pending patents on the use of immunotherapy in AML. M.B. and K.H. are past or present advisors to the Epicept Corporation, New York, and Meda, Stockholm, Sweden. The remaining authors declare no competing financial interests.
Correspondence: Kristoffer Hellstrand, Sahlgrenska Cancer Center, The Sahlgrenska Academy at University of Gothenburg, Box 425, SE-40530 Gothenburg, Sweden; e-mail: kristoffer.hellstrand@microbio.gu.se.