We recently demonstrated that megakaryocytes differentially express mRNA for MMPs and TIMPs and selectively transfer a subset of these transcripts to platelets.1 As a minor part of this report, we showed that megakaryocytes expressed MMP-9 mRNA while platelets contained only trace amounts of it and undetectable MMP-9 protein.1 In their letter to the editor, Mannello and Medda suggest that our results are inconsistent with previous reports showing MMP-9 zymographic activity in platelet lysates.2 Although the authors did not show that leukocyte-depleted platelets express MMP-9 protein, they argue that MMP-9 activity is detectable in platelets when EDTA and Na3VO4, that may inhibit MMP-9 activity, are removed from the lysis buffer.
The effects of EDTA on the activity of MMPs detected by zymography in biologic samples are controversial, with data showing partial inhibition,2 no influence,3 or enhancement of active MMP-9.4 In our current study1 and a previous report,5 we were unable to detect MMP-9 protein in platelets by ELISA, immunocytochemistry (ICC), and Western blotting (WB). EDTA and Na3VO4 are not used when detecting MMP-9 protein by ICC or WB analysis. Moreover, the presence of EDTA and Na3VO4 does not mask the detection of MMP-9 in ELISA-based assays.
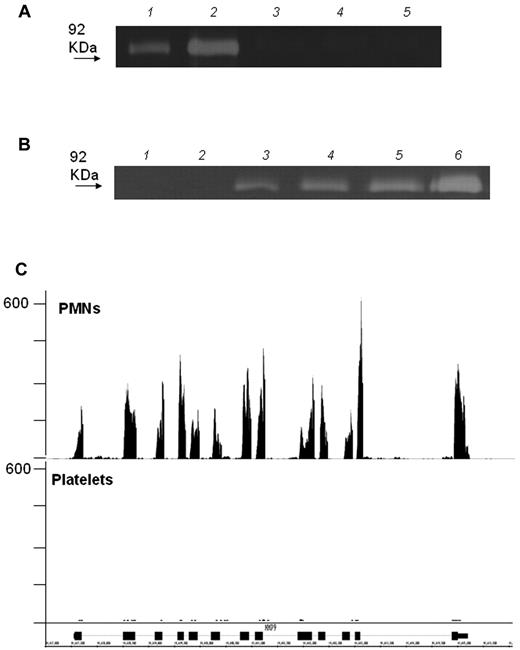
As duly noted in our report,1 it is possible that MMP-9 protein, if it exists in platelets, escaped the detection limits of our assays. However, we were unable to detect enzymatic activity in platelets using both zymography and a sensitive activity assay,1,5 whereas MMP-9 activity was readily detected in collagen-adherent monocytes that were costimulated with platelets.5 To address the influence of possible methodologic artefacts, we have now performed additional experiments in leukocyte-depleted human platelets using 3 different procedures: (1) complete lysis buffer, as detailed in our paper1 ; (2) the same buffer but without EDTA and Na3VO4; and (3) 3 cycles of freezing and thawing. We were unable to detect zymographic MMP-9 activity in any of the preparations (Figure 1A), while the addition of minimal amounts of leukocytes to purified platelets elicited MMP-9 activity (Figure 1B). This finding is consistent with next-generation RNA-sequencing data showing that human neutrophils express far higher amounts of MMP-9 transcripts than platelets (Figure 1C). Therefore, contamination by white blood cells may explain the finding of MMP-9 in platelets reported in previous studies.
Detection of activity and mRNA of MMP-9 in platelets. (A) Zymography of highly purified platelet lysates prepared according to 3 different methods (lane 3 = complete lysis buffer; lane 4 = the same lysis buffer but without EDTA and Na3VO4; lane 5 = 3 cycles of freezing in liquid nitrogen and thawing at 37°C). Two concentrations of MMP-9, used as standards, are shown in lanes 1 and 2. No MMP-9 is detectable in platelet lysates prepared by any of the 3 methods. (B) Zymography of highly purified platelets, alone (lane 1) or with added white blood cells at platelet leukocyte ratios of 789:1 (lane 4), 24 000:1 (lane 3), and 47 000:1 (lane 2). PMNs lysate and a concentration of MMP-9 used as standards, are shown in lanes 5 and 6, respectively. (C) RNA-seq analysis for MMP-9 mRNA in platelet and PMN lysates. Matched sequences are aligned to the MMP-9 gene using the Integrated Genome Browser (IGB). Gene map (bottom portion of the panel, oriented 5′-3′ direction) is represented by thick (exon) and thin (intron) lines. The transcript levels were quantified using RPKM (reads per kilobase of exon model per million mapped reads). The RPKM for adult PMNs without any treatment is 82.6916 compared with 0.4619 for platelets.
Detection of activity and mRNA of MMP-9 in platelets. (A) Zymography of highly purified platelet lysates prepared according to 3 different methods (lane 3 = complete lysis buffer; lane 4 = the same lysis buffer but without EDTA and Na3VO4; lane 5 = 3 cycles of freezing in liquid nitrogen and thawing at 37°C). Two concentrations of MMP-9, used as standards, are shown in lanes 1 and 2. No MMP-9 is detectable in platelet lysates prepared by any of the 3 methods. (B) Zymography of highly purified platelets, alone (lane 1) or with added white blood cells at platelet leukocyte ratios of 789:1 (lane 4), 24 000:1 (lane 3), and 47 000:1 (lane 2). PMNs lysate and a concentration of MMP-9 used as standards, are shown in lanes 5 and 6, respectively. (C) RNA-seq analysis for MMP-9 mRNA in platelet and PMN lysates. Matched sequences are aligned to the MMP-9 gene using the Integrated Genome Browser (IGB). Gene map (bottom portion of the panel, oriented 5′-3′ direction) is represented by thick (exon) and thin (intron) lines. The transcript levels were quantified using RPKM (reads per kilobase of exon model per million mapped reads). The RPKM for adult PMNs without any treatment is 82.6916 compared with 0.4619 for platelets.
We subscribe to the position that strict attention must be paid to methodologic issues when studying the pathophysiology of MMPs, which is why we screen MMPs by multiple assays that include in situ detection of the protein within individual platelets and broader assessment of mRNA, protein, and activity using platelet preparations devoid of leukocytes.1,5 Using these criteria, we were unable to detect appreciable amounts of MMP-9 in platelets. Similarly, Kälvegren et al recently demonstrated that collagen-stimulated platelets release MMP-1 and MMP-2, but not MMP-9.6 Moreover, they were unable to detect MMP-9 protein inside resting platelets by immunofluorescence microscopy.6 Thus, it is not obvious to us that platelets from healthy subjects possess appreciable amounts of MMP-9. Nevertheless, we encourage further scientific dialogue and evaluation of this issue as our group and others determine how platelet-derived MMPs participate in diseases.1,5-10
Authorship
Acknowledgments: This study was supported, in part, by grants from Fondazione Cassa di Risparmio di Perugia (Protocols 2011.0137.021, 2010.020.161, and 2009.010.0478) to P.G. E.F. is supported by a grant from Regione dell'Umbria (POR Umbria FSE 2007-2013 ASSE II).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Gresele, MD, PhD, Division of Internal and Cardiovascular Medicine, Department of Internal Medicine, University of Perugia, Via E dal Pozzo, 06126 Perugia, Italy; e-mail: grespa@unipg.it.