To the editor:
In the May 31, 2011 online issue of Blood, Cecchetti et al presented a transcriptome analysis of matrix metalloproteinase (MMP) expression in megakaryocytes and platelets.1 Their results demonstrate that megakaryocytes and platelets differentially express mRNAs and proteins for MMPs. The authors found that platelets constitutively expressed MMP-2 protein and released it on thrombin activation, although platelets lacked mRNA for MMP-2. On the other hand, they did not detect pro–MMP-9 protein in both inactivated and thrombin-stimulated platelet lysates, even though MMP-9 mRNA was present; moreover, the authors also detected MMP activity in megakaryocyte releasates but not in their lysates.
The data are incongruent with previous quantitative studies and the peculiar subcellular localization in platelets of both zymogen and activated forms of MMP-9 (also named Gelatinase B).2-5 Cecchetti et al suggested several hypothesis for this unclear discrepancy/variance: (1) MMP-9 protein is secreted during pro-platelet formation in lieu of being retained in mature platelets, (2) the lack of MMP-9 proenzyme is due to more efficient platelet preparations (clearing CD45+ leukocytes and thus limiting the MMP-9 contamination).
Although no MMP zymogram was displayed by the authors, we believe that there could be a simpler explanation for the incongruence with the literature data, evidencing a neglected methodologic aspect. Cecchetti et al performed all cell lysates with a buffer containing 1mM of EDTA and Na3VO4, chemicals with well-known inhibitory effects on MMP activity. In fact, the Ca/Zn-dependent gelatinases are sensitive to and partially inhibited by the EDTA chelation activity,6,7 whereas orthovanadate (classic phosphotyrosine phosphatase inhibitor) has also been characterized as an MMP inhibitor (eg, for gelatinase B8 and collagenase9 ).
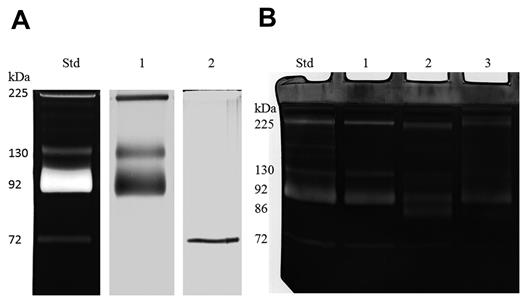
To reveal the possible partial inhibitory effect of EDTA and Na3VO4 on platelet gelatinases, in Figure 1 we show all gelatinolytic MMP forms present in whole cord blood10 and in purified mature platelets according to Cecchetti et al's procedure.1 Whole cord blood contained both MMP-2 proenzyme (at 72 kDa) and pro–MMP-9 forms (monomer at 92 kDa, and complexed forms at 130 and 225 kDa; lane standard); proforms are activable by 1mM APMA (lane 2). The EDTA/Na3VO4 treatment is able to partially inhibit MMP-9 in platelets lysates; in particular, both the proenzyme and APMA-activated MMP-9 forms showed a significant reduction of gelatinolytic activity (lane 3). Our observations may at least in part explain both the incongruent “absence” of MMP-9 activity in platelet lysates (similar to collagenase inhibition, like for MMP-3), as well as the presence of MMP-9 activity in only megakaryocyte releasates or untreated culture media (but not in their lysates with EDTA/Na3VO4).
Gelatin zymograms of cord blood and mature platelets from healthy individuals. Human platelets from peripheral blood and cord blood samples were obtained from the Blood Transfusion Center and the Gynecology Unit of the Hospital of Urbino. Umbilical cord blood samples were collected immediately after delivery from women with uncomplicated healthy pregnancies; peripheral blood samples were drawn from healthy volunteers. Washed platelets were freshly isolated according to CD45+ leukocytes depletion and lysis procedure detailed in Cecchetti et al1 (40mM Tris-HCl, 0.3M NaCl, 1mM EDTA, 1mM Na3VO4, 1mM NaF, NaN3 0.05%, NP-40 1%, pH 7.4). After centrifugation (20 000g, 4°C for 20 minutes) to remove cellular debris, the supernates of MMP standards from whole cord blood and the cleared platelet lysates were analyzed through Western blotting (using monoclonal antibody against MMP-2 and MMP-9) and gelatin zymography.5,10 Sample aliquots (containing 250 μg of total protein) were analyzed on 7.5% polyacrylamide gels containing 2 g/L gelatin 90 Bloom Type A from porcine skin. (A) Western blots of pro- and complexed forms of MMP-9, and proMMP-2 (lanes 1 and 2, respectively) recognized as gelatinases circulating in human cord blood from healthy subjects. Lane standard, cord blood lysates used as calibrator. (B) In lane 1, the MMP gelatinolytic activities in mature platelet lysates. All MMP forms activated by 1mM APMA are separated in lane 2, whereas the residual gelatinolytic activity of APMA-activated MMP in platelet lysates after the treatment with 1mM EDTA and orthovanadate is shown in lane 3. Lane standard, cord blood gelatinases.5,10 Molecular masses (kDa) are indicated.
Gelatin zymograms of cord blood and mature platelets from healthy individuals. Human platelets from peripheral blood and cord blood samples were obtained from the Blood Transfusion Center and the Gynecology Unit of the Hospital of Urbino. Umbilical cord blood samples were collected immediately after delivery from women with uncomplicated healthy pregnancies; peripheral blood samples were drawn from healthy volunteers. Washed platelets were freshly isolated according to CD45+ leukocytes depletion and lysis procedure detailed in Cecchetti et al1 (40mM Tris-HCl, 0.3M NaCl, 1mM EDTA, 1mM Na3VO4, 1mM NaF, NaN3 0.05%, NP-40 1%, pH 7.4). After centrifugation (20 000g, 4°C for 20 minutes) to remove cellular debris, the supernates of MMP standards from whole cord blood and the cleared platelet lysates were analyzed through Western blotting (using monoclonal antibody against MMP-2 and MMP-9) and gelatin zymography.5,10 Sample aliquots (containing 250 μg of total protein) were analyzed on 7.5% polyacrylamide gels containing 2 g/L gelatin 90 Bloom Type A from porcine skin. (A) Western blots of pro- and complexed forms of MMP-9, and proMMP-2 (lanes 1 and 2, respectively) recognized as gelatinases circulating in human cord blood from healthy subjects. Lane standard, cord blood lysates used as calibrator. (B) In lane 1, the MMP gelatinolytic activities in mature platelet lysates. All MMP forms activated by 1mM APMA are separated in lane 2, whereas the residual gelatinolytic activity of APMA-activated MMP in platelet lysates after the treatment with 1mM EDTA and orthovanadate is shown in lane 3. Lane standard, cord blood gelatinases.5,10 Molecular masses (kDa) are indicated.
To avoid misinterpretation and possible technical pitfalls (possibly because of the neglected peculiar MMP inhibition by EDTA/ Na3VO4), we believe that a more careful MMP analysis in megakaryocyte and platelet lysates should take into consideration that the MMP-2, -3, and -9 activities may be significantly inhibited by calcium chelators and phosphotyrosine phosphatase inhibitors. More cautious use of these MMP inhibitors will provide useful insights to understand how cytoplasm and nuclear MMPs (F.M. and V.M., unpublished observations, October 2011) may be transferred from megakaryocytes to platelets in physio-pathologic conditions.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ferdinando Mannello, Dept of Biomolecular Sciences, Section of Clinical Biochemistry, Unit of Cell Biology, University “Carlo Bo,“ Via O. Ubaldini 7, 61029 Urbino (PU), Italy; e-mail: ferdinando.mannello@uniurb.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal