Abstract
The atypical chemokine receptor D6 is a decoy and scavenger receptor for most inflammatory CC chemokines and prevents the development of exacerbated inflammatory reactions. Here we report that mice lacking D6 expression in the nonhematopoietic compartment have a selective increase in the number of Ly6Chigh monocytes in the circulation and in secondary lymphoid tissues. Under inflammatory conditions, Ly6Chigh monocytes accumulate in increased number in secondary lymphoid organs of D6−/− mice in a CCR2-dependent manner. Ly6Chigh monocytes derived from D6−/− mice have enhanced immunosuppressive activity, inhibit the development of adaptive immune responses, and partially protect mice from the development of GVHD. Thus, control of CCR2 ligands by D6 regulates the traffic of Ly6Chigh monocytes and controls their immunosuppressive potential.
Introduction
The chemokine system represents the main orchestrator of leukocyte recruitment.1–4 Among several regulatory mechanisms, a prominent role has been recently recognized to a set of chemokine receptors indicated as atypical in that structurally unable to directly mediate cell migration.5 Atypical chemokine receptors are structurally and functionally heterogeneous, but they share the biologic role of regulating signaling receptors' activity, by clearance, transport, or presentation of their cognate ligands.5–7 D6 is one of these atypical chemokine receptors, and its main function is to bind and drive to degradation the majority of inflammatory CC chemokines.8–10 By this chemokine scavenger function, D6 acts as a negative regulator of inflammation, as demonstrated by D6-deficient mice that are characterized by increased levels of inflammatory CC chemokines and development of exaggerated inflammatory reactions in different organs expressing D6 on lymphatic vessels,11 including skin,12,13 liver,14 colon,15,16 lungs,17,18 and placenta, where its expression is restricted to trophoblast cells.19 On the contrary, in certain conditions, the uncontrolled local inflammation observed in D6−/− mice has been shown to impair the development of specific immune responses, protecting mice from development of autoimmune diseases.20 Although D6−/− mice have been investigated extensively, several aspects of D6 biology, including the precise mechanisms by which it regulates inflammatory responses, are not yet fully understood.
In recent years, it became evident that inflammatory CC chemokines control not only monocyte recruitment at inflammatory sites but also regulate monocyte homeostasis and mobilization from the BM in infectious and hypercholesterolemia models.21–23 Based on the expression of the myeloid differentiation antigen Ly6C and the chemokine receptors CCR2 and CX3CR1, 2 phenotypic and functional monocyte subsets have been described in mice.24 Classic monocytes are Ly6Chigh/CCR2+/CX3CR1low and are also called inflammatory monocytes because they produce high levels of TNF-α and IL-1β in inflamed tissues and lymph nodes (LNs) during infection or tissue damage.25 The second subset is represented by nonclassic Ly6C−/CCR2−/CX3CR1high monocytes, also called patrolling monocytes because they crawl on the endothelium in homeostatic conditions, although they are the first extravasating cell type during an inflammatory response.26 Interestingly, cells with classic monocyte phenotype are a component of the heterogeneous population of myeloid cells with immunoregulatory function called myeloid-derived suppressor cells (MDSCs).27 This population consists of cells of myeloid origin (CD11b+) that are labeled by the specific antibody to Gr1 that recognizes both the Ly6C and the Ly6G markers on monocytes and neutrophils, respectively.28 Here we report that D6 expressed on nonhematopoietic compartment controls the traffic of monocyte subsets in a CCR2-dependent manner. In its absence, the immunosuppressive properties of Ly6Chigh monocytes are unleashed, resulting in a partial protection from the development of GVHD.
Methods
Reagents
Human CCL2 and Fluorokine MAP Mouse Multianalyte Profiling Base Kit or ELISA Kits were from R&D Systems. When not specified, reagents were purchased from Sigma-Aldrich. Anti-DEC205 OVA-conjugated was provided by F.B. Depleting anti-Gr1 (RB6-8C5) antibody and rat IgG2b isotype control (inoculated intraperitoneally 200 μg, every 3 days) were purchased from BioXCell.
Animals
The generation and genotyping of B6 D6−/− mice have been described previously13 ; mice were backcrossed onto the BALB/c background for 10 generations. B6 OVA-specific, TCR transgenic (OT-I) mice were purchased from The Jackson Laboratory and crossed with B6 CD45.1 mice (Ly5.1). B6 Ly5.1 wild-type (WT) and CCR2−/− mice were purchase from The Jackson Laboratory. All colonies were maintained in a specific pathogen-free/viral antibody-free barrier facility at Charles River Italia. Mice in each group were littermate controlled and used at 8 to 12 weeks of age. All procedures with the animals were in accordance with institutional guidelines and with national (DL 116, Gazzetta Ufficiale della Repubblica Italiana, supplement 40, 18-2-1992) and international law and policies (European Economic Community Council, 1987, Directive 86/609, Official Journal of European Communites L 358,1; and Institute of Laboratory Animal Resources, Committee on Life Sciences, National Research Council, 1996, Guide for the Care and Use of Laboratory Animals).
Phenotypic analysis and cell isolation
FITC-, PE-, allophycocyanin (APC)–, peridinin chlorophyll protein (PerCP)–, or biotin-conjugated anti–mouse mAbs to CD3e (145-2C11), CD4 (GK1.5) CD8a (53-6.7), CD11b (M1/70), CD19 (1D3), CD45 (30-F11), Ly6C (AL-21), Ly6G (1A8), Gr1 (RB6-8C5), I-A/I-E (2G9), CD11c (HL3), and NK-1.1 (PK136), as well as isotype-matched control antibodies and fluorescent dye-conjugated streptavidin, Trucount beads, and FITC BrdU Flow Kit were purchased from BD Biosciences PharMingen. Anti–mouse mAbs to CD115 (AFS98) PE, PDCA-1 (129c) APC, CD45.1 (A20) PECy7, CD45.2 (104) APC, FoxP3 (FJK-16s) AlexaFluor 647, and CD25 (PC61.5) PE were purchased from eBioscience. Anti–mouse F4/80 (A3-1) PE was purchased from Serotec. Dead cells were excluded by Violet Cell Death Kit (Invitrogen). Flow cytometry was performed using FACSCanto II instrument and FACSDiva Version 6.1.1 software (BD Biosciences). Blood was collected by eye extraction, BM was flushed from femurs and tibias, and LNs were enzymatically disaggregated with collagenase type 4 (1.6 mg/mL) and DNase (0.1 mg/mL) in RPMI for 30 minutes at 37°C. Spleen suspensions were obtained using MACS Dissociator (Miltenyi Biotec) and erythrocytes lysis (0.15M NH4Cl, 10 nM KHCO3, 1nM Na4EDTA, pH 7.2). Total T cells, CD4+ T cells, CD8+ T cells, and dendritic cells (DCs) were isolated from splenocytes using the Pan T-cell, CD4+ T-cell, and CD8+ T-cell Isolation Kit and Dendritic Cell Isolation Kit, respectively; granulocytic (CD11b+/Ly6Ghigh/Ly6Clow/int) and monocyte-like (CD11b+/Ly6G−/Ly6Chigh) cells were isolated from spleen by CD11b magnetic bead enrichment (Miltenyi Biotec) and sorting of stained CD11b+Ly6C+ cells with FACSAria cell sorter (BD Biosciences).
Quantitative RT-PCR
Total RNA was extracted from MDSCs with miRNA easy Mini kit (QIAGEN). Reverse transcription was done using High Capacity cDNA Reverse Trascription Kit (Applied Biosystems). Quantitative PCR was performed with TaqMan Gene Expression Assays in a 7900 HT Fast Real-Time PCR System (Applied Biosystems).
Mixed leukocyte reaction and in vitro T-cell proliferation
For mixed leukocyte reaction, B6 WT and D6−/− splenocytes or LN cells (5 × 105/well), and CD4+ T cells or CD8+ T cells (1 × 105/well) were cocultured with BALB/c DC (2 × 104/well) in triplicate in 96-well (Corning Life Sciences) containing complete DMEM for 72 hours. Gr1+ cells were depleted using biotin-conjugated anti–mouse Gr1 Ab followed by anti-biotin magnetic beads from Miltenyi Biotec. T-cell proliferation was tested stimulating OT-I splenocytes (2.5 × 105) with 0.25 mg/mL OVA peptide with indicated ratios of CD11b+/Ly6Ghigh/Ly6Clow/int and CD11b+/Ly6G−/Ly6Chigh cells in flat-bottom 96-well plates in complete RPMI medium for 72 hours. Supernatants were harvested after 72 hours to measure IFN-γ production. To evaluate the effect of inhibitors, OT-I splenocytes were diluted in B6 splenocytes (6 × 105/well) to achieve 2% of Ag-specific CD8+ T cells, and CD11b+ cells were added at 24% of total cells.29 Splenocytes were cultured for 48 hours with 0.25 mg/mL OVA peptide in presence of L-NMMA (20μM, Calbiochem), BEC (100μM; Enzo Life Sciences), or SC-58125 (5μM; Sigma-Aldrich). For all experiments, cells were pulsed with 1 μCi/well [3H] thymidine for 18 hours, and incorporation was measured using 1450 Microbeta Jet Counter (Perkin Elmer). For in vitro T-cell proliferation assay, CD11b+/Ly6G+, CD11b+/Ly6Chigh, and total CD11b+ cells were obtained from mice treated subcutaneously with complete Freund adjuvant (CFA) for 2 days (200 μL).
In vivo transgenic T-cell proliferation
For adoptive transfer experiments, OVA-specific TCR transgenic CD8+ T cells, obtained by spleens and LNs of OT-I mice using a CD8 isolation kit (Miltenyi Biotec), were labeled with 2μM CFSE (Invitrogen) for 10 minutes at room temperature and injected intravenously into B6 mice (1 × 106) that were immunized 18 hours later with recombinant anti-DEC205 OVA (50 ng/footpad) and lipopolysaccharide (100 ng/mouse). Three days after immunization, popliteal LNs were collagenase digested and CD8+ T-cell proliferation was assessed using flow cytometry.
Adoptive transfer of BM monocytes
BM monocytes were isolated from B6 Ly5.2 CCR2−/− and Ly5.1 WT mice. Cell suspensions were incubated with biotin anti-Ly6G, anti-CD3e, and anti-CD19 followed by incubation with MACS anti-biotin beads (Miltenyi Biotec). After depletion, cells were stained with biotin anti-Ly6C followed by MACS antibiotin beads. After positive selection, these cells (5 × 106/mL) were labeled with 1μM CFSE (Invitrogen). Recipient B6 Ly5.2 D6−/− and WT mice were treated subcutaneously with CFA (200 μL) 6 hours before intravenous injection of 5 × 106 CCR2−/− and 5 × 106 Ly5.1 WT cells (ratio 1:1). After 2 days, CFSE+ cells were analyzed in spleen.
BMDM
BM cells were obtained from femurs of B6 WT and CCR2−/− mice and incubated overnight at 37°C with complete medium. Nonadherent cells (5 × 105 cells/well) were then cultured in DMEM containing 5% FCS and 20 ng/mL mM-CSF adding hCCL2 (10 ng/mL) for 24 or 48 hours in 24-well ultra-low attachment plates (Corning Life Sciences).
BM transfer procedure
Recipient mice were maintained on antibiotic water (800 mg/mL gentamycin; Italfarmaco) for 1 week before and 1 week after lethal irradiation (900 cGy). Ly5.2 B6 D6−/− or WT mice were reconstituted with Ly5.1 B6 WT BM cells. Reverse chimeric mice were created by irradiating Ly5.1 mice and reconstituting them with either Ly5.2 D6−/− or WT BM cells. BM cells (4 × 106) were injected through the retro-orbital venous plexus 1 to 3 hours after irradiation. Blood was analyzed after 15 weeks of BM transfer.
Induction of GVHD
BALB/c D6−/− or WT mice received 2 doses of lethally irradiation (700 cGy) followed by intravenous infusion of donor B6 D6−/− or WT BM cells (1 × 107) and B6 D6−/− or WT splenocytes (1.5 × 107). The reverse experiments were performed using irradiated B6 WT or D6−/− mice (900 cGy) as recipients and BALB/c BM (1 × 107 cells) and splenocytes (3 × 107). Mice were monitored every 2 days after transplantation for survival and by a standard scoring system described previously.30
Statistical analysis
Data were analyzed by unpaired Student t test. Survival curves were compared using log-rank test with GraphPad Prism Version 4 software.
Results
Increased Ly6Chigh monocytes in blood and spleen of D6−/− mice
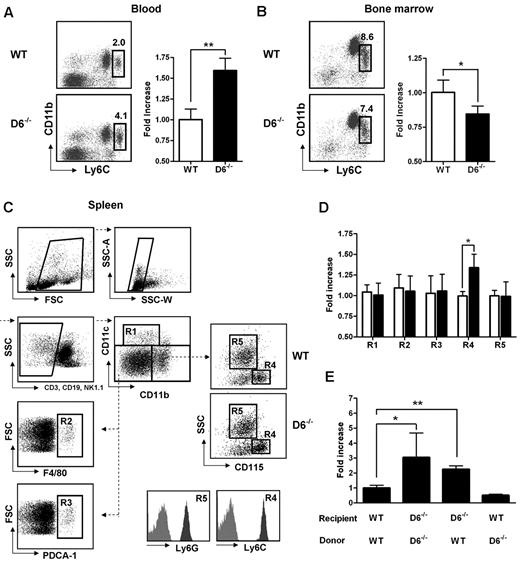
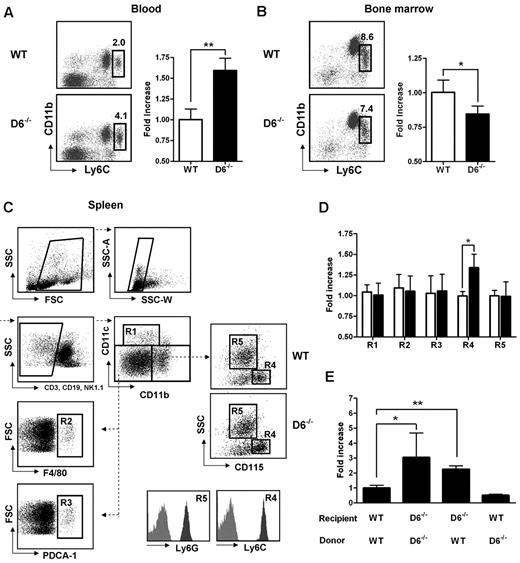
D6−/− mice display enhanced serum chemokine titers both in homeostatic and inflammatory conditions and show an increased inflammatory response in several in vivo models.12–19 To understand how inflammatory chemokine scavenging performed by D6 impacts on homeostatic leukocyte traffic, analysis of leukocytes in blood, spleen, and BM cells was performed. No differences were found between WT and D6−/− mice in the frequency and absolute number of T lymphocytes (CD3+/CD4+, CD3+/CD8+, CD3+/CD25+/Foxp3+), B lymphocytes (CD19+), and granulocytes (CD11b+/Ly6G+; supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Conversely, analysis of monocyte subsets indicated a selective increase in frequency and absolute number of classic monocytes (CD11b+/CD115+/Ly6Chigh) in blood (Figure 1A), whereas a slight but significant decrease of Ly6Chigh monocytes frequency was observed in BM of D6−/− compared with WT mice (Figure 1B), suggesting an increased rate of monocyte exit from this compartment. Analysis of the spleen using a multicolor gating strategy (Figure 1C)31 indicated that D6−/− mice have normal number of classic CD11chigh DC, F4/80high red pulp macrophages, PDCA-1+/B220+ plasmacytoid DCs, and Ly6G+ granulocytes, whereas they have increased percentage and absolute number of CD115+/Ly6Chigh monocytes (Figure 1D).
Lack of D6 on the nonhematopoietic compartments causes increased number of inflammatory monocytes. (A) Percentage in CD45+ gate (left panel) and fold increase of absolute number (right panel) of CD11b+/Ly6Chigh monocytes recovered from blood in D6−/− (black bar) versus WT mice (white bar). (B) Fold increase of percentage of CD11b+/Ly6Chigh monocytes in total BM CD45+ leukocytes of D6−/− (black bar) and WT mice (white bar). (C) Gating strategy for evaluating the percentage and absolute numbers of leukocytes in mouse spleen. Dead cells, doublets, and CD3+, CD19+, and NK1.1+ cells were excluded for further analysis. Cell labeling with anti-CD11b and CD11c allows identification of myeloid DCs (R1: CD11b+/CD11chigh), red pulp macrophages (R2: F4/80+), and plasmacytoid DCs (R3: PDCA-1+) in the CD11cdim/CD11bdim gate. Inflammatory monocytes (R4) and neutrophils (R5) are distinguished within the CD11bhigh population as SSClow/CD115+/Ly6Chigh and SSChigh/CD115−/Ly6Ghigh, respectively. Gates are based on isotype controls. (D) Fold increase of the absolute number of cells in spleen of D6−/− (black bar) versus WT (white bar) mice based on gated strategy used in panel C. (E) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6−/− (Ly5.2) mice reconstituted with WT Ly5.1 or D6−/− BM cells and in WT mice (Ly5.1) reconstituted with D6−/− BM cells versus WT mice (Ly5.1) reconstituted with WT mice (Ly5.2). In all panels, data are representative of 4 independent experiments (n = 10 mice/experiment). *P < .05. **P < .005.
Lack of D6 on the nonhematopoietic compartments causes increased number of inflammatory monocytes. (A) Percentage in CD45+ gate (left panel) and fold increase of absolute number (right panel) of CD11b+/Ly6Chigh monocytes recovered from blood in D6−/− (black bar) versus WT mice (white bar). (B) Fold increase of percentage of CD11b+/Ly6Chigh monocytes in total BM CD45+ leukocytes of D6−/− (black bar) and WT mice (white bar). (C) Gating strategy for evaluating the percentage and absolute numbers of leukocytes in mouse spleen. Dead cells, doublets, and CD3+, CD19+, and NK1.1+ cells were excluded for further analysis. Cell labeling with anti-CD11b and CD11c allows identification of myeloid DCs (R1: CD11b+/CD11chigh), red pulp macrophages (R2: F4/80+), and plasmacytoid DCs (R3: PDCA-1+) in the CD11cdim/CD11bdim gate. Inflammatory monocytes (R4) and neutrophils (R5) are distinguished within the CD11bhigh population as SSClow/CD115+/Ly6Chigh and SSChigh/CD115−/Ly6Ghigh, respectively. Gates are based on isotype controls. (D) Fold increase of the absolute number of cells in spleen of D6−/− (black bar) versus WT (white bar) mice based on gated strategy used in panel C. (E) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6−/− (Ly5.2) mice reconstituted with WT Ly5.1 or D6−/− BM cells and in WT mice (Ly5.1) reconstituted with D6−/− BM cells versus WT mice (Ly5.1) reconstituted with WT mice (Ly5.2). In all panels, data are representative of 4 independent experiments (n = 10 mice/experiment). *P < .05. **P < .005.
To understand whether these modifications are related to D6 expression on hematopoietic cells or lymphatic vessels, BM transfer experiments were performed. After 15 weeks of BM engraftment, the increased number of circulating monocytes was only observed when recipient mice were D6−/−, independently from the genotype of donor cells (Figure 1E), indicating that the phenotype was not the result of an intrinsic defect of D6−/− leukocytes but the absence of D6 expression on the nonhematopoietic compartment, possibly the lymphatic vessels, which express D6 at high levels.11
Mobilization of BM leukocytes under inflammatory conditions
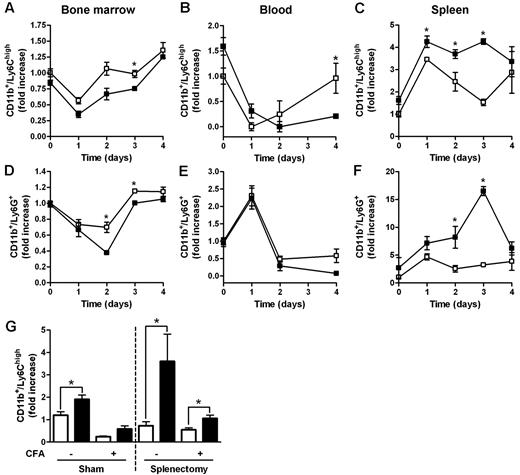
Myeloid populations in different compartments of D6−/− and WT mice were compared after subcutaneous injection of CFA, an inflammatory challenge previously shown to induce anticipated and exacerbated local inflammation, and enhanced LN reactivity in the absence of D6.12 In BM, the percentage of Ly6Chigh monocytes and Ly6G+ granulocytes declined with a similar kinetic at day 1 after injection, with no differences between WT and D6−/− mice (Figure 2A,D). However, starting from day 2 after CFA injection, the frequency of BM monocytes and granulocytes started to recover, reaching higher percentages (Figure 2A,D) in WT mice compared with D6−/− mice. A similar trend was observed in blood, with significantly higher number of circulating monocytes in WT compared with D6−/− mice at day 4 after CFA injection (Figure 2B). From day 1 after injection, a marked recruitment of monocytes and granulocytes into the spleen was observed in both WT and D6−/− mice, with absolute numbers of monocytes and granulocytes significantly higher in D6−/− compared with WT mice (Figure 2C,F). Similar results were obtained when draining LNs were analyzed (data not shown).
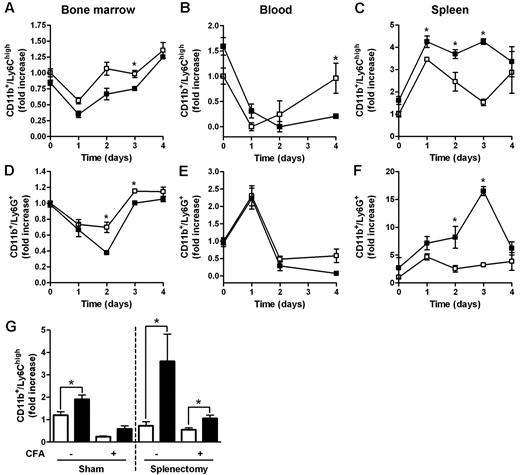
Increased accumulation of myeloid cells in the spleen of D6−/− mice after CFA injection. FACS analysis of BM, spleen, and blood harvested from WT and D6−/− mice injected subcutaneously with 200 μL of CFA in the right flank. BM CD11b+/Ly6Chigh monocytes (A) and CD11b+/Ly6G+ granulocytes (D), expressed as fold increase of the percentage of CD45+ leukocytes, recovered at indicated time points. Fold increase of the absolute number of monocytes (B-C) and granulocytes (E-F) in blood and spleen of D6−/− (■) versus WT (□) mice. (G) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6−/− (■) versus WT (□) mice after 4 weeks of splenectomy or sham operated before and after 48 hours CFA treatment. In all panels, data are representative of 2 independent experiments (n = 4 mice/time point). Data are mean ± SD. *P < .05 comparing D6−/− and WT animals.
Increased accumulation of myeloid cells in the spleen of D6−/− mice after CFA injection. FACS analysis of BM, spleen, and blood harvested from WT and D6−/− mice injected subcutaneously with 200 μL of CFA in the right flank. BM CD11b+/Ly6Chigh monocytes (A) and CD11b+/Ly6G+ granulocytes (D), expressed as fold increase of the percentage of CD45+ leukocytes, recovered at indicated time points. Fold increase of the absolute number of monocytes (B-C) and granulocytes (E-F) in blood and spleen of D6−/− (■) versus WT (□) mice. (G) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes in blood of D6−/− (■) versus WT (□) mice after 4 weeks of splenectomy or sham operated before and after 48 hours CFA treatment. In all panels, data are representative of 2 independent experiments (n = 4 mice/time point). Data are mean ± SD. *P < .05 comparing D6−/− and WT animals.
Both the proliferative potential of spleen Ly6Chigh monocytes, analyzed with propidium iodide dye method, the number, and the proliferation rate of spleen and BM myeloid progenitors (LSK: Lin−/IL-7R−/Sca-1+/c-Kit+; CMP: Lin−/IL7R−/Sca−/c-Kit+/CD34+/CD16/CD32+; GMP: Lin−/IL-7R−/Sca-1−/c-Kit+/CD34+/CD16/CD32high) analyzed in vivo by the use of BrdU uptake by FACS analysis, were superimposable between WT and D6−/− mice, ruling out a potential role of extramedullary hematopoiesis (data not shown). WT and D6−/− mice have no difference in the amount of plasma levels of GM-CSF and IL-6 (data not shown). The role of the spleen as a monocyte reservoir32 was further ruled out by the spontaneous increase of circulating monocytes and granulocytes observed in D6−/− mice, compared with WT mice, 4 weeks after splenectomy (Figure 2G, and data not shown). Of note, in splenectomized animals, D6−/− mice showed an increased amount of circulating monocytes also after CFA injection (Figure 2G).
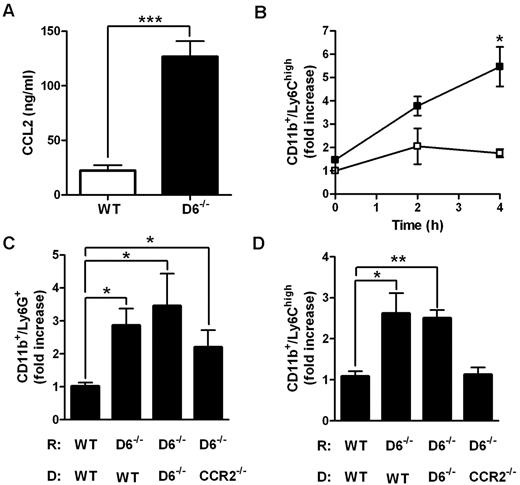
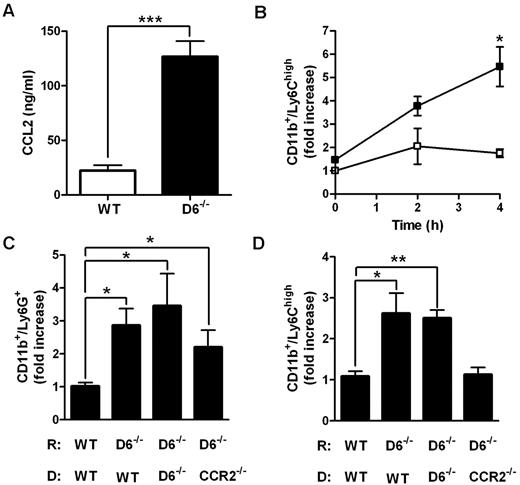
Accumulation of Ly6Chigh monocytes in lymphoid organs is a CCR2-dependent mechanism.21,22,33 To understand whether CCL2/CCR2 axis was involved in the increased accumulation of Ly6Chigh monocytes found in D6−/− mice, 2 parallel approaches were performed. First, CCL2 was intravenously injected in WT and D6−/− mice. After 4 hours, the amount of CCL2 was higher in the serum of D6−/− mice compared with WT mice (Figure 3A), indicating that the chemokine clearance is significantly delayed when D6 is missing. Under these experimental conditions, the total number of BM Ly6Chigh monocytes declined with no statistical differences between WT and D6−/− mice (data not shown), but a significant increase in the absolute number of circulating inflammatory monocytes (data not shown) and, consistent with observations after CFA injection, increased number of splenic monocytes in D6−/− compared with WT mice, was detected (Figure 3B). Then, BM transfer experiments with WT or CCR2−/− mice as donors were performed. After 15 weeks of BM engraftment, mice were treated with CFA for 2 days. Whereas increased accumulation of Ly6G+ neutrophils in the spleen was comparable in D6−/− recipients (Figure 3C), increased accumulation of Ly6Chigh monocytes in the spleen of D6−/− recipient mice was detected when mice were reconstituted with WT or D6−/− BM but not with CCR2−/− BM (Figure 3D), demonstrating that the accumulation of Ly6Chigh monocytes in D6−/− spleen is a CCR2-dependent mechanism. Finally, adoptive transfer experiments performed with an equal amount of differentially labeled CCR2−/− and WT Ly6Chigh monocytes indicate that they accumulate in the spleen with the same CCR2/WT ratio in WT (0.76 ± 0.06) and D6−/− (0.66 ± 0.03) recipients, indicating that the increased monocyte accumulation found in D6−/− spleen mostly relies on their increased BM release.
Role of CCL2/CCR2 axis in the increased mobilization of inflammatory monocytes in D6−/− mice spleen. (A) CCL2 concentrations in the serum of D6−/− (black bar) and WT (white bar) mice 4 hours after intravenous injection of 2 μg hCCL2. Data are mean ± SD of 2 experiments with 4 mice/group. (B) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes recovered from spleen of D6−/− (■) versus WT (□) mice at indicated time points after injection (n = 8 mice/time point). *P < .05, D6−/− versus WT mice. (C-D) Fold increase of the absolute number of CD11b+/Ly6G+ neutrophils (C) and CD11b+/Ly6Chigh monocytes (D) in spleen of D6−/− mice reconstituted with WT or D6−/− or CCR2−/− BM cells versus WT mice reconstituted with WT BM cells. Spleens were analyzed 2 days after subcutaneous injection of CFA (200 μL). Data are mean ± SD (n = 8 mice/group). *P < .05. **P < .005. ***P < .0005.
Role of CCL2/CCR2 axis in the increased mobilization of inflammatory monocytes in D6−/− mice spleen. (A) CCL2 concentrations in the serum of D6−/− (black bar) and WT (white bar) mice 4 hours after intravenous injection of 2 μg hCCL2. Data are mean ± SD of 2 experiments with 4 mice/group. (B) Fold increase of the absolute number of CD11b+/Ly6Chigh monocytes recovered from spleen of D6−/− (■) versus WT (□) mice at indicated time points after injection (n = 8 mice/time point). *P < .05, D6−/− versus WT mice. (C-D) Fold increase of the absolute number of CD11b+/Ly6G+ neutrophils (C) and CD11b+/Ly6Chigh monocytes (D) in spleen of D6−/− mice reconstituted with WT or D6−/− or CCR2−/− BM cells versus WT mice reconstituted with WT BM cells. Spleens were analyzed 2 days after subcutaneous injection of CFA (200 μL). Data are mean ± SD (n = 8 mice/group). *P < .05. **P < .005. ***P < .0005.
Reduced T-cell priming in D6−/− mice
To understand whether Ly6Chigh monocytes and Ly6G+ neutrophils present in higher frequency in the spleen and LNs of D6−/− mice affect specific immune responses, the ability of D6−/− whole splenocytes (WSs) and LN cells to respond to allogeneic stimulation was assessed using BALB/c DC as stimulators in a 1-way mixed leukocyte reaction. D6−/−-derived cells proliferated very poorly compared with WT splenocytes or LN cells (Figure 4A-B), and this was not the result of a T cell–intrinsic defect in that isolated CD4+ and CD8+ cells either from WT or D6−/− B6 mice displayed the same ability to proliferate to BALB/c DCs (supplemental Figure 1D-E). Removal of Ly6Chigh monocytes and Ly6G+ neutrophils with the depleting antibody to Gr1+, which recognizes both the Ly6C and the Ly6G antigens, slightly increased the proliferation of WT splenocytes and fully restored the ability of D6−/− splenocytes to proliferate (Figure 4A).
Reduced T-cell proliferation in D6−/− mice. (A) WS, Gr1+-depleted WS and (B) LNs taken from B6 D6−/− (black bar) versus WT (white bar) mice were used as responders in a 1-way mixed leukocyte reaction. Graphs represent the total [3H]-thymidine uptake in the presence of BALB/c DCs as allogeneic stimulators. Data are mean ± SD and are representative of 3 independent experiments. *P < .05. **P < .005. (C) Absolute number ± SD of CD8+ OT-I in draining LN of D6−/− (black bar) and WT (white bar) mice immunized with DEC205-OVA plus lipopolysaccharide treated with anti-Gr1 or isotypic antibody (200 μg) at the time of transfer and at day 1 after immunization. (D) CFSE dilution profile of transferred OT-I cells in LN of D6−/− and WT mice for the indicated treatment (isotype or anti-Gr1). (E) Percentage of proliferating OT-I cells in LN of D6−/− (black bar) and WT (white bar) mice. Data are representative of 2 independent experiments (n = 4 mice/group). *P < .05. **P < .005.
Reduced T-cell proliferation in D6−/− mice. (A) WS, Gr1+-depleted WS and (B) LNs taken from B6 D6−/− (black bar) versus WT (white bar) mice were used as responders in a 1-way mixed leukocyte reaction. Graphs represent the total [3H]-thymidine uptake in the presence of BALB/c DCs as allogeneic stimulators. Data are mean ± SD and are representative of 3 independent experiments. *P < .05. **P < .005. (C) Absolute number ± SD of CD8+ OT-I in draining LN of D6−/− (black bar) and WT (white bar) mice immunized with DEC205-OVA plus lipopolysaccharide treated with anti-Gr1 or isotypic antibody (200 μg) at the time of transfer and at day 1 after immunization. (D) CFSE dilution profile of transferred OT-I cells in LN of D6−/− and WT mice for the indicated treatment (isotype or anti-Gr1). (E) Percentage of proliferating OT-I cells in LN of D6−/− (black bar) and WT (white bar) mice. Data are representative of 2 independent experiments (n = 4 mice/group). *P < .05. **P < .005.
To evaluate the relevance of Ly6Chigh monocytes and Ly6G+ neutrophils present in D6−/− mice lymphoid organs on the induction of an adaptive immune response in vivo, B6 WT and D6−/− mice were adoptively transferred with CFSE-labeled OVA-specific TCR transgenic T cells (OT-I) followed by immunization with a recombinant anti-DEC205 antibody fused to OVA that is cross-presented in the context of MHC class I mainly by CD8+ LN-resident DCs.34 OT-I cells transferred in D6−/− mice showed reduced accumulation (Figure 4C) and proliferation (Figure 4D-E) within draining LNs compared with OT-I cells primed in WT mice. When mice were treated with a depleting anti-Gr1 antibody during the immunization protocol, OT-I cell proliferation was increased in both WT and D6−/− recipients, and OT-I accumulation and proliferation in D6−/− recipients were fully restored, being comparable with that observed in WT recipients (Figure 4D-E), increased immunosuppressive activity of D6−/− Ly6Chigh monocytes.
Gr1+ myeloid cells with immunosuppressive functions, collectively indicated as MDSCs, compose a granulocytic (CD11b+/Ly6Ghigh/Ly6Clow/int) and a monocyte-like (CD11b+/Ly6G−/Ly6Chigh) subset.28 To identify the precise nature of the Gr1+ myeloid population responsible for the increased immunosuppressive activity observed in the absence of D6, these 2 subsets were sorted from WT or D6−/− spleens after CFA injection and tested for their ability to suppress antigen-specific T-cell responses. Granulocytic Ly6Ghigh cells either from WT or D6−/− mice inhibited T-cell proliferation and IFN-γ production only when added at high concentrations, and no differences were observed between cells obtained from WT or D6−/− mice (data not shown). On the contrary, Ly6Chigh monocytes very effectively inhibited both proliferation and IFN-γ production, and most importantly cells taken from D6−/− mice were consistently more potent than cells obtained by WT mice (Figure 5A-B). To investigate the molecular mechanisms supporting the increased immunosuppressive activity of Ly6Chigh monocytes taken from a D6−/− mice, analysis of myeloid differentiation markers was performed. Compared with cells obtained from WT mice, Ly6Chigh/CD115+ splenic monocytes (corresponding to gate R5 in Figure 1C) from D6−/− mice showed a significant reduction in the expression levels of several differentiation markers, including CD115, CD11b, and F4/80 (Figure 5C). CD11b was also down-regulated in circulating monocytes in D6−/− mice, both in basal conditions and after CFA challenge, whereas the opposite was observed for monocytes derived from CCR2−/− mice (Figure 5D). Collectively these data indicate that, under homeostatic conditions, lack of D6 results in the release of Ly6Chigh monocytes characterized by an immature phenotype, similar to those mobilized as a consequence of CCR2 activation under inflammatory conditions, and that this phenomenon is further increased when D6−/− are challenged with inflammatory stimuli.
Increased immunosuppressive phenotype and activity of CD11b+/Ly6Chigh monocytes from D6−/− mice. [3H]-thymidine incorporation (A) and IFN-γ production (B) by OVA peptide-stimulated OT-I splenocytes cultured alone (gray bar) or in the presence of D6−/− (black bar) and WT (white bar) CD11b+/Ly6Chigh monocytes at indicated ratio. *P < .05, OT-I treated with D6−/− versus WT cells. #P < .05, treated OT-I versus basal proliferation. (C) Mean fluorescence intensity fold increase of markers on gated CD11b+/Ly6Chigh monocytes from spleen of D6−/− (black bar) versus WT (white bar) mice (n = 7 mice/group). (D) Mean fluorescence intensity fold increase of CD11b expression on circulating CD11b+/Ly6Chigh monocytes of D6−/− (black bar) and CCR2−/− (gray bar) versus WT (white bar) mice (n = 7 mice/group) in basal conditions or 2 days after CFA. #P < .05, CFA versus basal condition. (E) Fold increase of ARG1 and COX2 mRNA expression in CD11b+/Ly6Chigh monocytes sorted from spleen of D6−/− (black bar) versus WT (white bar) mice. (F) Recovery of OT-I splenocyte proliferation in the presence of D6−/− (black bar) and WT (white bar) CD11b+ cells after treatment with indicated inhibitors. Data are mean ± SD of 3 independent experiments. *P < .05, D6−/− versus WT cells. #P < .05, inhibitors treatment versus medium. (G) Induction of ARG1 and COX2 expression in BMDM from WT (white bar) and CCR2−/− (gray bar) mice cultured for indicated times in presence of hCCL2 (10 ng/mL). *P < .05, CCL2-treated versus control cells. **P < .005, CCL2-treated versus control cells.
Increased immunosuppressive phenotype and activity of CD11b+/Ly6Chigh monocytes from D6−/− mice. [3H]-thymidine incorporation (A) and IFN-γ production (B) by OVA peptide-stimulated OT-I splenocytes cultured alone (gray bar) or in the presence of D6−/− (black bar) and WT (white bar) CD11b+/Ly6Chigh monocytes at indicated ratio. *P < .05, OT-I treated with D6−/− versus WT cells. #P < .05, treated OT-I versus basal proliferation. (C) Mean fluorescence intensity fold increase of markers on gated CD11b+/Ly6Chigh monocytes from spleen of D6−/− (black bar) versus WT (white bar) mice (n = 7 mice/group). (D) Mean fluorescence intensity fold increase of CD11b expression on circulating CD11b+/Ly6Chigh monocytes of D6−/− (black bar) and CCR2−/− (gray bar) versus WT (white bar) mice (n = 7 mice/group) in basal conditions or 2 days after CFA. #P < .05, CFA versus basal condition. (E) Fold increase of ARG1 and COX2 mRNA expression in CD11b+/Ly6Chigh monocytes sorted from spleen of D6−/− (black bar) versus WT (white bar) mice. (F) Recovery of OT-I splenocyte proliferation in the presence of D6−/− (black bar) and WT (white bar) CD11b+ cells after treatment with indicated inhibitors. Data are mean ± SD of 3 independent experiments. *P < .05, D6−/− versus WT cells. #P < .05, inhibitors treatment versus medium. (G) Induction of ARG1 and COX2 expression in BMDM from WT (white bar) and CCR2−/− (gray bar) mice cultured for indicated times in presence of hCCL2 (10 ng/mL). *P < .05, CCL2-treated versus control cells. **P < .005, CCL2-treated versus control cells.
To understand the mechanism by which lack of control of inflammatory chemokines in the absence of D6 could increase Ly6Chigh monocyte suppressive activity, sorted CD11b+/CD115+/Ly6Chigh monocytes were analyzed for expression of genes responsible for immunosuppressive activity. Although no significant difference between the 2 groups was found for inducible nitric oxide synthases (data not shown), Ly6Chigh monocytes from D6−/− mice displayed increased expression of arginase 1 (ARG1) and COX2 (Figure 5E). The role of COX2 and ARG1 in the suppressive activity of D6−/− monocytes was further demonstrated by the use of specific inhibitors (SC-58125 and BEC, respectively) that were able to significantly recover proliferation of T cells cultured with D6−/− but not WT monocytes (Figure 5F). CCL2 was previously reported to sustain an autocrine pathway leading to COX2 up-regulation in human monocytes.35 Similarly, in BM-derived macrophages (BMDM) from WT but not CCR2−/− mice, CCL2 up-regulated COX2 and ARG1 expression levels (Figure 5G). These data indicate that delayed inflammatory chemokine clearance in the absence of D6 not only induces accumulation of immature Ly6Chigh monocytes in blood and secondary lymphoid organs but also increases their suppressive capacity by inducing COX2 and ARG1 expression.
Delayed GVHD development in D6−/− mice
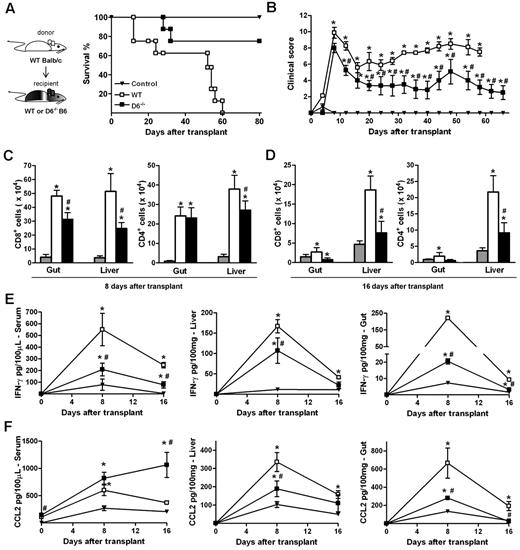
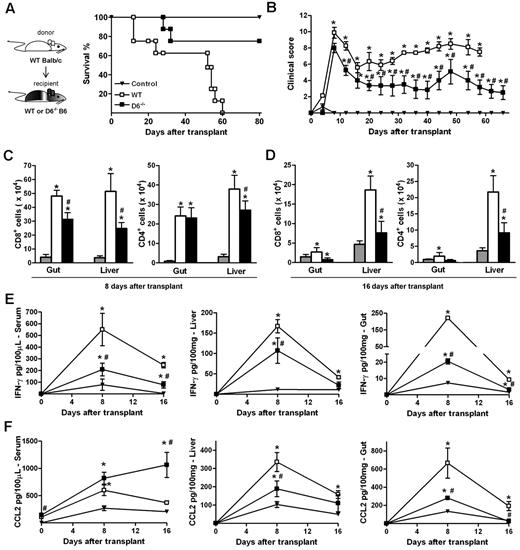
The ability of D6−/− Ly6Chigh monocytes to suppress specific immune responses was further assessed in vivo in a GVHD model induced by the transfer of WS and BM cells from allogeneic (WT BALB/c) donors in lethally irradiated WT or D6−/− B6 recipients. The 2 cohorts exhibited similar disease activity at early time points, when acute GVHD is typically at its peak. At later time points, WT B6 mice developed a severe GVHD and died by day 60, whereas D6−/− recipient mice showed a significant reduction in GVHD manifestations and an increased survival rate (Figure 6A-B). Consistent with this, both the number of infiltrating CD4+ and CD8+ effector cells (Figure 6C-D) as well as IFN-γ and CCL2 levels (Figure 6E-F) were significantly lower in GVHD target organs (liver and gastrointestinal tract) of D6−/− mice compared with WT mice. Similarly, IFN-γ serum concentrations were reduced (Figure 6E). On the contrary, circulating levels of CCL2 were higher in D6−/− compared with WT mice, being significantly different at the steady state and at day 16 after transplantation (Figure 6F).
D6−/− mice are partially protected from fully mismatched GVHD. (A) Survival curves and (B) clinical scores of WT (□) or D6−/− (■) B6 mice treated with BM and WS cells from BALB/c mice after myeloablation. Control mice (▾) are B6 recipients treated with syngeneic BM and WS. Data are representative of 2 independent experiments (n = 8 mice/group). (C-D) Absolute number of CD8+ and CD4+ cells in gut and liver of mice killed at day 8 and day 16 after transplantation in control group (gray bar), WT (white bar), and D6−/− (black bar) B6 recipients. Concentration of mIFN-γ (E) and mCCL2/JE (F) in serum, liver, and gut of control (▾), WT (□), and D6−/− (■) B6 recipients. Data are mean ± SD of 2 independent experiments (n = 10 mice/group). *P < .05, D6−/− or WT mice versus control mice. #P < .05, D6−/− versus WT mice.
D6−/− mice are partially protected from fully mismatched GVHD. (A) Survival curves and (B) clinical scores of WT (□) or D6−/− (■) B6 mice treated with BM and WS cells from BALB/c mice after myeloablation. Control mice (▾) are B6 recipients treated with syngeneic BM and WS. Data are representative of 2 independent experiments (n = 8 mice/group). (C-D) Absolute number of CD8+ and CD4+ cells in gut and liver of mice killed at day 8 and day 16 after transplantation in control group (gray bar), WT (white bar), and D6−/− (black bar) B6 recipients. Concentration of mIFN-γ (E) and mCCL2/JE (F) in serum, liver, and gut of control (▾), WT (□), and D6−/− (■) B6 recipients. Data are mean ± SD of 2 independent experiments (n = 10 mice/group). *P < .05, D6−/− or WT mice versus control mice. #P < .05, D6−/− versus WT mice.
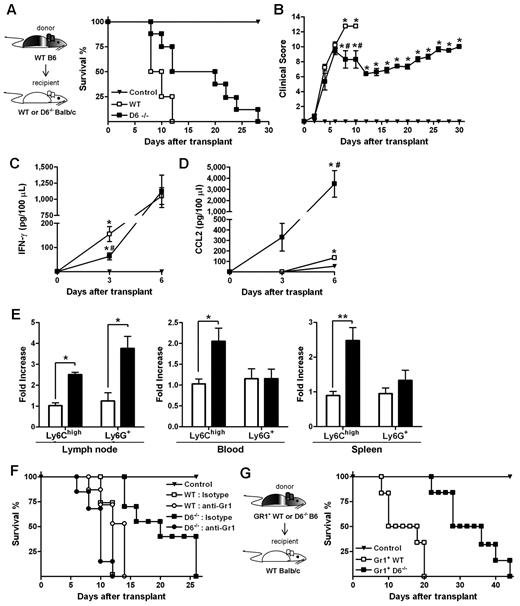
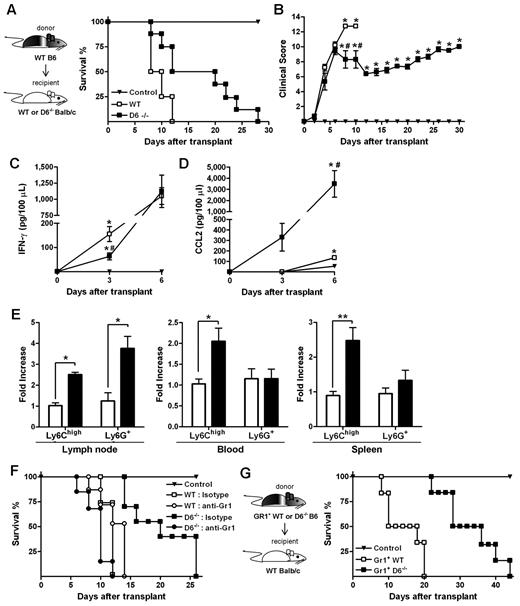
When an acute GVHD model was performed, using BALB/c mice as recipients of allogeneic WS and BM cells from B6 donors, animals developed a more severe and rapid GVHD compared with WT B6 recipients.36 Consistent with results obtained in the D6−/− B6 background, the absence of D6 in recipient mice resulted in prolonged survival and a significant delay in the disease (Figure 7A-B), and correlated with decreased concentrations of IFN-γ (Figure 7C) and increased concentrations of CCL2 (Figure 7D) in the serum. Analysis of leukocytes in LNs, spleen, and blood at day 3 after transplantation indicated that Ly6Chigh monocytes were increased in D6−/− recipients compared with control and WT BALB/c recipients (Figure 7E). The protective role of these leukocytes in GVHD was demonstrated by depletion experiments performed by the use of the anti-Gr1 antibody, which did not affect the mortality rate of WT mice but completely abrogated the disease delay observed in D6−/− BALB/c mice (Figure 7F).
Role of D6−/− Gr1+ cells in GVHD protection. (A) Survival curves and (B) clinical scores of WT (□) or D6−/− (■) BALB/c mice transplanted with BM and WS taken from WT B6. Serum level of mIFN-γ (C) and mCCL2/JE (D) measured at day 3 and day 6 after transplantation (2 independent experiments, n = 6 mice/group). Data are mean ± SD. *P < .05, D6−/− and WT versus control mice. #P < .05, D6−/− versus WT mice. (E) Fold increase of absolute number ± SD of CD11b+/Ly6Chigh and CD11b+ Ly6G+ cells of D6−/− (black bar) mice versus WT (white bar) BALB/c recipients (2 independent experiments, n = 6 mice/group). *P < .05, D6−/− versus WT mice. **P < .005, D6−/− versus WT mice. (F) Survival curves of WT (open symbols) or D6−/− (closed symbols) BALB/c mice transplanted with BM and WS taken from WT B6, treated with isotype (squares) or anti-Gr1 (circles). (G) WT BALB/c transplanted with BM and CD3+ cells taken from WT B6, treated with CD11b+/Gr1+ cells from WT (□) or D6−/− (■) B6 mice. In all experiments, control mice (▾) were transplanted with syngeneic BM and WS. Data are representative of 2 independent experiments (n = 8 mice/group).
Role of D6−/− Gr1+ cells in GVHD protection. (A) Survival curves and (B) clinical scores of WT (□) or D6−/− (■) BALB/c mice transplanted with BM and WS taken from WT B6. Serum level of mIFN-γ (C) and mCCL2/JE (D) measured at day 3 and day 6 after transplantation (2 independent experiments, n = 6 mice/group). Data are mean ± SD. *P < .05, D6−/− and WT versus control mice. #P < .05, D6−/− versus WT mice. (E) Fold increase of absolute number ± SD of CD11b+/Ly6Chigh and CD11b+ Ly6G+ cells of D6−/− (black bar) mice versus WT (white bar) BALB/c recipients (2 independent experiments, n = 6 mice/group). *P < .05, D6−/− versus WT mice. **P < .005, D6−/− versus WT mice. (F) Survival curves of WT (open symbols) or D6−/− (closed symbols) BALB/c mice transplanted with BM and WS taken from WT B6, treated with isotype (squares) or anti-Gr1 (circles). (G) WT BALB/c transplanted with BM and CD3+ cells taken from WT B6, treated with CD11b+/Gr1+ cells from WT (□) or D6−/− (■) B6 mice. In all experiments, control mice (▾) were transplanted with syngeneic BM and WS. Data are representative of 2 independent experiments (n = 8 mice/group).
Delayed GVHD is mediated by Gr1+ myeloid cells from D6−/− spleen
Data obtained in vitro with isolated myeloid populations from WT and D6−/− B6 mice indicated that CD11b+/ Ly6Chigh monocytes taken from D6−/− mice have an increased immunosuppressive potential (Figure 5A). To confirm this observation in vivo, lethally irradiated WT BALB/c mice were transferred with BM and WS from allogeneic WT or D6−/− B6 mice. As expected, recipient mice receiving cells from WT B6 mice developed a severe GVHD and died before day 15. Conversely, mice receiving allogeneic D6−/− cells were significantly protected against GVHD in terms of mortality and clinical score (supplemental Figure 2A, and data not shown). A similar protection was achieved when irradiated WT BALB/c mice were transferred with allogeneic WT BM cells and WS taken from D6−/− mice, indicating that the protective effect was conferred by cells present in the D6−/− spleen (supplemental Figure 2A). Importantly, protection was lost on depletion of Gr1+ cells from WS taken from D6−/− mice (supplemental Figure 2B). Furthermore, WT BALB/c mice receiving only CD3+ cells from WT or D6−/− B6 mice died before day 15, similarly to mice transferred with WT WS (supplemental Figure 2C), indicating that D6 expression is not required for proper function of CD3+ cells. Finally, transfer of Gr1+ myeloid cells purified from D6−/− spleen to irradiated and transplanted WT BALB/c mice conferred a more effective protection compared with the transfer of the same number of Gr1+ myeloid cells purified from WT B6 mice (Figure 7G).
Discussion
The atypical chemokine receptor D6 exerts a nonredundant role in the control of inflammation, as D6−/− mice are more susceptible to infections and inflammation in several models of pathology compared with WT littermates.12–19 This phenotype has been ascribed to the exacerbation of the local inflammatory response caused by the delayed clearance and consequent increase in concentration of inflammatory CC chemokines observed in the absence of D6, which operates as a decoy and scavenger receptor for its ligands.37 Differently from the well established role of D6 in the negative regulation of inflammatory responses, its relevance for the onset and development of adaptive immune responses is less defined, and evidence suggests that in this context it may even play an opposite role, acting as a promoter of the immune response.20 Here we report that the control of CC chemokines by D6 has a selective impact on the traffic and differentiation/activation properties of Ly6Chigh monocytes, resulting in a significant inhibition of the development of adaptive immune responses.
Plasticity and diversity are hallmarks of the mononuclear phagocyte lineage, and macrophage heterogeneity and its impact on the development of innate and adaptive immune responses have long been recognized.38,39 Along the same line, heterogeneity in circulating monocytes is also increasingly appreciated.26 In mice, at least 2 different monocyte populations can be distinguished, based on the expression of the marker Ly6C.25 Among these, Ly6Chigh monocytes, also called classic or inflammatory monocytes for their ability to produce primary inflammatory cytokines, also represent a component of the so-called MDSCs, which in different pathologic conditions accumulate in blood and lymphoid organs and exert a suppressive effect on adaptive immune responses.27,40 Several reports have clearly demonstrated that the CCR2 agonists CCL2 and CCL7 control systemic levels of monocyte subsets by inducing their mobilization from BM.21,22,41 In the tumor context, CCL2 and its receptor CCR2 were found to be relevant for monocyte recruitment and survival and to promote their differentiation toward tumor-associated macrophages.42–44 As mentioned, D6−/− mice have a delay in the clearance of inflammatory CC chemokines induced during an inflammatory response,19 and data presented here demonstrate that this also has an impact on the traffic properties of Ly6Chigh monocytes. Under inflammatory conditions, D6−/− mice displayed a significant increase in Ly6Chigh monocytes in secondary lymphoid organs compared with WT littermates. Similar observations were made after direct intravenous injection of CCL2, suggesting its direct action on BM precursors. Consistent with the observation that Ly6Chigh monocytes traffic is also altered in seemingly homeostatic conditions, here we also show that D6-deficient animals have increased concentrations of CCL2 in serum (Figure 6F), and we have previously reported similar data for CCL11,19 suggesting, for the first time, a homeostatic function of this receptor. Despite the well-recognized role of the spleen as a site of extramedullary hematopoiesis and a reservoir of a fast mobilizing pool of monocytes,32 experiments in splenectomized animals demonstrate that these mechanisms are not involved in our experimental setting. Conversely, data obtained in CCR2−/− BM chimera demonstrate that D6 regulates Ly6Chigh monocyte mobilization from BM via the control of CCR2 ligand concentrations in the bloodstream, both in steady-state conditions and in the course of a reactive immune response.
D6 is expressed by different leukocyte subsets, mainly marginal zone B cells, DCs, and cardiac graft-infiltrating macrophages,45–47 but its function on these cell types has not been defined.6 In this context, it is worth mentioning that we did not detect D6 expression on Ly6Chigh monocytes (data not shown), consistent with the hypothesis that the effect of D6 on their mobilization from BM is mediated by its control on the chemokine milieu. Data presented here are in agreement with previous observations15 indicating that chemokine clearance is mainly exerted by D6 expressed on stromal cells, presumably lymphatic vessels in barrier tissues, including skin, gut, lung, and placenta.7
Compared with cells from WT animals, Ly6Chigh monocytes derived from D6−/− mice presented a more immature phenotype, as indicated by lower expression of maturation markers, including CD11b, CD115, and F4/80, a phenotype resembling the one observed under inflammatory conditions and absent in CCR2−/− mice. In addition, Ly6Chigh monocytes derived from D6−/− mice presented a significant increase in their immunosuppressive potential both in vitro and in vivo. This is correlated with increased expression of the immunosuppressive genes COX2 and ARG1. It was previously reported that CCL2 increase COX2 expression in human monocytes, and here we report that also in murine BMDM CCL2 induces COX2 and ARG1 expression that give rise to the increased immunosuppression that indeed was blocked by specific inhibitors.
In an immunization model based on transfer of OVA-specific CD8+ cells and challenge with the cognate antigen, D6−/− recipient mice showed a significant defect in proliferation and LN accumulation of antigen-specific T lymphocytes; and when tested in fully MHC-mismatched allogeneic GVHD models, both B6 and BALB/c D6−/− recipients were partially but significantly protected from GVHD. In both experimental conditions, the immune response was restored after depletion of Gr1+ cells; and despite the fact that the use of anti-Gr1 has some limitations,48 it is well assessed that this antibody ablates the suppressor activity of Gr1+ myeloid cells. Thus, in the absence of D6, the BM releases increased amounts of immature Ly6Chigh monocytes that infiltrate lymphoid organs and exert an immunosuppressive activity.
GVHD is the most significant clinical problem that arises after allogeneic BM cell transplantation. It has been shown that T-cell migration into GVHD target tissues is mediated by local chemokine production induced by the conditioning treatment. Interestingly, there is a rapid and transient chemokine increase in GVHD target tissues before T-cell infiltration, and chemokine production is then amplified by the allogeneic reaction.49 Inhibition of inflammatory chemokines30,50 has been associated with protection from GVHD mortality because of decreased effector cell recruitment to target tissues, and chemokines have been proposed as molecular targets for the treatment of acute GVHD. On the contrary, here we show that, in the absence of the control of inflammatory chemokines by D6, increased circulating levels of CCL2 are detectable few days after the myeloablation and transplantation procedure and that this results in increased recruitment of monocytes to lymphoid organs that give rise to partial protection from the GVHD. Indeed, although immature or M2 polarized myeloid cells have been mainly characterized as a mechanism of tumor escape, they regulate immune responses in many different pathologic conditions, including infections, traumatic stress, surgical sepsis, and transplantation.27 In transplantation, there is increasing evidence suggesting that myeloid cells have great potential as a novel immune intervention modality for prevention of graft rejection,51 and the research is focused on molecular targets that can be exploited to generate CD11b+/Gr1+ cells with high suppressive function.29 The results presented here indicate D6 as a key player in the differential regulation of the traffic of monocyte subsets through a CCR2-dependent mechanism. In addition, D6 controls the acquisition of an immunosuppressive phenotype by Ly6Chigh myeloid cells, and its deficiency results in partial protection against GVHD.
From a therapeutic viewpoint, inhibition of D6 activity could be used in combination with standard therapies to further delay disease progression. Moreover, inhibiting D6 might be useful to implement new approaches for the treatment of GVHD patients, in particular steroid-refractory patients, which rely on the adoptive transfer and/or expansion of immunosuppressive and regulatory leukocyte populations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Ministero dell'Istruzione dell'Università e della Ricerca (PRIN project 2009JP9WTS; FIRB projects RBFR08CW8G and RBAP11H2R9), the Ministero della Salute (Ricerca finalizzata), The European Community's Seventh Framework Programme (FP7-2007-2013) under grant agreement n° HEALTH-F4-2011-281608 TIMER and Italian Foreign Affair Ministry (General Direction for Cultural Cooperation and Promotion); Coordenação de aperfeiçoamento de pessoal de nível superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasil); and the Italian Association for Cancer Research. This work was conducted in the context and with the support of the Fondazione Humanitas per la Ricerca. B.S. is an FIRC fellowship recipient.
Authorship
Contribution: B.S. and M.G.C. designed the research, performed experiments, analyzed results, and made the figures; N.C., A.A., C.B., and A.S. performed experiments and analyzed results; V.P. and F.B. contributed new reagents; M.M.T. and A.M. designed the research and wrote the paper; and M.L. and R.B. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Massimo Locati, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, I-20089 Rozzano, Italy; e-mail: massimo.locati@humanitasresearch.it.
References
Author notes
B.S. and M.G.C. contributed equally to this study.

![Figure 4. Reduced T-cell proliferation in D6−/− mice. (A) WS, Gr1+-depleted WS and (B) LNs taken from B6 D6−/− (black bar) versus WT (white bar) mice were used as responders in a 1-way mixed leukocyte reaction. Graphs represent the total [3H]-thymidine uptake in the presence of BALB/c DCs as allogeneic stimulators. Data are mean ± SD and are representative of 3 independent experiments. *P < .05. **P < .005. (C) Absolute number ± SD of CD8+ OT-I in draining LN of D6−/− (black bar) and WT (white bar) mice immunized with DEC205-OVA plus lipopolysaccharide treated with anti-Gr1 or isotypic antibody (200 μg) at the time of transfer and at day 1 after immunization. (D) CFSE dilution profile of transferred OT-I cells in LN of D6−/− and WT mice for the indicated treatment (isotype or anti-Gr1). (E) Percentage of proliferating OT-I cells in LN of D6−/− (black bar) and WT (white bar) mice. Data are representative of 2 independent experiments (n = 4 mice/group). *P < .05. **P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/22/10.1182_blood-2011-10-388082/5/m_zh89991291460004.jpeg?Expires=1764970135&Signature=3~7Z6tAhTlBCuDC6q~XZwVdvDn8eJedhUiI8-28Yg2DBku98hepaWY-QIe3GL~Y6nglCszrQfKAjwzGzYZBYH1aHKiUXPvX81BOpjYUIRHRXrfVwliF44Jz0pSNDBs~iPIzBXRMi6d7JRHDMf8TdyzaQooUTbViCmxzSVe9R-YSokVN9ZuObBuu98SZ9r2zBTGSH1C9K86cKkMWI7dwHMXKLb3Twpvh13idL8fVIO~cxskRlHEizbzPEpYYNdNRoaFBamXxjOLBeUC21f5HiG09KkykP1zNQhtpk-8vUsYWfNE7iflG49kaIea6s4k-Cyk-Nh~mroglqTmd408v~wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Increased immunosuppressive phenotype and activity of CD11b+/Ly6Chigh monocytes from D6−/− mice. [3H]-thymidine incorporation (A) and IFN-γ production (B) by OVA peptide-stimulated OT-I splenocytes cultured alone (gray bar) or in the presence of D6−/− (black bar) and WT (white bar) CD11b+/Ly6Chigh monocytes at indicated ratio. *P < .05, OT-I treated with D6−/− versus WT cells. #P < .05, treated OT-I versus basal proliferation. (C) Mean fluorescence intensity fold increase of markers on gated CD11b+/Ly6Chigh monocytes from spleen of D6−/− (black bar) versus WT (white bar) mice (n = 7 mice/group). (D) Mean fluorescence intensity fold increase of CD11b expression on circulating CD11b+/Ly6Chigh monocytes of D6−/− (black bar) and CCR2−/− (gray bar) versus WT (white bar) mice (n = 7 mice/group) in basal conditions or 2 days after CFA. #P < .05, CFA versus basal condition. (E) Fold increase of ARG1 and COX2 mRNA expression in CD11b+/Ly6Chigh monocytes sorted from spleen of D6−/− (black bar) versus WT (white bar) mice. (F) Recovery of OT-I splenocyte proliferation in the presence of D6−/− (black bar) and WT (white bar) CD11b+ cells after treatment with indicated inhibitors. Data are mean ± SD of 3 independent experiments. *P < .05, D6−/− versus WT cells. #P < .05, inhibitors treatment versus medium. (G) Induction of ARG1 and COX2 expression in BMDM from WT (white bar) and CCR2−/− (gray bar) mice cultured for indicated times in presence of hCCL2 (10 ng/mL). *P < .05, CCL2-treated versus control cells. **P < .005, CCL2-treated versus control cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/22/10.1182_blood-2011-10-388082/5/m_zh89991291460005.jpeg?Expires=1764970135&Signature=Ld4BTQNs1uf8BpLTIcaDk4hOdFUVTAPZ~qFq0Oh3VJuW1PeXVDIZ67bvj189CIPuVe3PxMy6SGlYaa8pMT~XYty2fPAeNcxxMzXuvLxUI~kmN57GVT1qcF-eo0~TF9SNA3pruC~hPGrktYS5KYKSnUZso398unIWdNlmkghM6qvKDVJljpqQuSb9LBRVxj9qEFMkBPkw66x85AHBBtiWcfEJl2GFZ1Rzza68FfUrqxJOFKfjbTk93dYk1dmm6RUN3CjW4K9FcgH-dPVpaxMr5nWE-gcKAzLD4jxHI-PGPO~1hZwM3waLDCrlsPwgxImUZFlgIZbpKN7X6HWVA47ivw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Reduced T-cell proliferation in D6−/− mice. (A) WS, Gr1+-depleted WS and (B) LNs taken from B6 D6−/− (black bar) versus WT (white bar) mice were used as responders in a 1-way mixed leukocyte reaction. Graphs represent the total [3H]-thymidine uptake in the presence of BALB/c DCs as allogeneic stimulators. Data are mean ± SD and are representative of 3 independent experiments. *P < .05. **P < .005. (C) Absolute number ± SD of CD8+ OT-I in draining LN of D6−/− (black bar) and WT (white bar) mice immunized with DEC205-OVA plus lipopolysaccharide treated with anti-Gr1 or isotypic antibody (200 μg) at the time of transfer and at day 1 after immunization. (D) CFSE dilution profile of transferred OT-I cells in LN of D6−/− and WT mice for the indicated treatment (isotype or anti-Gr1). (E) Percentage of proliferating OT-I cells in LN of D6−/− (black bar) and WT (white bar) mice. Data are representative of 2 independent experiments (n = 4 mice/group). *P < .05. **P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/22/10.1182_blood-2011-10-388082/5/m_zh89991291460004.jpeg?Expires=1765129847&Signature=vtoeDSUawwMNHVL0n1-oL-jS0wVg5CYSP4S9jQLc8VQYfwFF~G~ELMabPN9jXfUJQQGNQW327g~5Wa3RtEj6QtRsWoIJwiT7MIltw5q4t9yAVTIFAxhPDjcFqoqUcAChQdWDWHTftAETng2n8ttIpdo8JeQ9YzzbO-Hqjfq3NQr~YjMHshT81yjM6IHcn-B~az~ManwGhhw4O0cggFRUfHeo3czgl2W4BbWUOb2WAA16cwRs1ECJ2YpirbexxDvmB-4t5GP8iTD2RLfffebUMaUo7WR1oUcis94Pkr7-0-ppd7Av2G4TAV1YkPaqOTb0U4LI4XvCrinW66JOL6WOpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Increased immunosuppressive phenotype and activity of CD11b+/Ly6Chigh monocytes from D6−/− mice. [3H]-thymidine incorporation (A) and IFN-γ production (B) by OVA peptide-stimulated OT-I splenocytes cultured alone (gray bar) or in the presence of D6−/− (black bar) and WT (white bar) CD11b+/Ly6Chigh monocytes at indicated ratio. *P < .05, OT-I treated with D6−/− versus WT cells. #P < .05, treated OT-I versus basal proliferation. (C) Mean fluorescence intensity fold increase of markers on gated CD11b+/Ly6Chigh monocytes from spleen of D6−/− (black bar) versus WT (white bar) mice (n = 7 mice/group). (D) Mean fluorescence intensity fold increase of CD11b expression on circulating CD11b+/Ly6Chigh monocytes of D6−/− (black bar) and CCR2−/− (gray bar) versus WT (white bar) mice (n = 7 mice/group) in basal conditions or 2 days after CFA. #P < .05, CFA versus basal condition. (E) Fold increase of ARG1 and COX2 mRNA expression in CD11b+/Ly6Chigh monocytes sorted from spleen of D6−/− (black bar) versus WT (white bar) mice. (F) Recovery of OT-I splenocyte proliferation in the presence of D6−/− (black bar) and WT (white bar) CD11b+ cells after treatment with indicated inhibitors. Data are mean ± SD of 3 independent experiments. *P < .05, D6−/− versus WT cells. #P < .05, inhibitors treatment versus medium. (G) Induction of ARG1 and COX2 expression in BMDM from WT (white bar) and CCR2−/− (gray bar) mice cultured for indicated times in presence of hCCL2 (10 ng/mL). *P < .05, CCL2-treated versus control cells. **P < .005, CCL2-treated versus control cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/22/10.1182_blood-2011-10-388082/5/m_zh89991291460005.jpeg?Expires=1765129847&Signature=TfdONHEHluh-cC9Lpo4SVGIYHDG51kDWSEfdXjy~Of9pnH4H90Tg15QvoQjisGoqRontkq77B9hs-a6oqsekBWxL5F12~31WKMl3ctAkMuhYZ3pIDGkyVR2GntUzX-wv~ZuHzdcFV8XhMHmK~0Ewsf7oTgX0PryfuluCSLQ7Qs6yXh~~Ybgb0XrIYkGVdNNCs9T8pI~0dq52ZerHfKu1tnYc9XFYHUTICDav112n7dooiFYS4CIFQLHCNJ2ky330wvl240gk4mnjpj6hhKwEw6pELnBYFY54~7qijRgQzLYV1~bDOLlHWEILeLsPqTissn309tb-p3SoP07J~29qBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)