Delayed recovery of mature blood cells poses a serious, expensive, and often life-threatening problem for many stem cell transplantation recipients, particularly if heavily pretreated and serving as their own donor, or having a CB transplantation as the only therapeutic option. Importantly, the different cells required to ensure a rapid, as well as a permanent, hematopoietic recovery in these patients remain poorly defined. We now show that human CB and mobilized peripheral blood (mPB) collections contain cells that produce platelets and neutrophils within 3 weeks after being transplanted into sublethally irradiated NOD/scid-IL-2Rγc-null mice. The cells responsible for these 2 outputs are similarly distributed between the aldehyde dehydrogenase–positive and –negative subsets of lineage marker-negative CB and mPB cells, but their overall frequencies vary independently in individual samples. In addition, their total numbers can be seen to be much (> 30-fold) lower in a single “average” CB transplantation compared with a single “average” mPB transplantation (normalized for a similar weight of the recipient), consistent with the published differential performance in adult patients of these 2 transplantation products. Experimental testing confirmed the clinical relevance of the surrogate xenotransplantation assay for quantifying cells with rapid platelet regenerative activity, underscoring its potential for future applications.
Introduction
Improved understanding of transplantable cells that can rapidly restore acceptable platelet and neutrophil levels is an outstanding clinical need. Failure to obtain adequate outputs of these cells is associated with profound morbidity and mortality, and also poses a huge financial burden on health care systems worldwide. One of the most common settings where blood count recovery remains problematic is in myeloablated adult recipients of HSC transplants where the only donor options are either an allogeneic CB cell harvest or an autologous one where the patient has been heavily pretreated. In either case, the pace of platelet and neutrophil recovery after transplantation is typically delayed, sometimes dangerously so.1 Interestingly, both the posttransplantation administration of G-CSF and the transplantation of multiple CB units have helped these situations but both of these strategies remain inadequate.2,3 These findings point to a need for new methods to anticipate when a transplantation will be inadequate or to generate sufficient numbers of cells ex vivo that have rapid in vivo neutrophil and platelet-producing potential.
Various lines of evidence suggest that transplantable cells with these properties should be different from HSCs that can reconstitute the entire system permanently. In mice, it is now well established that the first mature blood cells to be produced in lethally irradiated recipients transplanted with unseparated BM cells are derived from a multiplicity of lineage-restricted progenitor subsets. Only later are the same mature blood cell types derived from cells with a larger range of lineage options and greater self-renewal potential.4 Accumulating evidence indicates a high degree of parallelism in this hierarchy of primitive hematopoietic subsets with the human system.5,–7 A suitable approach to designing and validating an in vivo assay for such short-term repopulating human cells was made possible by the development of the NOD/scid/IL-2Rγc−/− (NSG) mouse as a recipient of human xenografts.8 Earlier studies had suggested that human cells with short-term early repopulating activity cannot be investigated in NOD/scid (NS) mice because these human cells are selectively eliminated by the low levels of natural killer (NK) cells still present in NS hosts. Conversely, genetic or immunologic strategies that reduce or eliminate this host NK activity selectively enable such rapidly reconstituting human cells to engraft more efficiently.5,7,9,,,–13
The present study was undertaken to determine whether a short-term xenografting assay using NSG mice as recipients could be developed to enable the robust and quantitative detection of transplantable human cell types capable of rapidly producing platelets and neutrophils (within 2-4 weeks after transplantation). Our findings show that such functionally defined populations are readily quantified in transplanted NSG mice, are phenotypically heterogeneous, and also differ both from each other and from HSCs. Those responsible for platelet production also appear to have clinically predictive correlates.
Methods
Human cells
CB cells from anonymized normal, full-term infants delivered by cesarean section were collected in heparin, pooled, and the low-density (< 1.077 g/cm3) fraction isolated by centrifugation on Ficoll-Hypaque (Pharmacia Biotech AB). Mobilized peripheral blood (mPB) cells were collected by leukapheresis from chemotherapy-treated patients who then received a daily subcutaneous injection of 5 μg/kg G-CSF and subsequently underwent an autologous transplantation with their collected cells. All samples were cryopreserved until use. mPB samples used to characterize the cells they contained were from samples that gave rapid clinical recoveries. Additional samples that gave poor platelet recoveries were used exclusively to correlate this activity with the results of the xenograft assay.
Cell staining and sorting
Thawed CB or mPB cells were first depleted of cells expressing CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A lineage (Lin) markers using the EasySep kit, according to the manufacturer's directions (StemCell Technologies). These Lin− cells were then stained for aldehyde dehydrogenase (ALDH) activity using the ALDEFLUOR kit, according to the manufacturer's directions (Aldagen Inc). FACS was used to isolate Lin−ALDH+ cells.
Colony-forming cell assays
Cells were cultured in 1.1-mL volumes of Methocult H4435 (STEMCELL Technologies) containing 30% FBS, 3 U/mL human erythropoietin, 50 ng/mL human steel factor, and 20 ng/mL each of human IL-3, IL-6, GM-CSF, and G-CSF. Cultures were evaluated 14-18 days later for the presence of colonies of maturing erythroblasts (from burst-forming units [BFU-E]), or granulocytes and macrophages (from CFU-GM) or colonies containing mixtures of these lineages (from CFU-GEMM). To enumerate clonogenic precursors of megakaryocytes (CFU-Mks), cells were cultured in collagen-based MegaCult-C (STEMCELL Technologies) containing 50 ng/mL human thrombopoietin and 10 ng/mL each of human IL-3 and IL-6. Cultures were then fixed and stained 12 days later according to the manufacturer's directions to detect and enumerate the presence of megakaryocyte colonies of different sizes as indicated in Table 1.
Transplantation assays in NSG mice
Test cells were injected intravenously along with 106 irradiated (15 Gy) human BM cells into 6- to 10-week-old NSG mice within 24 hours after the mice had been irradiated with 315 cGy 137Cs γ rays. To detect human neutrophils in the BM, mature RBCs in BM aspirates were lysed with 0.8% NH4OH (StemCell Technologies) on ice for 10 minutes. Nonspecific staining with Abs was then blocked by incubating the cells with 10% human serum and anti–mouse FcR Ab (2.4G2; StemCell Technologies) for 10 minutes on ice before adding anti–human CD45 (2D1), CD15 (HI98), CD66b (G10F5; all from BD Pharmingen) for 30 minutes on ice. Cells were then washed and resuspended in Hanks buffer with 2% FBS and 1 μg/mL propidium iodide (PI) for FACS analysis. To detect circulating human platelets in the PB, a 20-μL sample of blood was collected from each mouse. Total mouse platelet counts were recorded with an automated animal blood cell counter (Scil Vet ABC). Blood samples were stained directly with human-specific anti-CD41a (HIP8) and mouse-specific anti-CD41 (MWreg30; both from BD Pharmingen) for 30 minutes at room temperature. Two hundred microliters of 0.8% NH4OH were then added to each sample to lyse the RBCs present during a 5-minute period of incubation at room temperature. Samples were then diluted with 200 μL of HEPES-Tyrode buffer (10mM HEPES, 137mM NaCl, 268mM KCl, 0.42mM NaH2PO4, 1.7mM MgCl2, 11.9mM NaHCO3 and 5mM glucose) and analyzed by FACS immediately. Mice were classified as repopulated with human neutrophils if 5 or more human CD45+CD15/66+ events were detected per 20 000 PI− cells analyzed (ie, ≥ 0.025% of total viable mouse BM cells). To detect human neutrophils in the PB, 50-100 μL of blood was collected from each mouse, blocked, and stained directly with the same Abs described for neutrophil detection in the BM for 30 minutes on ice. After staining, mature RBCs were lysed with 0.8% NH4OH on ice for 10 minutes, and the remaining cells were then washed and resuspended in Hanks buffer with 2% FBS and 1 μg/mL PI for FACS analysis.
Biotin labeling of circulating mouse and human platelets in NSG mice
To measure the average platelet half-life in NSG mice, 0.75 mg of Sulfo-NHS-biotin (Pierce) dissolved in 200 μL of PBS was injected intravenously into mice. The first bleeding was considered as time 0 (t0) and set as the initial level of biotinylation. Blood samples were diluted 10 times with Tyrode HEPES buffer and centrifuged at 100g for 5 minutes at room temperature. The platelet-enriched supernatant was collected and centrifuged again at 1500g for 15 minutes at room temperature. The platelet pellet was then resuspended in 100 μL of Tyrode HEPES buffer and stained with human-specific anti-CD41a (HIP8), mouse-specific anti-CD41 (MWreg30) and streptavidin-allophycocyanin (BioLegend) for 30 minutes at room temperature, and the cells then analyzed by flow cytometry. The level of biotinylated platelets was then expressed as the percentage of the level of biotinylated platelets detected at t0.
All human and animal studies were approved by the Research Ethics Board and the Animal Care Committee of the University of British Columbia.
Results
Transplanted NSG mice can be used to detect and quantify human cells capable of rapidly producing circulating platelets
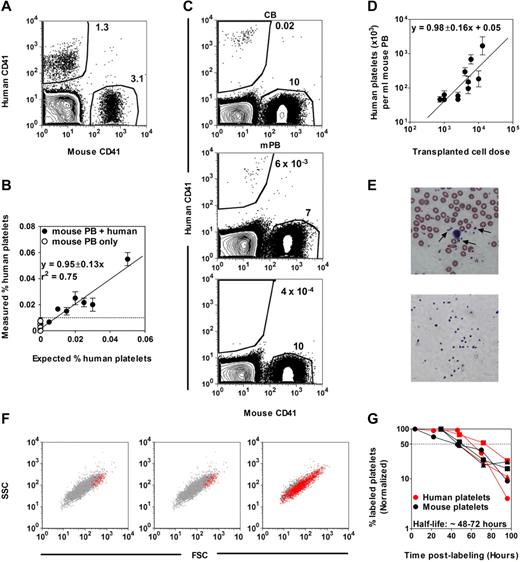
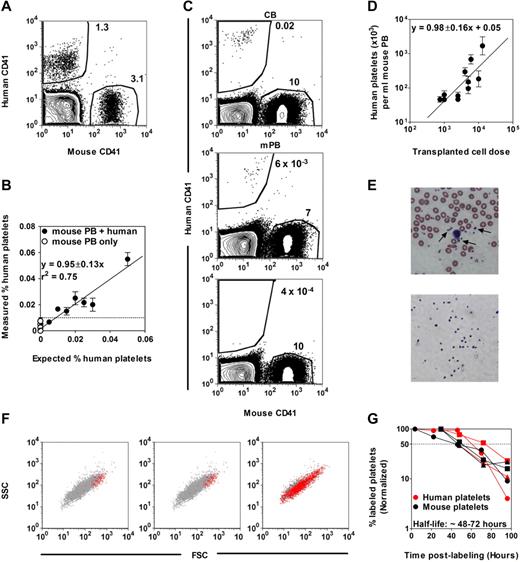
Figure 1 shows the results of a first baseline experiment designed to establish the feasibility and sensitivity of detecting human platelets admixed in the peripheral blood (PB) of NSG mice. Both human and mouse platelets were readily identified by flow cytometric analysis as unique and separable populations by their small size, and could be further distinguished from one another by their staining (or not) with an anti–human CD41 Ab (Figure 1A). Analysis of mouse PB samples containing decreasing numbers of human platelets showed that the presence of ≥ 5 human CD41+ platelets per 5 × 104 mouse CD41+ platelets represented a significant signal above background events (Figure 1B). Circulating human platelets could also be readily detected in the PB of NSG mice within 2-3 weeks of their being transplanted with Lin− cells isolated from either human CB or mPB harvests (Figure 1C). This suggested the suitability of the NSG host to detect human cells with rapid platelet-producing activity in vivo.
Detection of human platelets in NSG mouse blood. (A) FACS distinction of human and mouse CD41+ platelets in a mixture of human and mouse blood cells after staining with human- and mouse-specific anti-CD41 Abs and gating on low forward and side light scattering events. (B) The minimum number of human platelets detectable in mouse blood determined from an analysis of serial dilutions of human blood cells in undiluted mouse blood (= 0.01%). (C) Representative FACS plots of human platelets produced in NSG mice transplanted with human lin− CB (top panel) or lin− mPB (middle panel) cells. The bottom panel shows the blood of a nontransplanted NSG mouse. (D) Linear relationship between the dose of Lin−ALDH+ CB cells transplanted and the level of circulating human platelets detected 3 weeks later. Shown are the mean ± SEM of data pooled from 7 experiments (2-5 mice per cell dose per experiment). (E) Comparison of Wright-Giemsa–stained human platelets (arrows) present in normal human blood (top panel) and isolated by FACS (as small human CD41+ cells) from the blood of NSG mice 3 weeks after transplantation of human CB cells (bottom panel). (F) Representative FACS profiles of circulating human and mouse platelets in NSG mice 3 weeks after transplantation of human CB (left panel) or mPB cells (middle panel), or in a fresh mixture of human and mouse blood cells (right panel). Red and gray dots are human CD41+ and mouse CD41+ platelets, respectively. SSC indicates side light scattering activity; and FSC, forward light scattering activity. (G) Half-life determinations of human and mouse platelets in NSG mice transplanted with human CB cells. Platelets were labeled by injecting the mice IV with sulfo-NHS-biotin and the changing percentage thereafter of the initial level of biotin-labeled mouse and human platelets was then determined. Each symbol type represents a single mouse.
Detection of human platelets in NSG mouse blood. (A) FACS distinction of human and mouse CD41+ platelets in a mixture of human and mouse blood cells after staining with human- and mouse-specific anti-CD41 Abs and gating on low forward and side light scattering events. (B) The minimum number of human platelets detectable in mouse blood determined from an analysis of serial dilutions of human blood cells in undiluted mouse blood (= 0.01%). (C) Representative FACS plots of human platelets produced in NSG mice transplanted with human lin− CB (top panel) or lin− mPB (middle panel) cells. The bottom panel shows the blood of a nontransplanted NSG mouse. (D) Linear relationship between the dose of Lin−ALDH+ CB cells transplanted and the level of circulating human platelets detected 3 weeks later. Shown are the mean ± SEM of data pooled from 7 experiments (2-5 mice per cell dose per experiment). (E) Comparison of Wright-Giemsa–stained human platelets (arrows) present in normal human blood (top panel) and isolated by FACS (as small human CD41+ cells) from the blood of NSG mice 3 weeks after transplantation of human CB cells (bottom panel). (F) Representative FACS profiles of circulating human and mouse platelets in NSG mice 3 weeks after transplantation of human CB (left panel) or mPB cells (middle panel), or in a fresh mixture of human and mouse blood cells (right panel). Red and gray dots are human CD41+ and mouse CD41+ platelets, respectively. SSC indicates side light scattering activity; and FSC, forward light scattering activity. (G) Half-life determinations of human and mouse platelets in NSG mice transplanted with human CB cells. Platelets were labeled by injecting the mice IV with sulfo-NHS-biotin and the changing percentage thereafter of the initial level of biotin-labeled mouse and human platelets was then determined. Each symbol type represents a single mouse.
Because the sensitivity of detecting human platelet production in NSG mice is dependent on the number of mouse platelets present in the same sample, it was important to quantify the extent and time course of changes anticipated in circulating mouse platelet levels in the irradiated recipients. This analysis showed an early drop in circulating mouse platelet levels, which were never fully restored, but did return to stable, near-normal levels between 3 and 4 weeks after irradiation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Using this information, we then examined the relationship between the number of Lin−ALDH+ CB cells transplanted (over a 20-fold range) and the number of circulating human platelets present 3 weeks later (Figure 1D). The linear relationship linking these 2 parameters indicates a quantitative input-output function supporting the use of limiting dilution methods for absolute frequency determinations.
Microscopic and flow cytometric examination of the human platelets appearing in the circulation at the 3-week time point showed these to be morphologically similar to the platelets found in normal human PB (Figure 1E) but lacked the smaller subsets (Figure 1F). To determine whether this might be related to a different half-life in the NSG host, we monitored their rate of disappearance after in vivo biotin labeling (Figure 1G). The result of this experiment showed that both mouse and human platelets have a comparable half-life in NSG mice of between 48 and 72 hours.
Human neutrophil chimerism in the PB is more than 10-fold lower than in the BM of xenotransplanted NSG mice
In previous studies, we had shown that multiple human hematopoietic tissues (BM, mPB, and CB) contain cells that will engraft NS-β2-microglobulin-null mice and produce detectable levels of CD15/66b+ (mature myeloid) cells in their BM within 3 weeks.5 In these experiments, we further showed that the majority of such cells are found within the CD38+ subset of CD34+ cells. Given our observation that human platelet outputs could be followed in the more readily monitored PB of engrafted NSG mice, we next asked whether PB could also be used to evaluate human neutrophil production in the same xenograft model.
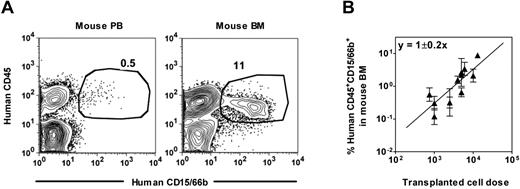
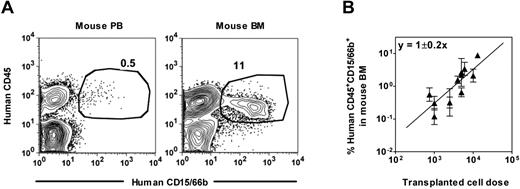
Preliminary measurements of mixtures of human CD15/66b+ neutrophils with NSG mouse PB cells showed that the sensitivity of detecting human neutrophils was again 0.01% (supplemental Figure 2A) and human neutrophils could likewise be detected in the PB as well as in the BM of NSG mice transplanted with human Lin−ALDH+ CB or mPB cells. Comparison of the representation of human neutrophils in the PB and BM (percentage of total viable nucleated cells in each case) of individual transplanted mice covering a range of engraftment levels showed that these values were highly correlated (Pearson correlation coefficient = 0.91, supplemental Figure 2B). However, PB values were consistently > 10-fold lower than the BM values (Figure 2A, supplemental Figure 2B), which alters the transplantation dose required to achieve detectable outputs (data not shown). Accordingly, for the rest of our experiments, we chose to stay with a BM end point to analyze cells with human neutrophil output activity after confirming that this end point is linearly related to the dose of input cells transplanted (Figure 2B).
Detection of human neutrophils in NSG mouse blood. (A) Representative FACS plots showing the distinction of human CD45+CD15/66b+ neutrophils in the blood (left panel) and BM (right panel) of a NSG mouse transplanted with human Lin−ALDH+ CB cells. (B) Linear relationship between the dose of Lin−ALDH+ CB cells transplanted and the level of human neutrophils detected 3 weeks later in the BM. Shown are the mean ± SEM of data pooled from 7 experiments (2-5 mice per cell dose per experiment).
Detection of human neutrophils in NSG mouse blood. (A) Representative FACS plots showing the distinction of human CD45+CD15/66b+ neutrophils in the blood (left panel) and BM (right panel) of a NSG mouse transplanted with human Lin−ALDH+ CB cells. (B) Linear relationship between the dose of Lin−ALDH+ CB cells transplanted and the level of human neutrophils detected 3 weeks later in the BM. Shown are the mean ± SEM of data pooled from 7 experiments (2-5 mice per cell dose per experiment).
Human cells that rapidly regenerate platelets and neutrophils in vivo are phenotypically heterogeneous and independently variable in number
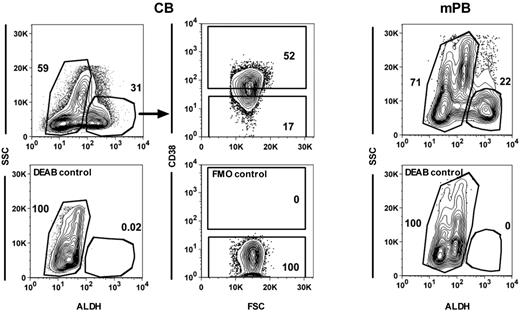
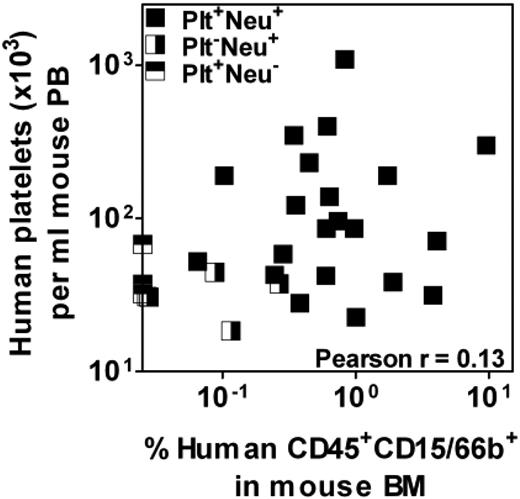
Human HSCs and closely related primitive progenitor cells have been previously shown to be ALDH+ as well as CD34+CD38−, whereas many, but not all, human progenitors detectable by standard in vitro colony-forming cell (CFC) assays are CD34+CD38+ and ALDH−.14,,,–18 Therefore, we next sought to determine the extent to which ALDH activity might serve as a discriminating feature of CB and mPB cells with short-term platelet and/or neutrophil-producing activity, and the extent to which their distribution of ALDH activities might mirror those of the corresponding lineages of CFCs in the same samples. Accordingly, the ALDH+ and ALDH− fractions of Lin− low-density CB and mPB samples were isolated (Figure 3) and proportional aliquots transplanted into NSG mice. Aliquots of the same fractions were also plated into collagen or methylcellulose cultures to assess their frequency (and hence content) of megakaryocytic and granulopoietic CFCs. Table 1 summarizes the data obtained. It can be seen that for both 3-week platelet and neutrophil production, the majority of transplantable activity was found to be associated with the rarer ALDH+ fraction (≥ 71% of the total 3-week platelet and neutrophil production was derived from ALDH+ cells). However, the ALDH− fractions also contained consistent rapid platelet and neutrophil-producing activity, with somewhat more of the total 3-week platelet-producing activity (19% in CB and 29% in mPB) in this fraction compared with the total 3-week neutrophil-producing activity (12% in CB and 5% in mPB). This difference in the distribution of 3-week platelet and neutrophil-producing activity between the ALDH+ and ALDH− fractions provided the first indication that these functions might be attributes of different cells. Comparing rapid platelet and neutrophil-producing activities in individual recipients of Lin−ALDH+ cells provided further evidence that these are attributes of different progenitor subtypes. As shown in Figure 4, the output of human platelets and neutrophils in each of these mice was highly variable and uncorrelated (Figure 4).
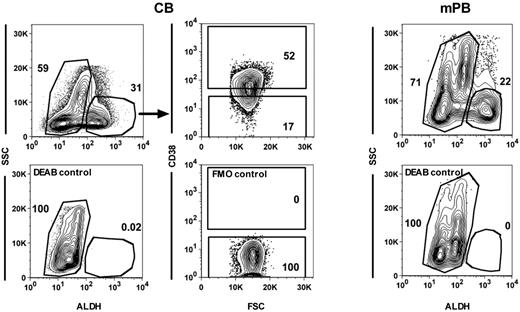
Isolation of different subsets of human CB and mPB cells. Representative FACS plots showing gates used to isolate ALDH+ and ALDH− CB (top left panel) and mPB cells (top right panel) and the CD38+ and CD38− subsets within the ALDH+ CB cells (top middle panels). Bottom panels show the controls for each of these staining.
Isolation of different subsets of human CB and mPB cells. Representative FACS plots showing gates used to isolate ALDH+ and ALDH− CB (top left panel) and mPB cells (top right panel) and the CD38+ and CD38− subsets within the ALDH+ CB cells (top middle panels). Bottom panels show the controls for each of these staining.
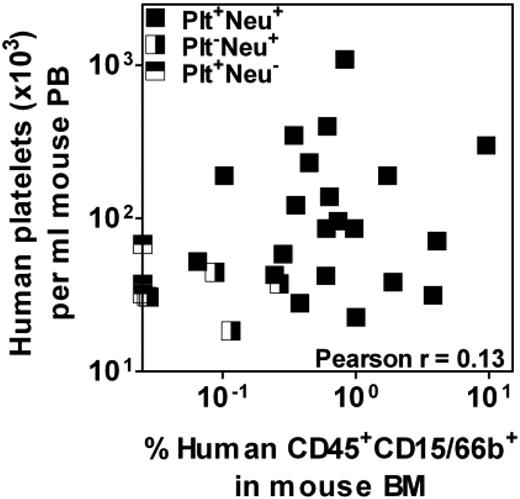
Lack of correlation between rapid platelet- and neutrophil-producing activity in individually analyzed mPB samples. Low numbers of Lin−ALDH+ cells from 3 different mPB preparations were transplanted into NSG mice and the levels of human platelets and neutrophils present 3 weeks later in the PB and BM, respectively, were determined. Each data point indicates an individual mouse and mice positive for only one of the 2 lineages or both are shown separately.
Lack of correlation between rapid platelet- and neutrophil-producing activity in individually analyzed mPB samples. Low numbers of Lin−ALDH+ cells from 3 different mPB preparations were transplanted into NSG mice and the levels of human platelets and neutrophils present 3 weeks later in the PB and BM, respectively, were determined. Each data point indicates an individual mouse and mice positive for only one of the 2 lineages or both are shown separately.
Examination of the distribution of CFU-megakaryocytes (Mks) and CFU-GMs between the ALDH+ and ALDH− subsets of Lin− CB and mPB cells showed a parallel set of differences to those observed for the cells with corresponding lineage outputs in vivo (Table 1). Particularly notable is the similarity in the distributions of the more primitive subset of CFU-Mks (defined by their greater output of Mks/colony) and cells with rapid platelet-producing activity in vivo between the ALDH+ and ALDH− fractions, in contrast to the predominance of all types of CFU-GMs and cells with rapid neutrophil-producing activity in vivo in the ALDH+ fraction. Taken together, these findings suggest some overlap between the 3-week platelet-producing cells and primitive CFU-Mks, and between 3-week neutrophil-producing cells and total CFU-GMs.
In 2 additional experiments, the CD38+ and CD38− fractions of Lin−ALDH+ cells were isolated by FACS from each of 2 separate CB pools (Figure 3) and proportional aliquots of these fractions were then transplanted in NSG mice or plated in vitro as before. As shown in Table 2, the 3-week platelet and neutrophil-producing activities of these 2 CB pools were again dissociated. In the first CB pool (no. 1), the 3-week assays for neutrophil-producing cells were uninformative but the data for platelet production was robust and showed approximately equal output from both the CD38+ and CD38− fractions of the transplanted Lin−ALDH+ cells. In the second CB pool (no. 2), the platelet-producing activity was similar, and although robust neutrophil-producing activity was also detected, its distribution between the donors' CD38+ and CD38− subsets was opposite to that of the platelet-producing cells (∼ 6:1 and 1:2, respectively). Interestingly, these distributions were mirrored by a similar heterogeneity in the distributions of CFU-Mks and CFU-GMs in the subsets of CD38+ and CD38− Lin−ALDH+ cells, although the latter did not track as closely with the corresponding repopulating cells as seen for the ALDH+ and ALDH− subsets.
In summary, these findings show that platelet and neutrophil-producing activities vary independently between CB and mPB, between different CB preparations, and between different subsets of cells within these sources, but track similarly with their corresponding primitive subsets of lineage-restricted progenitors with in vitro clonogenic activity. Together, they argue in support of the rapid platelet- and neutrophil-producing cells detected in transplanted NSG mice being vested in phenotypically heterogeneous progenitor populations that are more closely related to their corresponding lineage-restricted progenitor counterparts than to each other.
Human cells that rapidly regenerate platelets and neutrophils in vivo are present at different frequencies in CB and mPB
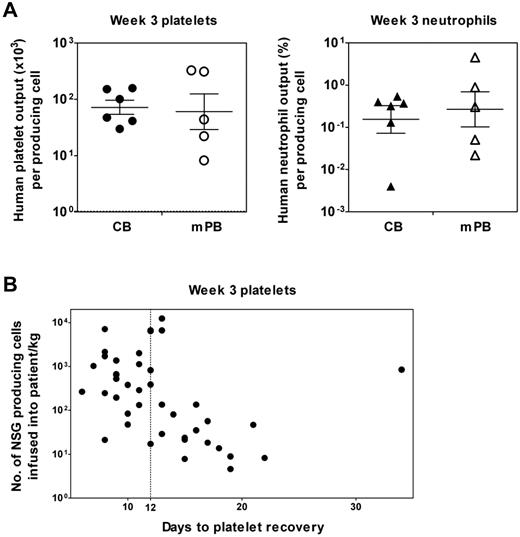
To determine the frequency in different CB and mPB samples of cells with rapid platelet and/or neutrophil-producing ability, we performed limiting dilution assay (LDA) transplantation experiments (supplemental Table 1A-D). The results show that their frequency in the Lin−ALDH+ fraction of CB cells is ∼ 2.5- and 4.5-fold higher, respectively, than in mPB (Table 3). From these frequency data, knowledge of the corresponding levels of repopulation achieved for a given transplantation dose (eg, Tables 1–2) and the established linearity between the number of cells transplanted and output (eg, Figures 1D, 2B), we then calculated the average 3-week platelet and neutrophil outputs per producing platelet- and neutrophil-producing cell, assuming each mouse has 3 mL of blood and a total BM cellularity of 2 × 108 cells. The results of these calculations show no significant difference in the 3-week platelet outputs of the original cells in CB or mPB that were responsible for their production in transplanted NSG mice and a similar equivalence was evident in the outputs of neutrophils from their precursors in CB and mPB (Figure 5A, left and right panels, respectively).
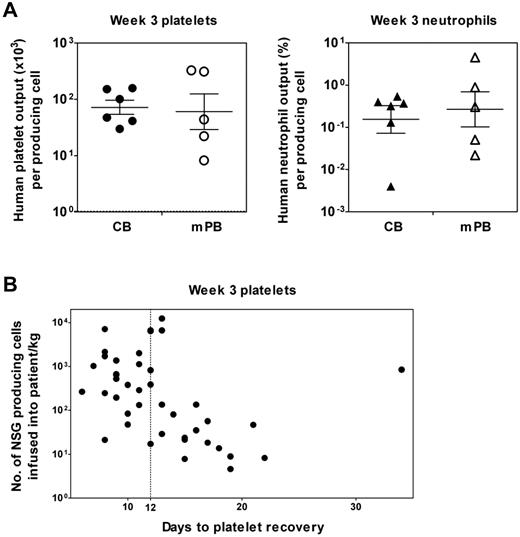
Similar 3-week platelet and neutrophil outputs from Lin−ALDH+ progenitors in human CB and mPB detected by a clinically relevant xenotransplantation assay using NSG mice. (A) The individual values shown are average outputs calculated for different samples of CB and mPB based on limiting dilution analysis of the frequency of rapid platelet- and neutrophil-producing cells present in each sample tested and their measured average outputs at doses that were nonlimiting but also nonsaturating. The crossbars represent the geometric mean ± SEM of the sample values for CB and mPB separately. (B) Poor rapid human platelet production in NSG mice correlates with delayed platelet recovery in clinical transplantations. Shown is a plot of the number of rapid human platelet-producing cells (measured using the 3-week NSG transplantation assay) determined to be present in 42 mPB autotransplantations (based on the frequency of these multiplied by the cellularity of the transplantation and then normalized per kilogram of body weight of the patient) versus the time taken by each patient to recover their platelet count (defined as > 20 × 109/L after transplantation). The dotted line shows the definition of delayed recovery chosen here to be 12 days to correspond to the earliest time when human platelets would appear from the cells assayed in NSG mice.
Similar 3-week platelet and neutrophil outputs from Lin−ALDH+ progenitors in human CB and mPB detected by a clinically relevant xenotransplantation assay using NSG mice. (A) The individual values shown are average outputs calculated for different samples of CB and mPB based on limiting dilution analysis of the frequency of rapid platelet- and neutrophil-producing cells present in each sample tested and their measured average outputs at doses that were nonlimiting but also nonsaturating. The crossbars represent the geometric mean ± SEM of the sample values for CB and mPB separately. (B) Poor rapid human platelet production in NSG mice correlates with delayed platelet recovery in clinical transplantations. Shown is a plot of the number of rapid human platelet-producing cells (measured using the 3-week NSG transplantation assay) determined to be present in 42 mPB autotransplantations (based on the frequency of these multiplied by the cellularity of the transplantation and then normalized per kilogram of body weight of the patient) versus the time taken by each patient to recover their platelet count (defined as > 20 × 109/L after transplantation). The dotted line shows the definition of delayed recovery chosen here to be 12 days to correspond to the earliest time when human platelets would appear from the cells assayed in NSG mice.
However, from estimates of the average cellularities of a single unit of 100 mL of CB and that of a minimally acceptable mPB transplantation (set at 63 × 109 cells assuming a frequency of 1% CD34+ cells and a required 63 × 107 CD34+ cells for an average 70-kg recipient), we also calculated the total number of rapid platelet and neutrophil-producing cells in each of these products (Table 4). Using these estimates, it can be seen that CB contains ∼ 25- to 50-fold fewer cells with either rapid neutrophil- or platelet-producing activity.
Low numbers of human cells that regenerate platelets in 3 weeks in NSG mice predict delayed platelet recovery in clinical transplantations
To further test the relevance of our NSG assays, we obtained 42 mPB samples of cells that had been used as clinical autotransplants with subsequent variable rates of platelet and neutrophil recovery (supplemental Table 2). Eighteen of the patients transplanted with these cells showed a delayed platelet recovery (> 12 days to reach a platelet count of 20 × 109/L). Twelve of these also showed a neutrophil recovery that was similarly delayed (> 12 days to reach a neutrophil count of 0.5 × 109/L). We then transplanted aliquots of each of these samples into NSG mice to determine the content of rapid platelet and neutrophil-producing cells present in each, based on the outputs of platelets and neutrophils measured in the PB and BM, respectively, 3 weeks later. We then used these numbers to calculate the number of repopulating cells of each type that had been infused into the patients (per kilogram of body weight). Figure 5B shows a plot of this second set of numbers against the time taken for each corresponding patient to recover a platelet count of 20 × 109/L after transplantation. Excluding the one significant outlier with a very prolonged delay in platelet recovery (5 weeks, which is > 3 SD from the mean number of days to platelet recovery), the results show a significant inverse correlation between these 2 parameters for patients with delayed platelet recoveries (Pearson r = −0.58, P = .02). This correlation is also independent of the number of CD34+ cells/kg body weight infused into the patients (P = .2) and not improved by inclusion of the latter parameter (P = .9). However, a similar inverse correlation was not discernible between the dose of cells with rapid neutrophil-producing activity in NSG mice and the delay in neutrophil recovery apparent in the more limited set of patients thus affected (data not shown).
Outputs of platelets and neutrophils in individual CB- or mPB-transplanted NSG mice remain uncorrelated until 20 weeks after transplantation
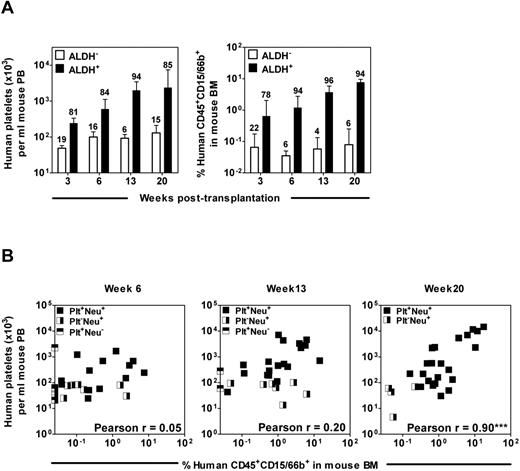
We also monitored the kinetics of human platelet and neutrophil production in NSG mice transplanted with Lin−ALDH+ and Lin−ALDH− CB and mPB cells over more prolonged periods (up to 20 weeks) after transplantation. Detectable levels of circulating human platelets and BM neutrophils were sustained in mice transplanted with 5000 Lin−ALDH+ cells and, by week 20, their progeny were contributing ∼ 85% of the total human platelet output and ∼ 94% of the total human neutrophil output (Figure 6A). Consistent with previous reports,5,19 the majority of the original CB and mPB cells responsible for these 20-week outputs also had a CD38− phenotype (supplemental Figure 3). The output of platelets and neutrophils was also linearly related to the number of cells transplanted at all time points after transplantation (supplemental Figure 4). Limiting dilution analyses showed that the frequency of cells with more prolonged platelet and neutrophil-producing activity in the Lin−ALDH+ fraction of CB remained relatively constant but decreased progressively in the same fraction of mPB (> 100-fold for the platelet producers and ∼ 7-fold for the neutrophil producers by week 20, Table 3). Interestingly, however, although most repopulation after 6 weeks was historically assumed to be derived from multipotent cells,5,19 we found that a significant correlation between platelet and neutrophil outputs was not obtained until 20 weeks after transplantation (Figure 6B).
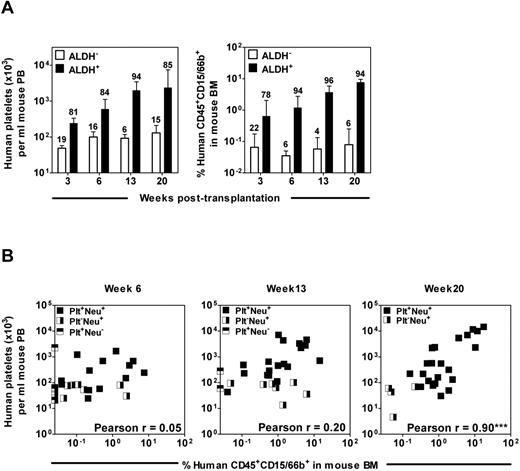
Long-term (20 weeks) but not intermediate (6-13 weeks) human platelet- and neutrophil-producing activities in different samples of CB correlate with one another. (A) NSG mice were each transplanted with 5000-10 000 Lin−ALDH+ CB cells or the cells from the matching fraction of Lin−ALDH− cells and the number of circulating human platelets (left panel) or human neutrophils in the BM (right panel) was determined up to 20 weeks later. The number above each bar indicates the proportion of total platelet- or neutrophil-producing cell activity in that cell fraction. Values shown are the mean ± SEM of data from 3 experiments. (B) Lin−ALDH+ cells from 7 different CB preparations were transplanted into NSG mice and the outputs of human platelets (in PB) and neutrophils (in BM aspirates) in each mouse were determined at various time points later. Pearson correlation analyses showed that these values were significantly correlated only for the week 20 posttransplantation end point.
Long-term (20 weeks) but not intermediate (6-13 weeks) human platelet- and neutrophil-producing activities in different samples of CB correlate with one another. (A) NSG mice were each transplanted with 5000-10 000 Lin−ALDH+ CB cells or the cells from the matching fraction of Lin−ALDH− cells and the number of circulating human platelets (left panel) or human neutrophils in the BM (right panel) was determined up to 20 weeks later. The number above each bar indicates the proportion of total platelet- or neutrophil-producing cell activity in that cell fraction. Values shown are the mean ± SEM of data from 3 experiments. (B) Lin−ALDH+ cells from 7 different CB preparations were transplanted into NSG mice and the outputs of human platelets (in PB) and neutrophils (in BM aspirates) in each mouse were determined at various time points later. Pearson correlation analyses showed that these values were significantly correlated only for the week 20 posttransplantation end point.
Discussion
The development of highly immunodeficient mice has revolutionized our ability to isolate and characterize many previously inaccessible primitive subtypes of human hematopoietic cells with transplantable in vivo regenerative potential. However, assays for cells that are responsible for rapid count recovery have remained poorly developed. This issue is of critical importance in the clinical transplantation field where platelet and even neutrophil recovery can often be sufficiently delayed to cause a serious risk of morbidity and even mortality, in spite of the use of platelet transfusions and G-CSF administration. Indeed, this issue is one of the major drawbacks to the wider use of CB transplants in adults where combining several units has not provided a solution. Studies in mice predict that the cells required to produce platelets and neutrophils within 2-3 weeks will do so only transiently and be phenotypically quite different from those responsible for permanent rescue of blood production.20,21 Further support for this has come from limited studies of human cells after the recognition that such cells also show heightened sensitivity to residual innate immune mechanisms when they are transplanted into immunodeficient mice.5,13 And when these are overcome, the transient, myeloid-restricted repopulating activity of CD34+CD38+ cells can then be detected, whereas all repopulating cells were previously reported to be CD34+CD38−.
NSG mice are particularly well suited for the development of assays for human cells able to rapidly regenerate platelet and neutrophil outputs because these mice have no detectable NK activity and a normal life expectancy of 2+ years.22 We now describe the use of these mice to detect and quantify the presence in CB and mPB samples of quantitatively distinct populations of transplantable human cells with rapid platelet and neutrophil-producing activity. Using this assay, we further show that these functionally defined populations from both sources are phenotypically similar, although different from each other in their relative content of ALDH+ low-density cells or the CD38− subset of ALDH+ low-density cells. In addition, we show that low numbers of these rapid NSG repopulating cells in mPB samples correlate with a reduced content of CFU-Mks and CFU-GMs and delayed platelet recovery in patients who received transplants.
Over the years, much effort has been unsuccessfully invested in attempts to develop ex vivo culture treatments that can accelerate hematopoietic recovery in recipients of CB or inadequate mPB transplants. Interestingly, only recently has some likelihood of success using factors like Delta-1 ligand23 or HOX-related proteins24,–26 emerged, although several other possibilities are on the horizon. The present introduction of a predictive assay for the specific cell type(s) of interest may facilitate progress in this area. In this regard, it is interesting that our findings confirm a similar HSC content of CB and mPB collections with a large discrepancy in rapid platelet and neutrophil-producing cell numbers as previously anticipated from less specific end points.5 This reinforces the notion that the delayed count recovery associated with the clinical use of CB transplantations is largely because of a profound, selective quantitative insufficiency of cells with rapid repopulating activity. Similarly, they explain why, at the same time, long-term, sustained donor cell chimerism is likely to be achieved if complications in the initial phase after transplantation are resolved.
The evidence presented here that the cells required for rapid platelet and neutrophil recovery are different also explains why the rates of recovery of both of these lineages in individual transplantation recipients are not necessarily concordant. As shown, the numbers of each of these types of rapid repopulating cells may vary quite differently from one sample to another and also within the different phenotypes of cells that possess these activities.
In summary, the introduction of an assay for clinically relevant and distinct cells required for rapid platelet and neutrophil outputs after transplantation should now enable many future and critical avenues of investigation. These include the more efficient development of methods for expanding the numbers of these cells ex vivo, characterizing their precise relationship with in vitro clonogenic cells with the same lineage differentiation capacity, delineating the mechanisms that regulate their production under differing conditions of demand, and elucidating the mechanisms regulating their homing, mobilization and sensitivity to innate immune attack in nonautologous recipients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the British Columbia Women's Hospital and Health Center and of the British Columbia Cancer Agency Stem Cell Assay Laboratory for assistance in procuring, processing, and cryopreserving CB samples. They also acknowledge the importance of the Hematology Cell Bank of British Columbia and its many contributors, supporters, and staff for enabling access to patients' cell samples used in this study.
This work was supported by grants from the Stem Cell Network (SCN) of Canada, The Terry Fox Foundation and the Terry Fox Research Institute, and the Canadian Institutes of Health Research. A.M.S.C. held a Fellowship from the Croucher Foundation (Hong Kong) and K.D. held a Co-Op Award from the SCN of Canada. R.R.B. was supported in part by a Scholar Award from the Michael Smith Foundation for Health Research and also holds a Terry Fox Foundation New Investigator Award. D.C.R. was supported in part by the Fonds de Recherche du Quebec-Santé (FRQS).
Authorship
Contribution: A.M.S.C. and C.J.E. designed the experiments; A.M.S.C., D.L., S.R., K.D., and P.H.M. performed the experiments; R.D. and R.R.B. performed the statistical analysis; D.H. and D.C.R. reviewed the clinical data on the mPB transplantation samples; and A.M.S.C., D.H., D.C.R., and C.J.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Connie J. Eaves, Terry Fox Laboratory, 675 West 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: ceaves@bccrc.ca.