Abstract
Heparin-induced thrombocytopenia is a prothrombotic adverse drug effect induced by platelet-activating antibodies against multimolecular complexes of platelet factor 4 and heparin. Diagnosis rests on a clinical assessment of disease probability and laboratory testing. Management involves immediate discontinuation of heparin and initiation of an alternative anticoagulant. Because of the frequency of thrombocytopenia among heparinized patients, the limited specificity of widely available immunoassays, the limited availability of more specific functional assays, and clinicians' fears of missing a case of true disease, overtesting, overdiagnosis, and overtreatment have become common. As a result, a substantial number of thrombocytopenic patients are unnecessarily exposed to costly alternative anticoagulants and their attendant risk of bleeding. In this review, we describe not only our approach to the evaluation and management of patients with heparin-induced thrombocytopenia, but also the measures we use to minimize misdiagnosis and unnecessary treatment of patients without the disease. In addition, we propose areas of investigation for improvement of the diagnosis and management of this potentially fatal disorder.
Introduction
Heparin-induced thrombocytopenia (HIT), formerly HIT type II, is a prothrombotic and potentially lethal disorder caused by platelet, endothelial, and monocyte-activating antibodies that target multimolecular complexes of platelet factor 4 (PF4) and heparin.1 Although HIT was first described more than 50 years ago,2 not until the 1990s was the target antigen identified, the now widely used anti-PF4/heparin ELISA developed,1 and the first effective therapies approved. Since that time, a major emphasis of training and contemporary literature has been early disease recognition and prompt initiation of therapy.3,4
This educational campaign has fueled a sea change in clinical practice. One of the most frequently encountered clinical problems in HIT is no longer under-recognition, but overdiagnosis, leading to exposure of thrombocytopenic patients without HIT to costly alternative anticoagulants and their attendant risk of bleeding.5,6 In this review, we highlight not only our approach to patients with HIT, but also measures we use and research we think is needed to minimize misdiagnosis and unnecessary and potentially harmful treatment of persons without the disorder.
Who develops HIT?
HIT is reported to occur in 0.2% to 5% of heparin-treated adults, although precise estimates are limited by the challenges of disease recognition and diagnosis. Risk factors fall into 2 categories: heparin-related and host-related variables (Table 1).
Heparin-related risk factors include type of heparin and duration of exposure. Low molecular weight heparin (LMWH) is associated with a 5- to 10-fold lower risk of HIT than unfractionated heparin (UFH)7,8 but accounts for a growing proportion of cases because of its increasing use. In theory, there may be a gradation of risk among LMWHs, with smaller, less charged species being associated with a lower incidence of disease. The risk is smaller still, and may be negligible, with the pentasaccharide fondaparinux.9 With respect to duration of therapy, a meta-analysis of 3529 medical and surgical patients receiving UFH thromboprophylaxis for 6 or more days demonstrated an incidence of HIT of 2.6%.7 A hospital database review suggested that briefer courses are associated with a substantially lower incidence (∼ 0.2%).10 The impact of heparin dose and route of administration are less certain. Several studies demonstrate a greater frequency of HIT among patients receiving intravenous therapeutic-dose than subcutaneous prophylactic-dose UFH,11,12 although confounding because of differences in indication precludes determination of the contribution of these variables to disease risk.
Host-related variables, including age, sex, and patient population, are also important. In an analysis of national hospital discharge data involving more than 10 million admissions for venous thromboembolism, heparin-associated thrombocytopenia was vanishingly rare among patients younger than 40 years of age.13 In a large systematic review, female sex was associated with an odds ratio of 2.37.8 The same study demonstrated a 3-fold greater risk among surgical than medical patients. Type of surgery also appears to be important. Patients who undergo surgery on cardiopulmonary bypass (CPB) show a rate of anti-PF4/heparin antibody formation that approaches 50% by postoperative day 514 but only a 2% to 3% incidence of HIT.15 In contrast, HIT occurs in approximately 5% of patients who receive UFH thromboprophylaxis after major orthopedic surgery, although seroconversion is much less frequent.15,16 The basis for the disparate rates of seroconversion and HIT among these 2 populations is unknown but may in part relate to differences in extent of platelet activation and PF4 release triggered by the respective surgeries. The potential importance of platelet activation is further supported by a randomized controlled trial of heparin thromboprophylaxis in trauma patients, in which a greater incidence of seroconversion and HIT was observed in persons with major trauma than minor trauma.17 The incidence of HIT is less than 1% in critically ill patients18 and those undergoing chronic hemodialysis19 and less than 0.1% in pregnancy.20 Prevalent antecedent infections, such as periodontitis, predispose to anti-PF4/heparin antibody formation and may increase risk.21 Notwithstanding a growing understanding of risk factors, clinicians have little ability to forecast which persons will develop HIT. Epidemiologic and mechanistic studies aimed at identifying novel risk factors and biomarkers are needed to achieve this goal. In the absence of such data, risk factors do not figure prominently in our assessment of the probability of HIT in individual patients.
Clinical diagnosis: determining the clinical (pretest) probability of HIT
The cardinal clinical manifestation of HIT, a platelet count fall in the setting of a proximate heparin exposure, is common among inpatients and has poor diagnostic specificity. This is underscored by a multicenter registry of 2420 hospitalized patients who received heparin for 4 or more days. Fully 36.4% developed thrombocytopenia,22 although the incidence of HIT in this predominantly medical population was probably on the order of 1%.7 We therefore carefully consider other salient features, including timing and relative fall in platelet count, severity of thrombocytopenia, presence of thrombosis or hemorrhage, and plausibility of alternative explanations for thrombocytopenia (Table 2) when assessing pretest probability.
Timing of platelet count fall in relation to heparin exposure
In heparin-naive patients, HIT classically presents with a platelet count fall beginning 5 to 14 days after initial exposure while drug is still present.23 Rapid-onset HIT, in which the platelet count drops within hours of heparin administration, may occur in patients with preexisting anti-PF4/heparin antibodies. Such patients invariably have a history of recent heparin exposure, generally within the preceding 30 days.23 Rarely, manifestations of HIT may develop after heparin is discontinued. This phenomenon, referred to as delayed-onset HIT, occurs a median of 10 to 14 days after heparin withdrawal and is often associated with disseminated intravascular coagulation (DIC).24 A prothrombotic thrombocytopenic disorder reminiscent of HIT occurring in the absence of known heparin exposure has been described in a few patients.25 The pathogenesis and clinical implications of this observation are uncertain.
Relative fall in platelet count
Percentage fall is measured from peak platelet count after heparin exposure to its nadir. A 50% or greater reduction occurs in the large majority of patients, although approximately 10% evince a more modest decline of 30% to 50%.26
Severity of thrombocytopenia
The nadir platelet count need not meet the traditional laboratory criterion for thrombocytopenia (< 150 × 109/L). For instance, surgical patients with postoperative thrombocytosis may subsequently experience a marked (> 50%) platelet count decline because of HIT that does not fall below this threshold.27 Indeed, in contradistinction to other drug-induced thrombocytopenias, the median nadir platelet count is approximately 60 × 109/L and seldom falls below 20 × 109/L in the absence of concomitant DIC.26
Thrombosis and hemorrhage
The most dreaded and frequent clinical complication of HIT is thrombosis, which may be limb- or life-threatening. New thromboembolism is the presenting feature in approximately half of cases28 and may precede the onset of thrombocytopenia.29 In historical series, as many as 40% of patients without clinically apparent thrombosis at presentation (ie, “isolated HIT”) developed thromboembolism in the 10 days after heparin cessation if an alternative parenteral anticoagulant was not administered.30 Lower extremity deep vein thrombosis and pulmonary embolism are the predominant thrombotic manifestations, outnumbering arterial events by approximately 2:1.29 Deep vein thrombosis may be complicated by phlegmasia and compartment syndrome, leading to ischemic myonecrosis. Arterial thromboembolism, most frequently involving the extremities, is also common, particularly after cardiovascular surgery.29 Cyanosis and ischemic gangrene of digits may occur in the setting of preserved proximal pulses, presumably because of diffuse microvascular disease. Thrombosis of other vascular beds, including cerebral sinuses, visceral vessels, and bilateral adrenal veins with resultant hemorrhage and adrenal failure,31 as well as occlusion of vascular grafts, fistulas, and extracorporeal circuits is well documented. HIT-associated thrombosis shows a propensity to occur in areas of vessel injury (eg, at sites of central venous catheter or arterial line insertion or other vascular interventions).29,32
In contrast to most other drug-induced thrombocytopenias, significant spontaneous bleeding is rare, even when thrombocytopenia is severe. In prospective studies, bleeding complications were not increased in HIT patients over nonthrombocytopenic controls.16 We consider petechiae or hemorrhage as evidence against HIT and carefully contemplate alternative diagnoses before initiating an alternative anticoagulant.
Unusual clinical manifestations
Rare clinical sequelae, which curiously may occur in the absence of thrombocytopenia, include anaphylactoid reactions after intravenous heparin bolus, transient global amnesia, and skin necrosis at injection sites.33 Erythematous non-necrotizing injection site lesions are generally the result of delayed type IV hypersensitivity rather than HIT and do not require HIT-specific therapy.34
Alternative causes of thrombocytopenia
As important as remaining vigilant for characteristic clinical features is deliberate assessment of the likelihood of other etiologies of thrombocytopenia. Other thrombotic thrombocytopenias, such as antiphospholipid syndrome and malignancy-associated microangiopathy, may masquerade as HIT. Common causes of hospital-acquired thrombocytopenia include infection, medications other than heparin, DIC, hemodilution, and intravascular devices, such as balloon pumps, ventricular assist devices, and extracorporeal circuits. Such causes are especially common in patients with critical illness and those recovering from surgery on CPB, contributing to particularly frequent overdiagnosis in these settings.
Incident thrombocytopenia occurs in as many as half of all patients admitted to intensive care units, a substantial fraction of which arises during heparin administration.35 Nevertheless, in a prospective study, HIT was identified in only 0.4% of patients in this setting.18 Therefore, in critically ill patients suspected of having HIT, we are disinclined to reflexively recommend suspension of heparin or initiation of an alternative anticoagulant before return of laboratory test results unless characteristic clinical features (Table 2) are present and alternative causes of thrombocytopenia are absent or unlikely.
In patients undergoing surgery on CPB, platelet counts decline by a mean of 40% over the ensuing 72 hours.36 The nearly universal development of thrombocytopenia, coupled with the low specificity of laboratory assays after CPB (see “Laboratory diagnosis”) and prevalence of cyanotic digits because of hypotension, vasopressors, and underlying peripheral arterial disease, cultivates a climate of frequent overdiagnosis, although the overall incidence of HIT is only 2% to 3% in this setting.15 When we assess patients after CPB, we pay particular attention to the pattern of fall in platelet count. Recovery of platelets after surgery followed by a secondary fall between postoperative days 5 to 14 is suspicious for HIT, whereas thrombocytopenia that persists beyond 4 days without recovery is almost always the result of another etiology unless a further fall in the platelet count is observed.37
Synthesizing clinical data: clinical scoring models versus standard diagnosis
Several clinical scoring systems have been developed to assist clinicians in synthesizing the complex clinical data necessary to estimate pretest probability.38-40 The most extensively studied of these, the 4Ts,39 has demonstrated high sensitivity in a variety of clinical settings but is limited by modest interobserver agreement and positive predictive value.18,39,40 Recent studies have called into question the utility of the 4Ts in patients with critical illness.41,42 The HIT Expert Probability Score, a model based on the opinions of 26 clinical HIT experts, exhibited improved reliability and operating characteristics in a single institution series, but has not been prospectively evaluated.40 Neither scoring system has been compared directly with other approaches to clinical diagnosis nor subjected to a formal impact analysis on clinical behavior and outcomes. In our practice, we use a gestalt approach to diagnosis based on the criteria outlined in Table 2 and caution against the use of scoring models until these methodologic requirements for validation have been met.43 Nevertheless, model-guided management holds the promise of substantially curbing unnecessary treatment, as suggested by a recent study in which use of the HIT Expert Probability Score was associated with a potential 41% reduction in the number of patients receiving a direct thrombin inhibitor (DTI),40 and we eagerly await their continued refinement and validation.
Laboratory diagnosis
Because of the challenges of clinical diagnosis, physicians rely heavily on laboratory testing. Standard tests fall into 2 categories: functional assays, which detect patient antibodies that induce heparin-dependent platelet activation; and immunologic assays, which identify circulating anti-PF4/heparin antibodies, irrespective of their capacity to activate platelets (Table 3).
Immunologic assays
The most widely used immunologic assay is the polyspecific solid-phase ELISA, in which PF4/heparin or PF4/polyanion complexes are coated onto microtiter plate wells and dilute patient serum is added. If serum contains anti-PF4/heparin IgG, IgA, and/or IgM, antibodies will bind the complex and can be detected by addition of secondary anti-isotype antibodies. The sensitivity of the assay approaches 100%,44 although rare cases of anti-PF4/heparin-negative HIT in which the target antigen is a complex of heparin and a cationic cytokine other than PF4 have been reported.45
Notwithstanding its excellent negative predictive value, 2 key limitations of the ELISA, its turnaround time and poor positive predictive value, contribute to misdiagnosis and unnecessary treatment. Although the analytic turnaround time of the polyspecific ELISA is approximately 2 hours, it is most cost-efficient when multiple patient samples are accommodated in a single run. Many laboratories therefore batch samples and perform the assay no more than once or twice a week, frequently leaving clinicians to make critical initial management decisions without benefit of laboratory results. We have pushed for daily testing in our institution to provide results in a relevant time frame to inform such decisions. We think this change will improve care and reduce costs by curbing the number of ELISA-negative patients who receive empiric treatment with an alternative anticoagulant before return of laboratory results. Several rapid fluid phase immunologic assays, which are designed to accommodate single patient samples and yield results in minutes, have been developed and offer another means of improving turnaround time. The best studied of these tests, the particle gel immunoassay, may be helpful in emergency settings but appears to have slightly lower sensitivity than the ELISA, a critical limitation in light of the potential risks of delayed or missed diagnosis.46 Other rapid test systems, including a lateral flow immunoassay46 and latex particle-enhanced immunoturbidimetric assay,47 have shown promise in preliminary studies.
The major shortcoming of the ELISA and, indeed, of all marketed immunoassays, is limited specificity.44 False-positive results are common and may result from detection of nonpathogenic anti-PF4/heparin antibodies48 or antiphospholipid antibodies against either PF449 or PF4-bound β2 glycoprotein I.50 Immunologic assays perform particularly poorly in post-CPB patients, approximately half of whom test positive within a week after surgery.14 The specificity of the polyspecific ELISA in this setting is reported to be as low as 26%, contributing to frequent misdiagnosis and unnecessary and potentially deleterious therapy.51
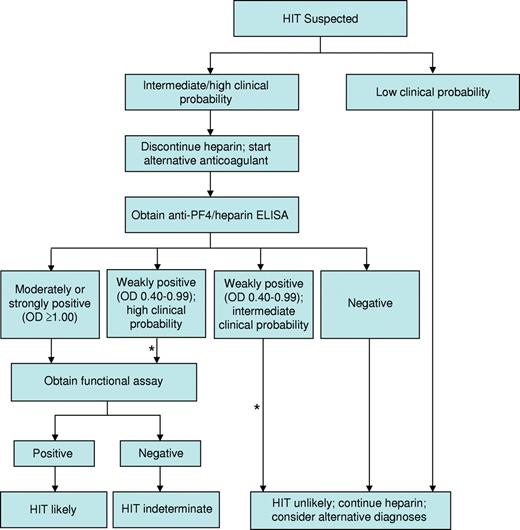
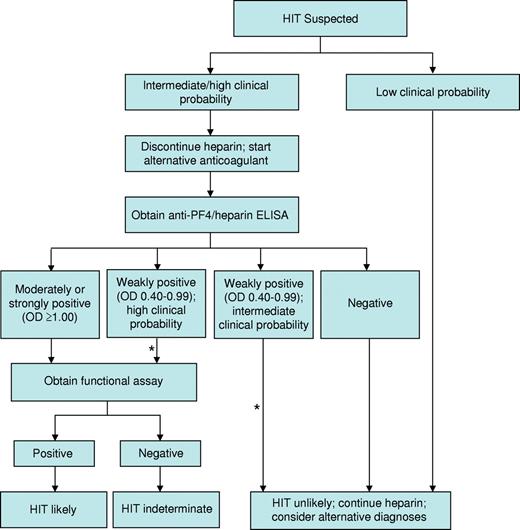
Several strategies have been implemented to improve the specificity of the ELISA for the diagnosis of HIT. First, the magnitude of a positive optical density (OD) result is directly associated with the odds of a positive functional assay,52 4Ts score,53 and risk of thrombosis.54 In one study, only 1 of 37 patient samples exhibiting a weakly positive OD (0.40-0.99) demonstrated heparin-dependent platelet activation in contrast to 33 of 37 samples with a strongly positive ELISA (OD ≥ 2.00).52 We use OD in conjunction with an assessment of clinical probability to guide management (Figure 1). A second strategy, based on evidence that IgG antibodies carry the greatest potential to cause HIT, is use of IgG-specific ELISAs. Such assays exhibit greater specificity than their polyspecific counterparts,55 but at the possible expense of slightly reduced sensitivity.56 A third strategy involves a confirmatory step, in which inhibition of a positive ELISA result by more than or equal to 50% by high-dose heparin (100 units) is considered to affirm the presence of heparin-dependent antibodies. Although this method enhances specificity,57 false-positive results remain common and false-negative results may also occur, particularly at high OD values.58 We continue to use the polyspecific ELISA to exclude HIT pending new information regarding the frequency and impact of false-negative results with alternative approaches. A new generation of immunoassays with high negative and high positive predictive values is needed to reduce overdiagnosis while avoiding the unacceptable scenario in which a patient with HIT continues to receive heparin based on false-negative results. Development of such assays requires a deeper understanding of biologic properties that distinguish pathogenic antibodies from their nonpathogenic counterparts.
Our approach to the diagnosis and initial management of patients with suspected HIT. Our approach is based primarily on clinical assessment, however imprecise, and complemented by the results of HIT laboratory tests, which are also limited by technical imprecision and uncertain specificity. *Estimates of the clinical probability of HIT in an individual patient may change over time. Therefore, it is our practice to frequently reevaluate patients in whom diagnostic uncertainty exists and to adjust treatment accordingly.
Our approach to the diagnosis and initial management of patients with suspected HIT. Our approach is based primarily on clinical assessment, however imprecise, and complemented by the results of HIT laboratory tests, which are also limited by technical imprecision and uncertain specificity. *Estimates of the clinical probability of HIT in an individual patient may change over time. Therefore, it is our practice to frequently reevaluate patients in whom diagnostic uncertainty exists and to adjust treatment accordingly.
Functional assays
The most extensively studied functional tests for the diagnosis of HIT are the 14C-serotonin release assay (SRA) and heparin-induced platelet activation assay (HIPA). In the SRA, various concentrations of heparin and patient serum or plasma, depleted of residual heparin, are added to washed donor platelets radiolabeled with 14C-serotonin.59 A positive test is signified by heparin-dependent release of 14C-serotonin. The HIPA is similar but uses platelet aggregation as an endpoint.60 Both tests are significantly more specific than existing immunoassays and are useful for confirming a positive ELISA. Unfortunately, technical requirements, including need for reactive donor platelets, radioisotope for the SRA, and impeccable technique for the HIPA, restrict their use to a small number of reference laboratories. As such, they are available to most clinicians only as send-outs and do not provide results in real time necessary to guide initial management. Even among centers that perform functional assays, methods vary, raising concerns about interlaboratory reproducibility.61 Novel approaches that facilitate standardization and do not pose the technical barriers associated with the HIPA and SRA are needed to increase accessibility of specific functional assays.
Diagnostic and initial treatment algorithm
Synthesizing the complex clinical and laboratory information necessary to establish a diagnosis is often challenging. Even experts exhibit substantial variation in diagnostic approach.40 The algorithm we use for incorporating clinical and laboratory data into an initial management strategy is shown in Figure 1.
Parenteral anticoagulation
Treatment of HIT entails immediate withdrawal of all heparin, including heparin-containing flushes and catheters. Heparin cessation alone, however, is often insufficient to prevent thrombosis. Historical series of untreated patients document a striking 5% to 10% daily risk of thrombosis in the 2 days after discontinuing heparin and a 30-day cumulative risk of new thromboembolism as high as 50%.30,62 Prompt initiation of a nonheparin parenteral anticoagulant is therefore standard of care.63 Several parenteral anticoagulants are available for the treatment of HIT (Table 4) and are discussed in “Argatroban.”63,64
Argatroban
Argatroban is a hepatically cleared DTI labeled for the management of HIT. The pivotal studies that led to its approval (ARG-911 and ARG-915) were not randomized controlled trials but single-arm open-label studies in which argatroban-treated subjects were compared with untreated historical controls.65,66 A limitation of these studies is that serologic confirmation was not required for inclusion and 36% of enrollees were found to be antibody-negative on posthoc testing.67 Moreover, a subset of argatroban-treated subjects had a remote history of HIT but did not have acute disease at study entry. With these limitations in mind, which could bias results in favor of argatroban, the hazard ratio of new thrombosis in a pooled analysis of ARG-911 and ARG-915 was approximately 0.3 in argatroban-treated subjects. There was no significant difference in bleeding between subjects and controls.68 Argatroban is administered as a continuous intravenous infusion and monitored with the activated partial thromboplastin time (aPTT). Reduction in initial dose in the setting of liver dysfunction, heart failure, anasarca, and recent cardiac surgery is recommended by the American College of Chest Physicians63 and may be appropriate in other patients with critical illness (Table 4).69
Lepirudin
Lepirudin is a renally cleared DTI licensed for the treatment of HIT. Like argatroban, its approval was based on single-arm studies with untreated historical controls as comparator, but serologic confirmation was required before enrollment.28,70,71 In a pooled analysis of these studies, the incidence of new thromboembolic events was significantly lower in the lepirudin-treated cohort (11.9% vs 32.1%). This benefit was offset by a 29.4% rate of major bleeding.71 A posthoc analysis showed that a dose of lepirudin exceeding 0.07 mg/kg per hour was an independent risk factor for hemorrhage without offering superior antithrombotic efficacy.72 We therefore advocate a lower initial infusion rate than indicated in the FDA label with bolus dosing reserved for life- or limb-threatening thrombosis (Table 4).63 Antilepirudin antibodies, which form in up to 56% of recipients,73 may potentiate its anticoagulant activity. In one study, approximately half of antibody-positive patients required a reduction in lepirudin dose to maintain the aPTT in the therapeutic range.74 Dose reduction is also necessary in renal insufficiency (Table 4). Anaphylaxis with lepirudin re-exposure occurs in 0.16% of patients, primarily after bolus dosing.75 We prefer to use an anticoagulant other than lepirudin in those with prior exposure. When re-exposure is necessary, we avoid bolus dosing and monitor closely for infusion reactions.
The manufacturer has announced that production and distribution of lepirudin will be discontinued in April 2012. Nevertheless, experience with this agent may be helpful in guiding use of similar drugs, including hirudin RB, a recombinant hirudin variant available in parts of Africa and Asia.
Bivalirudin
In contrast to other DTIs, bivalirudin undergoes partial enzymatic inactivation and has a shorter half-life. This property renders it potentially attractive in clinical settings in which rapid dose titration is desired, such as percutaneous intravascular procedures, cardiac surgery, and critical illness with multiorgan failure. Bivalirudin has been studied extensively in percutaneous coronary intervention76 where it is licensed for patients with and without HIT. It has been used successfully in patients undergoing cardiac surgery with and without cardiopulmonary bypass.77,78 Published evaluation of bivalirudin in patients with critical illness and organ failure is limited to case series,79 and additional investigation in these contexts is warranted. In the operating room and catheterization laboratory, dosing is monitored with the activated clotting time. Elsewhere, bivalirudin is monitored with the aPTT. Dose reductions may be appropriate in patients with renal and/or hepatic dysfunction (Table 4).79
Desirudin
Desirudin is a subcutaneously administered, renally cleared DTI licensed for thromboprophylaxis after hip arthroplasty. In the open-label PREVENT-HIT trial, the only prospective head-to-head comparison of DTIs, patients were randomized to receive argatroban or fixed-dose desirudin. Although the study closed because of poor accrual after only 16 subjects had been randomized, results were promising with no new thrombotic events or major bleeding among the 8 desirudin-treated subjects. Moreover, cost per treatment course was substantially lower in the desirudin arm ($1688 vs $8250).80 More data are needed to define the role of this promising agent.
Danaparoid
Danaparoid is an antithrombin-dependent inhibitor of activated factor X (FXa) composed of low molecular weight glycosaminoglycans. It is approved for treatment of HIT in multiple jurisdictions, although it is no longer marketed in the United States and drug shortages have limited its availability elsewhere. Efficacy was demonstrated in an open-label randomized trial of intravenous danaparoid versus dextran-70 in 42 patients with HIT complicated by thrombosis. Significantly more danaparoid-treated subjects were judged to have complete clinical recovery from thrombosis at discharge (56% vs 14%, P = .02).81 A subsequent analysis showed that low-dose subcutaneous danaparoid is less effective for preventing thromboembolism.82 Danaparoid should be given intravenously by bolus followed by accelerated initial infusion and then maintenance infusion (Table 4).63 In vitro cross-reactivity of HIT antibodies with danaparoid occurs in a minority of patients, although the clinical significance of this phenomenon is unproven and routine testing is not required.63,83
Fondaparinux
Fondaparinux, an indirect inhibitor of FXa, is administered subcutaneously and licensed for prevention and treatment of venous thromboembolism. Although it has not been systematically investigated in HIT, several case series have been published.84-86 In a pooled analysis involving a total of 71 patients, no new thrombotic events were reported. Four patients had major hemorrhage, 3 of whom had a creatinine clearance less than 30 mL/minute. In a serologic study of nearly 4000 patients presenting with acute venous thromboembolism, 14 were found to have heparin-dependent platelet activating antibodies at baseline, presumably because of recent heparin exposure, without overt clinical manifestations of HIT. Ten of these subjects were treated with fondaparinux and did not develop HIT. The remaining 4 received LMWH or UFH, and all developed HIT.87
Although the incidence of anti-PF4/heparin antibody formation after fondaparinux and LMWH is similar,88 fondaparinux-induced HIT is very rare. Several cases of HIT induced or exacerbated by fondaparinux have been described, although the attribution to fondaparinux in these cases remains uncertain.84 Overall, a favorable risk/benefit profile, ease of once daily subcutaneous administration, and potential lack of need for monitoring have contributed to our growing use of this agent in stable patients.
Limitations of currently available agents
Although currently available parenteral anticoagulants have improved outcomes, important drawbacks remain. First, management is complex, cumbersome, and prone to error. Approved agents require continuous intravenous infusion, serial monitoring, and frequent dose adjustments. Indeed, these drugs are sufficiently complex that many institutions have reassigned primary responsibility for their management to dedicated pharmacist-directed anticoagulation services.89 Desirudin and fondaparinux offer hope of reducing therapeutic complexity. Neither appears to require routine laboratory monitoring or dose adjustment. Both are administered subcutaneously and have negligible effect on the International Normalized Ratio (INR), thereby facilitating transition to outpatient therapy. In theory, new oral inhibitors of thrombin and FXa constitute rational therapies for HIT and also offer the promise of simplifying management. Dabigatran, apixaban, and rivaroxaban do not induce platelet aggregation or PF4 release in the presence of HIT-positive sera in vitro90 and warrant further investigation, although no published clinical data are available to support their use.
Second, DTIs are associated with incomplete antithrombotic efficacy and narrow therapeutic indices. Argatroban and lepirudin decrease thrombosis by approximately 50% to 65% but do not substantially reduce dismemberment or mortality.68,71 Moreover, these drugs are associated with a 10% to 20% incidence of major hemorrhage,68,71,91 a risk further compounded by the absence of effective reversal agents. Monitoring DTIs by aPTT may be confounded by HIT-associated consumptive coagulopathy, in which protocol-driven reduction in DTI therapy resulting from a “supratherapeutic” aPTT may result in subtherapeutic dosing and thrombus progression. Novel drugs that target pathways proximal to activation of coagulation may provide effective therapy without the degree of bleeding risk associated with currently available anticoagulants.64 Examples include a desulfated form of heparin with minimal anticoagulant activity92 ; PF4 antagonists, which interfere with formation of PF4/heparin complexes93 ; inhibitors of FcγRIIA-mediated platelet activation by HIT immune complexes; and inhibitors of splenic tyrosine kinase (Syk) and Ca2+ and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI), which disrupt intracellular transduction triggered by immune complex binding.94-96
Finally, there is growing appreciation of the financial burden of HIT, a major driver of which is cost of approved agents and the increased length of hospital stay their use entails.97 Future studies of therapeutics should include economic analyses that consider not only direct drug costs, but also costs associated with monitoring, length of stay, and toxicity.
Transitioning to oral anticoagulation
Patients with acute HIT are at risk for venous limb gangrene, a severe thrombotic complication of the microvasculature. As with warfarin-induced skin necrosis, this phenomenon is thought to result from the differential half-lives of vitamin K–dependent proteins and the relatively rapid onset of protein C deficiency after therapy with a vitamin K antagonist (VKA) is initiated.98 In addition to administering an alternative parenteral anticoagulant, we take the following precautions to guard against this complication63 :
For patients receiving a VKA at the time HIT is diagnosed, we stop the VKA and reverse its effects with vitamin K.
We do not start a VKA in patients with acute HIT until the platelet count has recovered to a stable plateau.
We avoid large loading doses (eg, warfarin > 5 mg), and we overlap the VKA with a parenteral anticoagulant for 5 days or more and until the INR has reached its intended target.
Another potential challenge unique to transitioning patients to a VKA from argatroban is posed by its INR-raising effect. If this effect is neglected, patients may be inadequately anticoagulated after argatroban is discontinued. We use a validated algorithm to guide this transition so that under-anticoagulation during a period of great thrombotic risk is avoided (Table 5).99
Duration of anticoagulation
We request bilateral lower extremity compression ultrasonography in all patients with acute HIT, even in the absence of signs and symptoms, because silent deep vein thrombosis is common100 and its presence may influence the recommended duration of anticoagulation. As with other provoked thromboses ascribed to a transient risk factor, we generally maintain patients with HIT-associated thromboembolism on dose-adjusted warfarin for 3 to 6 months. The optimal duration of anticoagulation in persons with HIT without thrombosis is unknown. In a historical series of 62 untreated patients with isolated HIT, the cumulative incidence of thromboembolism at 30 days was 53%.30 Most events occurred within 10 days of heparin discontinuation, a period corresponding to platelet recovery. We therefore continue anticoagulation in this population until the platelet count has recovered to a stable plateau at a minimum, and we consider extending therapy to a month after heparin cessation if additional thrombotic risk factors are present.
Platelet transfusion
Because HIT generally presents as a thrombotic rather than hemorrhagic diathesis, platelet transfusion is rarely necessary. Furthermore, there is a long-held, but largely theoretical, concern that transfusion of platelets may precipitate thrombosis. This dogma has been challenged by 2 recent case series of 41 platelet-transfused patients with suspected HIT, none of whom developed thrombosis during extended follow-up.101,102 It remains our practice to avoid prophylactic platelet transfusion in patients with confirmed or strongly suspected HIT, although we consider transfusion in the setting of clinically significant bleeding, high bleeding risk, or diagnostic uncertainty.
Heparin re-exposure for cardiovascular procedures
Despite the growing number of available anticoagulants, heparin remains the agent of choice for indications such as intraoperative anticoagulation during cardiac and vascular surgery, because of its favorable pharmacokinetics, ease of monitoring, reversibility with protamine, and familiarity to clinicians. The HIT immune response wanes over time. Functional assays become negative at a median of 50 days after heparin cessation, whereas anti-PF4/heparin antibody titers decline more slowly and are no longer detectable in 60% of patients by day 100.23 We use laboratory testing to identify a patient's position along this sequence and to assess the safety of intraoperative heparin re-exposure (Table 6).
The safety of intraoperative heparin in patients with remote HIT (negative immunologic and functional assays) was first demonstrated in a series of 10 patients undergoing cardiac surgery, none of whom developed clinical recurrence or recrudescence of anti-PF4/heparin antibodies.103 The safety of heparin administration during surgery in persons with subacute HIT (a negative functional assay and a positive ELISA) is less certain. In 3 such patients requiring urgent heart transplantation, exposure to heparin during surgery was uneventful,104 and this has been our experience and practice. Even a brief course of heparin should be avoided in patients with a positive functional assay (ie, acute HIT). If surgery cannot be delayed, an alternative anticoagulant, such as bivalirudin, should be used.63,77,78 In light of its documented efficacy and safety in large coronary angiography trials,76 we recommend bivalirudin over heparin for percutaneous vascular procedures in all patients with a history of HIT, be it acute, subacute, or remote. Whenever a patient with a history of HIT is re-exposed to heparin for an invasive procedure or surgery, use should be strictly limited to the intraoperative setting and meticulously avoided before and after surgery.
Hemodialysis
HIT affects less than 1% of persons undergoing chronic hemodialysis,19 although the incidence may be higher in patients receiving hemodialysis for acute indications. Among chronic hemodialysis patients without thrombocytopenia, approximately 10% test positive by polyspecific ELISA. Prospective studies have not demonstrated an association between seropositivity and increased risk of thromboembolism or vascular access occlusion.105,106 We do not advocate HIT laboratory testing in patients on hemodialysis unless there is an intermediate or high suspicion based on clinical criteria (Table 2). Ongoing heparin exposure during hemodialysis is contraindicated in patients with a history of HIT. Alternative strategies, including saline flushing, citrate, danaparoid, lepirudin, argatroban, and long-term VKA use, have been reported but not compared or systematically investigated.63
Conclusion
The management of HIT over its history can be separated into 3 distinct eras. In the first, dating from its initial description in 1958 until the 1990s, the mere existence of the entity was questioned and therapy was ad hoc, consisting primarily of supportive care and antithrombotic agents with uncertain efficacy, such as dextran and aspirin. A time of hope then emerged with the advent of new diagnostic tests and more effective treatments, which pushed clinicians to raise the specter of HIT earlier and to institute therapy promptly. We have now entered a third era, one in which the limited specificity of widely available diagnostic tools and physicians' fears of missing a case of true disease have conspired to foster a climate of frequent overdiagnosis and unnecessary exposure of thrombocytopenic patients without HIT to costly alternative anticoagulants and their potential harms. It is our hope and conviction that development of validated clinical scoring models, improved laboratory assays, and targeted therapies will usher in a new era of more exacting diagnosis and safer treatment.
Authorship
Contribution: A.C. and D.B.C. searched the literature and wrote the manuscript.
Conflict-of-interest disclosure: A.C. has provided consulting services to Bayer, Biogen-Idec, Canyon, CSL Behring, and Genzyme; has received research funding from Baxter, Bayer, and Novo Nordisk; and has provided expert witness testimony relating to heparin-induced thrombocytopenia. The remaining author declares no competing financial interests.
Correspondence: Adam Cuker, Hospital of the University of Pennsylvania, Penn Comprehensive Hemophilia and Thrombosis Program, 3 Dulles, 3400 Spruce St, Philadelphia, PA 19104; e-mail: adam.cuker@uphs.upenn.edu.