A recognized paradigm for the therapeutic action of intravenous immunoglobulin (IVIG) in immune thrombocytopenia (ITP) involves up-regulation of the inhibitory Fcγ receptor (FcγRIIB) in splenic macrophages. However, published data have indicated that opposing results are obtained when using FcγRIIB-deficient mice on different strain backgrounds. Herein we show BALB/c FcγRIIB−/− and wild-type, with or without spleens, all recover ITP with similar dynamics after IVIG (1 g/kg) treatment; however, this was not the case for C57BL/6 (B6) FcγRIIB−/−. In investigating this conundrum, we found that wild-type B6 mice are much less sensitive than BALB/c to IVIG-mediated amelioration of ITP, requiring approximately 2- to 2.5-fold more IVIG than BALB/c. When using 2.5 g/kg IVIG in FcγRIIB−/− B6 mice, amelioration of ITP was as in wild-type in all animals. Our findings led us to the conclusion that different strains of mice respond differently to IVIG and that FcγRIIB plays no role in the mechanism of effect of IVIG in experimental ITP.
Introduction
Intravenous immunoglobulin (IVIG) was first used to treat immune thrombocytopenia (ITP) in 1981, and its use to treat this and other autoimmune inflammatory diseases has continued to increase, particularly in relation to acute ITP in children.1,,,–5 The therapeutic benefit of IVIG has been ascribed to Fcγ receptor blockade, antibody-mediated autoantibody neutralization, inhibition of complement-mediated damage, modulation of cytokine production, down-regulation of B- or T-cell responses, effects on antigen-presenting cells, and modulation of dendritic cells.6,,,,–11
In 2001, it was reported that the inhibitory Fcγ receptor, FcγRIIB, is up-regulated in the splenic macrophages of immune thrombocytopenic mice after administration of IVIG and that FcγRIIB knockout (KO) mice fail to respond to IVIG therapy.12 These data raised the possibility that the therapeutic effect of IVIG reflects modulation of FcγRIIB inhibitory signaling, and this mechanism is now generally regarded as the explanation for IVIG-mediated amelioration of autoimmune inflammatory diseases.12,,–15 However, not all data from studies addressing this issue are consistent with this mechanistic paradigm.15,–17 Indeed, in one study, it was shown that FcγRIIB KO mice on a BALB/c background responded well to IVIG to ameliorate ITP.17 In contrast, other studies have used FcγRIIB-deficient mice on a C57BL/6 (B6) background and have not shown efficacy of IVIG to ameliorate ITP.15,17 Importantly, a study15 that used mice deficient in either the SHP-1 or SHIP-1 tyrosine phosphatases, responsible for FcγRIIB signaling and activation,18,–20 revealed these signaling molecules not to be required for IVIG amelioration of ITP, supporting a new hypothesis that FcγRIIB itself is not critical for the effects of IVIG. Using a newly described mouse model of ITP21 to reexamine the role of the FcγRIIB in IVIG therapy, we show herein, using both BALB/c and B6 background strains of FcγRIIB KO mice, that FcγRIIB is dispensable for IVIG efficacy to ameliorate ITP.
Methods
Mice
Wild-type BALB/cAnNTac (normal or splenectomized; BALB/c) and fully congenic C.129S4(B6)-Fcgr2btm1TtK/cAnNTac N12 KO mice on a BALB/c background (model 579; BALB/c KO) were purchased from Taconic Farms at 6 to 8 weeks of age. Fully congenic B6.129S4-Fcgr2btm1TtK N12 KO mice on a C57BL/6 background (TAC B6 KO) were also purchased from Taconic Farms. Wild-type C57BL/6J (stock 000664; B6), 129S4/SvJaeJ (stock 009104; 129S4), B6129SF2/J (stock 101045; F2), and mixed congenic B6;129S-Fcgr2btm1Ttk/J KO mice on a B6 background (stock 002848; JAX B6 KO) at 6 to 8 weeks of age were obtained from The Jackson Laboratory. Mice were kept under a natural light/dark cycle, maintained at 22 + 4°C, and fed with standard diet and water ad libitum. All experiments were performed following animal use protocols that were approved by the University Health Network Animal Research Committee in Toronto.
Mouse model of ITP
ITP was induced and sustained using passive administration of rat monoclonal anti–mouse glycoprotein IIb (CD41; clone MWReg30, rat IgG1κ) antibody purchased from BD Biosciences PharMingen. A dose-escalation protocol to maintain platelet nadir at day 2 and onwards was used as previously described.21 IVIG was obtained from Talecris/Grifols and used at 1.0 g/kg for Balb/c mice and 2.5 g/kg for B6 mice. IVIG was given by intraperitoneal injection when the platelet counts reached nadir, at day 2 after initial administration of antiplatelet antibody. Platelets in whole blood samples were quantified on a daily basis using a calibrated flow cytometer (FACSCalibur; BD Biosciences) as previously described.21
Data and statistical analysis
Group means and SDs of total platelet counts were determined and plotted. Student 2-tailed t test for equal variance was used to determine the significance (P < .05) of total platelet counts by comparing the counts of test mice with those of control mice.
Results and discussion
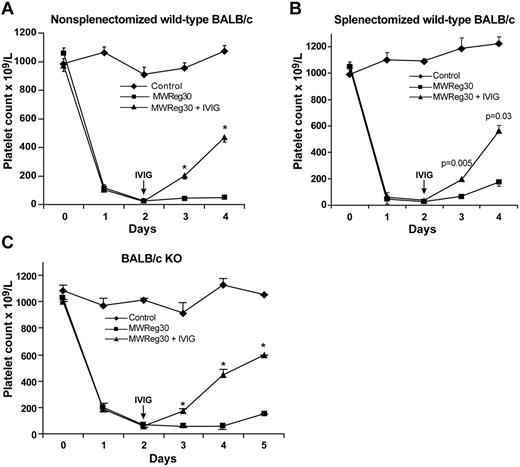
We have previously shown using quantitative real-time RT-PCR that FcγRIIB is not up-regulated in the spleen of mice after IVIG treatment.22 This is inconsistent with the previously proposed mechanism of IVIG amelioration of experimental ITP, which showed that up-regulation of FcγRIIB in the splenic macrophages was required.12 Thus, we wondered whether the spleen was even necessary for IVIG efficacy in ITP. Figure 1A-B shows that BALB/c splenectomized mice respond as well as nonsplenectomized mice to IVIG. Thus, in this mouse model of ITP, neither ITP induction nor its amelioration by IVIG depends on the spleen. Our results have been corroborated by a recent report that showed a spleen unnecessary for IVIG-mediated amelioration of ITP in a B6 mouse model.23 Taken together, we concluded from ours and previous results22,23 that the mechanism of IVIG in ITP does not involve FcγRIIB up-regulation in splenic macrophages.
Neither spleen nor inhibitory Fcγ receptor is required for IVIG amelioration of ITP in BALB/c mice. (A) Nonsplenectomized wild-type BALB/c mice respond to IVIG (1.0 g/kg) treatment; n = 8 per group. *P ≤ .0001, compared with untreated group. (B) Splenectomized wild-type BALB/c mice respond to IVIG (1.0 g/kg) treatment; n = 6 per group. P values are indicated. (C) FcγRIIB−/− KO BALB/c mice (BALB/c KO) respond to IVIG (1.0 g/kg); n = 9 per group. *P ≤ .0001, compared with untreated group.
Neither spleen nor inhibitory Fcγ receptor is required for IVIG amelioration of ITP in BALB/c mice. (A) Nonsplenectomized wild-type BALB/c mice respond to IVIG (1.0 g/kg) treatment; n = 8 per group. *P ≤ .0001, compared with untreated group. (B) Splenectomized wild-type BALB/c mice respond to IVIG (1.0 g/kg) treatment; n = 6 per group. P values are indicated. (C) FcγRIIB−/− KO BALB/c mice (BALB/c KO) respond to IVIG (1.0 g/kg); n = 9 per group. *P ≤ .0001, compared with untreated group.
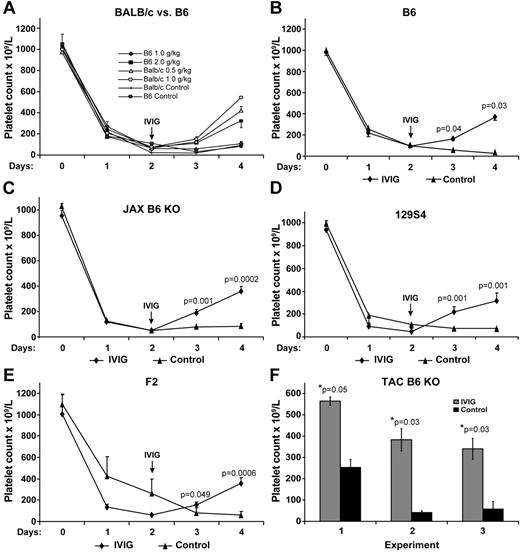
To further explore the role of FcγRIIB in ITP, we next used BALB/c mice deficient in FcγRIIB. We found that BALB/c KO mice responded as wild-type mice to IVIG (1 g/kg) with no differences in amelioration of their ITP (Figure 1C), supporting previously published findings.17 However, surprisingly, initial experiments in B6 mice using 1 g/kg IVIG showed no effect on ITP (data not shown). To address this apparent conundrum between the 2 background strains of mice, we thought that perhaps the B6 mouse strain may be less sensitive to the effects of IVIG administration. Therefore, we first performed a dose-response study of IVIG effect on ITP comparing wild-type BALB/c with B6 mice. Figure 2A shows that BALB/c mice are more sensitive to the effects of IVIG than B6 mice. Indeed, BALB/c mice respond well to IVIG to ameliorate ITP, even using 0.5 g/kg. In contrast, B6 mice failed to respond at all using 1 g/kg IVIG as did B6 KO mice (data not shown). For wild-type B6 mice to respond to IVIG required at least 2 g/kg; and even then, the response was less than that in BALB/c mice using 1 g/kg IVIG (Figure 2A). Indeed, for B6 mice to show a robust response to IVIG treatment, 2.5 g/kg was necessary (Figure 2B). Using 2.5 g/kg IVIG in B6 mice gave a response that was similar to BALB/c mice receiving 0.5 to 1 g/kg (Figure 2B compared with A). These results demonstrate that B6 mice are resistant to the effects of IVIG, requiring 2- to 2.5-fold more IVIG than BALB/c for clear amelioration of their ITP. Therefore, to examine the IVIG response in thrombocytopenic B6 KO mice, we decided to use 2.5 g/kg IVIG.
Response to IVIG treatment is strain background and dosage dependent and FcγRIIB independent. (A) Two- to 4-fold more IVIG is required for IVIG amelioration of ITP in B6 mice (n = 5 per group) compared with Balb/c mice (n = 6 in each group). (B) IVIG (2.5 g/kg) ameliorates ITP in wild-type B6 mice (n = 10). (C) IVIG (2.5 g/kg) ameliorates ITP using non-fully congenic JAX B6 KO mice (n = 9). (D) IVIG (2.5 g/kg) ameliorates ITP using 129S4 mice (n = 4). (E) IVIG (2.5 g/kg) ameliorates ITP using recommended control F2 wild-type mice (n = 7). Results represent the mean ± SEM. (F) IVIG (2.5 g/kg) ameliorates ITP using fully congenic TAC B6 KO mice. Three independent experiments are shown and indicate a 2.2-, 9.75-, and 6.6-fold, respectively, increase in the platelet count at day 2 after IVIG treatment when the platelet count was at nadir. Data are the mean ± SEM for n = 3, 4, and 5, respectively.
Response to IVIG treatment is strain background and dosage dependent and FcγRIIB independent. (A) Two- to 4-fold more IVIG is required for IVIG amelioration of ITP in B6 mice (n = 5 per group) compared with Balb/c mice (n = 6 in each group). (B) IVIG (2.5 g/kg) ameliorates ITP in wild-type B6 mice (n = 10). (C) IVIG (2.5 g/kg) ameliorates ITP using non-fully congenic JAX B6 KO mice (n = 9). (D) IVIG (2.5 g/kg) ameliorates ITP using 129S4 mice (n = 4). (E) IVIG (2.5 g/kg) ameliorates ITP using recommended control F2 wild-type mice (n = 7). Results represent the mean ± SEM. (F) IVIG (2.5 g/kg) ameliorates ITP using fully congenic TAC B6 KO mice. Three independent experiments are shown and indicate a 2.2-, 9.75-, and 6.6-fold, respectively, increase in the platelet count at day 2 after IVIG treatment when the platelet count was at nadir. Data are the mean ± SEM for n = 3, 4, and 5, respectively.
We first used mice from The Jackson Laboratory. These JAX B6 KO mice are not fully congenic, having a mixed background of B6 and 129S4, which we confirmed was approximately 50:50 using strain-specific SNP analysis (data not shown). To control for the mixed phenotype of these mice, we also tested 129S4 strain as well as F2 mice from the breeding of wild-type and 129S4 strains (recommended as control by The Jackson Laboratory). We found that in all mice IVIG used at 2.5 g/kg was able to ameliorate the ITP (Figure 2B-E). Thus, FcγRIIB is not required for IVIG effect in B6 mice when the dosage of IVIG used is sufficient.
To confirm these findings, we obtained B6 KO mice, fully congenic on a B6 background, from Taconic Farms. Again, in 3 independent experiments, we found that IVIG was able to ameliorate the ITP (Figure 2F). Thus, the conundrum of differing results for the role of FcγRIIB in ITP depending on the background strain of mice was the result of the differential sensitivity of the mouse strains to IVIG.
Our data contradict prior suggestions of a role for the FcγRIIB inhibitory receptor in the therapeutic effect of IVIG in ITP. However, our findings are consistent with previous results that showed IVIG able to ameliorate ITP despite lack of FcγRIIB signaling pathways,15 results with BALB/c KO mice,17 and lack of a role for FcγRIIB in patients treated with IVIG.16 Furthermore, our results provide a plausible explanation for the conundrum when using FcγRIIB KO mice on different background strains.
In conclusion, in our mouse model of ITP, we obtained consistent results in 2 different mouse backgrounds showing that FcγRIIB is not required for IVIG amelioration of the passive antibody-mediated thrombocytopenia. Our mouse model of ITP, although more closely resembling human ITP than other published mouse models,21,22 is still an imprecise model of the human disease for a variety of reasons. In addition, our results obtained using our experimental model of ITP may only be reflective of our particular mouse model; and, as such, the role of the inhibitory Fcγ receptor may be shown to be different in other mouse models of human diseases where IVIG shows efficacy,14 including other experimental ITP models. However, when FcγRIIB KO mice are used in studies to assess its role in the action of IVIG, it is best to test both BALB/c and B6 background strains of KO mice and show concordance of results obtained before defining a role for this receptor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Canadian Blood Services (grants XT00041 and XT00048). D.L. received a Canadian Blood Services Postdoctoral Fellowship award.
Authorship
Contribution: D.L. performed experiments, analyzed data, and wrote the manuscript; Y.K. performed experiments and analyzed data; and D.R.B. designed research, analyzed data, obtained grant funding, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald R. Branch, Research & Development, Canadian Blood Services, 67 College St, Toronto, ON, Canada M5G 2M1; e-mail: don.branch@utoronto.ca.