Abstract
CD52 is expressed on malignant cells in lymphoplasmacytic lymphoma (LPL), including IgM-secreting Waldenström macroglobulinemia (WM). We examined the activity of alemtuzumab in 28 symptomatic LPL (27 IgM and 1 IgA) patients. The median prior number of therapies for these patients was 2 (range, 0-5) and 43% had refractory disease. Patients received alemtuzumab at 30 mg IV 3 times weekly for up to 12 weeks after test dosing, and also received hydrocortisone, acyclovir, and Bactrim or equivalent prophylaxis. Patients had a complete response (n = 1), a partial response (n = 9), or a MR (n = 11) for an overall and major response rate of 75% and 36%, respectively. Median serum Ig decreased from 3510 to 1460 mg/dL (P < .001 at best response). With a median follow-up of 64 months, the median time to progression was 14.5 months. Hematologic and infectious complications, including CMV reactivation, were more common in previously treated patients and were indirectly associated with 3 deaths. Long-term follow-up revealed late-onset autoimmune thrombocytopenia (AITP) in 4 patients at a median of 13.6 months after therapy, which contributed to 1 death. Alemtuzumab is an active therapy in patients with LPL, but short- and long-term toxicities need to be carefully weighed against other available treatment options. Late AITP is a newly recognized complication of alemtuzumab in this patient population. This study is registered at www.clinicaltrials.gov as NCT00142181.
Introduction
Lymphoplasmacytic lymphoma (LPL) is a B-cell disorder characterized by BM infiltration with lymphoplasmacytic cells and an Ig monoclonal gammopathy.1,2 Inclusive in the diagnosis of LPL is Waldenström macroglobulinemia (WM), which is characterized by the secretion of IgM and in which other morbidities are more common, including hyperviscosity syndrome, cryoglobulinemia, cold agglutinemia, and sensory neuropathy.3,4 Despite advances in therapy, LPL remains incurable and novel therapeutic agents are urgently needed.
Monoclonal antibody therapy, particularly rituximab, has been an important mainstay in the therapy of symptomatic patients with WM, with overall response rates (ORRs) of 25%-45% and median durations of response of 8-29 months.5-8 No complete responses were observed in these previous studies. Ofatumumab is a novel, CD20-directed antibody that was recently evaluated as monotherapy in symptomatic WM patients and was associated with an ORR of 43%.9 Both rituximab and ofatumumab can produce an IgM flare, prompting hyperviscosity symptoms and/or exacerbation of IgM-related morbidities in WM patients.3,4,9-11
In view of these considerations, we and others have sought to develop other targeted therapies for LPL/WM patients. Alemtuzumab is a fully humanized human IgG1 monoclonal antibody that targets CD52 and has established efficacy in the treatment of other lymphomas.12,13 CD52 is widely expressed on BM lymphoplasmacytic cells in WM.14-16 CD52 is selectively expressed on WM patient mast cells which are found in excess numbers in their BMs, and support the growth and survival of WM cells through CD40 ligand stimulation.17,18 Alemtuzumab induces antibody-dependent cell-mediated cytotoxicity against BM mast cells from WM patients.19 Given these findings, we performed a prospective, phase 2 study of alemtuzumab in symptomatic patients with LPL/WM and present herein the long-term outcome of these studies.
Methods
Patient eligibility
Symptomatic patients with a clinicopathologic diagnosis of LPL, including patients with IgG, IgA, and IgM paraproteins (ie, WM) who were naive to alemtuzumab, had CD52-positive disease as determined by previous BM immunohistochemistry or flow cytometry, and required therapy based on consensus guidelines, were eligible for this study.20 A baseline platelet count of ≥ 25 000/μL, an absolute neutrophil count of ≥ 500/μL, a serum creatinine of < 2.5 mg/dL (unless nephropathy was attributable to their WM), a serum total bilirubin and serum glutamic oxalacetic transaminase of < 2.5 times the upper limit of normal, and an Eastern Cooperative Oncology Group performance status of 0-2 were required for entry. No other monoclonal antibody therapy within 3 months of study entry was permitted. No chemotherapy, steroid therapy, or radiation therapy within 30 days of study entry was permitted. Patients who were pregnant or lactating; had serious comorbid disease; had any uncontrolled bacterial, fungal, or viral infection; or had an active second malignancy were not eligible. All men and women of reproductive potential were required to agree to use an acceptable method of birth control before, during, and for 6 months after completion of study treatment.
Treatment
Intended therapy consisted of 3 test doses of alemtuzumab initiated using a gradual dose escalation schedule over 1 week (3, 10, and 30 mg), followed by 36 additional treatment phase infusions of alemtuzumab at the 30 mg dose given IV 3 times per week over 12 weeks. Patients received before their alemtuzumab infusions 1 L of normal saline, diphenhydramine 50 mg IV, acetaminophen 650 mg by mouth, as well as hydrocortisone 100-200 mg IV and cimetidine 300 mg IV (if they reacted to a previous alemtuzumab infusion) to prevent infusion-related reactions. Famciclovir 250 mg twice a day or the equivalent and sulfamethoxazole and trimethoprim (double strength) twice a day on 3 days per week were given for herpes zoster and Pneumocystis carinii pneumonia prophylaxis, respectively, for the duration of alemtuzumab treatment plus 3 months. Dapsone was permitted for patients who had sulfur allergy in lieu of sulfamethoxazole and trimethoprim. Baseline CMV viral loads were obtained on all patients, and treatment was halted and ganciclovir begun in patients who demonstrated CMV reactivation.
Patients were assessed for response after the first 18 treatment phase infusions and if they had stable disease or better, were eligible to continue treatment. Dose or schedule modification was permitted as follows: alemtuzumab was held for ≥ 2 nonhematologic and/or grade 3 or higher hematologic toxicities. For the first occurrence, alemtuzumab was restarted at 30 mg when toxicities resolved to < grade 2 for nonhematologic or < grade 3 for hematologic toxicities, respectively. For the second occurrence, alemtuzumab was restarted at 10 mg. For the third occurrence, alemtuzumab was permanently discontinued. If alemtuzumab was discontinued for > 7 days, alemtuzumab was restarted using the test dose escalation schedule noted above.
Study design
A Simon 2-stage design was used. Using 90% power and α set at 0.05, 13 patients were to be included in the first stage of the 2-stage design to test the null hypothesis that the probability of response was ≤ 20% versus the alternative that probability of response was ≥ 45%. For the first stage, after alemtuzumab was administered to 13 patients, the study would have been terminated if 3 or fewer patients responded. If 4 or more patients responded in the first stage of the trial, an additional 14 patients were to be enrolled and treated during stage II. If, after study completion, the total number of responding patients was > 8, then alemtuzumab would be deemed truly effective and the null hypothesis that alemtuzumab produces a response rate < 20% would be rejected.
Response determination
A baseline evaluation was obtained for enrollment within 30 days before the initiation of therapy. Patients underwent restaging studies at 6 and 13 weeks and thereafter every 6 months until progression of disease. As part of their response evaluation, all patients underwent history and physical examination, laboratory studies consisting of a complete blood count, differential quantitative total serum Igs, and serum immunoelectrophoresis. A BM biopsy and aspiration were only performed after treatment to confirm a complete response. Response determinations were made using modified consensus panel criteria from the Third International Workshop on Waldenström's Macroglobulinemia,21,22 and response rates were determined on an intention-to-treat basis. Patients with relapsed disease were defined as those who demonstrated progressive disease after previous treatment response, whereas patients with refractory disease were defined as nonresponders to their previous therapy. Median time to progression (TTP) was calculated from the start of therapy using the Kaplan-Meier method with consensus criteria for disease progression.21 Primary end points for this study were best categorical response attainment, determination of median progression-free survival, and toxicity. Changes in serum IgM levels and blood counts after alemtuzumab therapy were also assessed at best response. The study was approved by the Dana-Farber Cancer Institute/Harvard Cancer Center Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Analysis of peripheral blood effector cells
Serial changes in the absolute levels of peripheral blood effector cells were performed as described previously.23
Statistical analysis
Comparison of pretreatment and posttreatment parameters was performed using a 2-tailed Student t test and Excel Version 2003 software (Microsoft). P ≤ .05 was considered significant.
Results
Patient and disease characteristics
The clinical features of the 28 patients enrolled in this study are summarized in Table 1. Twenty-seven of the 28 patients had WM. One patient had an IgA-secreting LPL. Of the 28 patients enrolled in the study, 23 were previously treated. Eleven (36%) and 12 (43%) of previously treated patients had relapsed or refractory disease, respectively. Among previously treated patients, the median number of prior therapies was 2 (range, 1-5). The median number of alemtuzumab infusions received was 36, (7-36) with 15 (53.6%) patients completing the full course of 36 treatment infusions. Two patients did not return for response assessment and therefore were not evaluable for response.
Clinical response to therapy
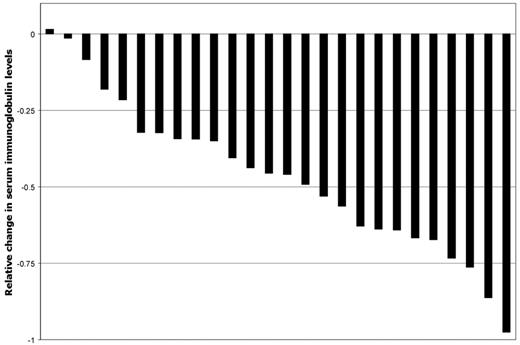
The individual changes in serum IgM levels at best response for all evaluable patients are shown in Figure 1. Median serum IgM levels for all evaluable patients with IgM-secreting LPL declined from 3510 mg/dL (range, 622-8620 mg/dL) to 1460 mg/dL (range, 105-8750 mg/dL) at best response (P < .001). IgA declined from 1210 to 529 at best response for the one patient with IgA LPL. On an intention-to-treat basis, the best ORR was 75%, with 10 (36%) and 11 (39%) patients achieving a major and a minor response, respectively. Seventeen of the 21 responders achieved at least a minor response by 13 weeks. Among major responders, one WM patient who received alemtuzumab as primary therapy achieved a complete response. The best ORR and major response rate for untreated patients was 100% and 80%, respectively, versus 76% (P = .54) and 29% (P = .054) for previously treated patients, respectively. No differences in response rates (ORR or major) were observed among previously treated patients with relapsed versus refractory disease (P = 1.0 and P = .63, respectively). Best ORR (80% vs 83.3%) and major responses (40% vs 33.3%) did not differ when all patients were stratified by baseline serum IgM level as < 6000 and ≥ 6000 mg/dL, respectively (P = 1.0 for both response determinations). For 5 patients with adenopathy (n = 5) or splenomegaly (n = 1), no change (n = 3), improvement (n = 1), or resolution (n = 1) occurred after alemtuzumab therapy. For responding patients, the median time to best response was 7.4 months (range, 1.3-54.4 months).
Relative changes in serum Ig levels for 26 evaluable LPL patients at best response after treatment with alemtuzumab.
Relative changes in serum Ig levels for 26 evaluable LPL patients at best response after treatment with alemtuzumab.
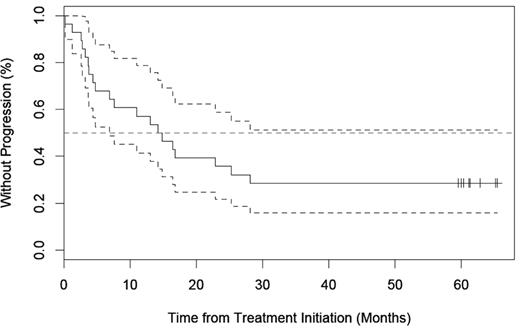
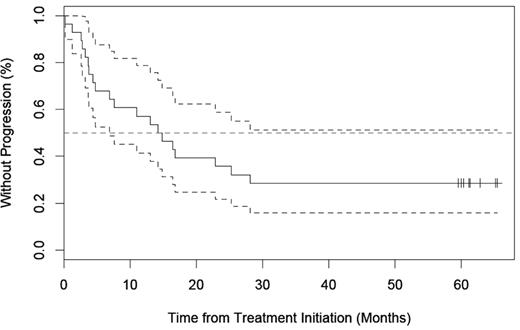
TTP
With a median follow-up of 64 months (range, 1.3-89.8), 14 of 21 (66.7%) responders had progressed. As shown in Figure 2, the median TTP for all 28 patients was 14.5 (range, 1.3-65.4 months) and for responding patients was 16.8 (range, 1.3-65.4 months).
Toxicities
Toxicities encountered were mainly hematologic and infectious and contributed indirectly to 3 deaths of patients while on therapy, and possibly to another death due to late emergence of immune thrombocytopenia. Encountered toxicities led to truncation of intended therapy in 13 patients due to: myelosuppression (n = 6) including pancytopenia (n = 3), neutropenia (n = 2), and thrombocytopenia (n = 1); CMV reactivation (n = 5); esophageal candidiasis (n = 1); and lack of prompt response in a patient who presented with hyperviscosity (n = 1). Grade 2 or higher toxicities are depicted in Table 2. Grade 3 or higher toxicities were more pronounced in previously treated (17 of 23; 73.9%) versus untreated (1 of 5; 20.0%) patients (P = .041), with rash constituting the only grade 3 toxicity encountered in an untreated patient (which resolved with a short course of systemic steroid therapy).
Grade 3 or higher hematologic toxicities included neutropenia, thrombocytopenia, and anemia, which occurred in 53.6%, 25.0%, and 10.7% of patients, respectively. One patient who relapsed after attaining a complete response with CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone/prednisolone)–rituximab developed autoimmune hemolytic anemia 4 weeks after discontinuation of alemtuzumab, which remitted after a short course of prednisone. Another patient whose disease was refractory to rituximab, thalidomide, and bortezomib developed grade 3 thrombocytopenia while on alemtuzumab therapy, which possibly contributed to a fatal hemorrhagic event (suspected on the basis of the patient's underlying acquired VWD).
CMV reactivation was the most common infectious event, occurring in 5 of 28 (17.8%) patients. This prompted discontinuation of alemtuzumab and was symptomatic in 4 of these 5 (80%) patients. Two patients who experienced CMV reactivation developed pancytopenia after institution of antiviral (ganciclovir or valganciclovir) therapy. Both succumbed to infectious complications unrelated to CMV, including one patient who developed coagulase-negative staphylococcal endocarditis leading to a fatal embolic stroke, and another patient with bronchoalveolar lavage culture–negative pneumonia that progressed to fatal acute respiratory distress syndrome. In both cases, prolonged neutropenia associated with anti-CMV therapy were deemed to have contributed to these events.
Late-onset alemtuzumab-related autoimmune thrombocytopenia
With a median follow-up of 64.0 months, we observed new-onset autoimmune thrombocytopenia in 4 (14.3%) patients, which occurred at a median of 12.9 months (range, 3.6-22.9) after completion of therapy. All 4 of these patients had WM, and all received 36 treatment infusions of alemtuzumab and no other interim therapy until the time of the AITP diagnosis. The median platelet count at the time of AITP diagnosis was 12 000 (range, 1000-31 000/mm3). The diagnosis of AITP was confirmed in all cases by a BM biopsy demonstrating adequate megakaryocytes and no other attributable causes, including WM or iatrogenic factors. One patient whose AITP was refractory to systemic treatment and splenectomy succumbed to an intracranial hemorrhage in the setting of severe thrombocytopenia 16 months after diagnosis of AITP. A second patient with refractory AITP to systemic therapy and splenectomy succumbed to rapidly metastasizing colon cancer. A third patient whose AITP responded to rituximab and steroids remains steroid dependent > 55.4 months after alemtuzumab therapy and > 30.8 months after the development of AITP. A fourth patient who developed alemtuzumab-related AITP achieved a complete response to treatment with steroids and rituximab.
Impact of alemtuzumab on peripheral blood immune cells and Ig levels
Median absolute peripheral blood cell levels for CD4+, CD8+, CD16+56+, CD19+, and monocytic cells (cells/mm3) are shown in Figure 3, and did not differ between previously untreated and treated patients (data not shown). As shown in Figure 3, significant decreases in CD4+ (P < .0001), CD8+ (P < .0001), CD16+56+ (P ≤ .02), CD19+ (P < .0001), and monocytic cells (P < .0001) occurred during the active treatment period, with persistent suppression of CD4+, CD8+, CD19+, and monocytic cells, but not CD16+56+ cells, noted throughout the 2-year follow-up period. No significant changes in serum IgA or IgG levels were observed in the 2-year follow-up period (data not shown).
Median absolute peripheral blood CD4+, CD8+, CD16+56+, CD19+, and monocytic cell levels after alemtuzumab therapy. Cell levels were measured at baseline (0), 6 and 12 weeks (W), and 6-24 months (M) after alemtuzumab therapy. The number of patients evaluated at each time point were as follows: 0, n = 26; 6W, n = 18; 12W, n = 22; 6M, n = 16; 9M, n = 17; 12M, n = 13; 15M, n = 10; 18M, n = 11; and 24M, n = 8.
Median absolute peripheral blood CD4+, CD8+, CD16+56+, CD19+, and monocytic cell levels after alemtuzumab therapy. Cell levels were measured at baseline (0), 6 and 12 weeks (W), and 6-24 months (M) after alemtuzumab therapy. The number of patients evaluated at each time point were as follows: 0, n = 26; 6W, n = 18; 12W, n = 22; 6M, n = 16; 9M, n = 17; 12M, n = 13; 15M, n = 10; 18M, n = 11; and 24M, n = 8.
Discussion
Given the wide expression of CD52 on LPL cells, we sought to define the efficacy and safety of alemtuzumab in a multicenter phase 2 study. The strength of this study was the prospective design with a relatively large number of patients with LPL, almost all of whom were subjects with WM, as well as the long-term median follow-up period of 64 months, which was essential in identifying long-term treatment-related complications. The results of this study have important implications for the care of patients with LPL. From a response perspective, our results clearly identify alemtuzumab as an active agent in LPL, with an ORR of 75%, which is inclusive of 36% of patients who achieved a major response (ie, a ≥ 50% decrease in disease burden). Responses were observed in symptomatic patients with both untreated and previously treated disease, although major responses were higher in untreated than in previously treated patients (80% vs 29%, respectively, P = .054).
A similar finding of higher response activity in untreated versus previously treated patients has been observed in CLL patients, and may be related to depletion or functional inactivation of effector cells by previous chemotherapy.24,25 In the present study, high response rates were observed in patients with relapsed or refractory disease and were not influenced by the baseline level of serum IgM, which has been reported previously to be an important determinant for response in WM patients receiving rituximab antibody.7,8 High rates of response with the use of alemtuzumab were also observed by Owen et al,26 who reported their preliminary experience in a small series of heavily pretreated WM patients. The median number of prior therapies in this series was 4 and, similar to the present study, patients received up to 12 weeks of therapy (at 30 mg IV 3 times weekly) after initial dose escalation. Among the 7 patients treated with alemtuzumab, 5 achieved a partial response and 1 a complete response.
Compared with previous experiences with rituximab in symptomatic WM patients, our studies and those by Owen et al26 suggest higher overall activity with alemtuzumab (ie, 75%-85% vs 25%-45%). These differences are unlikely to be dose related, because a smaller dose of alemtuzumab was administered during the 12-week treatment inclusive of test doses (ie, 1123 vs 1500 mg/m2 with standard and 3000 mg/m2 with extended course rituximab, respectively). Whereas several antibody-specific considerations could account for these differences, the possible elimination of CD52+-supportive microenvironmental cells such as mast cells, monocytes, or T cells could account for the more robust activity observed with alemtuzumab compared with rituximab.18-20,27,28 Also notable in the present study was the TTP of 15.6 months, which is as good as, if not better than, that reported with other active single agents in symptomatic LPL, including nucleoside analogs, rituximab, and bortezomib.3,4
Whereas the activity of alemtuzumab in this study of symptomatic LPL patients was notable, short- and long-term adverse events were remarkable as well. Neutropenia and AITP were particularly significant, with 53.6% and 25.0% of patients experiencing grade 3 or higher events, respectively, during active therapy. Whereas previous studies negated CD52 expression on neutrophils, a recent study showed that CD52 is expressed on neutrophils and that alemtuzumab leads to their direct complement-mediated lysis in vitro.29 Therefore, the presence of neutropenia after alemtuzumab therapy is explainable in this patient population. However, the production of AITP by alemtuzumab conflicts with megakaryocytes not being CD52 expressive and with the finding that megakaryopoiesis is actually enhanced by alemtuzumab in colony-forming assays.30 The precise mechanism for this finding remains speculative, but may involve depletion of CD52-expressing T cells, which autoregulate megakaryopoiesis. In addition to the myelosuppression that occurred during treatment, our studies also identified AITP as a late consequence of alemtuzumab therapy. Four cases of late alemtuzumab-related AITP were identified and one was related to the patient's death. In addition, one case of autoimmune hemolytic anemia was observed. A previous report identified AITP after treatment with alemtuzumab in a patient with CLL.31 One potential unifying hypothesis for these observations is the selection for phosphatidylinositolglycan-deficient cells. This was reported after alemtuzumab therapy and could result in the selection of complement defense antigen-deficient cells lacking CD55 and CD59, thereby rendering these cells susceptible to complement attack.32 In the present study, all grade 3 or higher hematologic events and late-AITP events occurred in previously treated individuals, raising the possibility that prior drug therapy may have contributed to alemtuzumab-related hematologic complications.
Routine surveillance permitted the identification of CMV reactivation, which occurred in 5 (18%) patients, all of whom were previously treated. CMV reactivation was not predicted by baseline effector cell levels, including CD4 counts (data not shown). The rate of CMV reactivation in this study was in agreement with that previously reported with the use of alemtuzumab as salvage therapy in CLL patients (20%),33 but lower than that reported by Owen et al26 in more heavily pretreated patients (43%). In our study, 2 patients who developed CMV reactivation died; however, the etiology of their deaths was related to CMV-directed therapy, which produced prolonged neutropenia and contributed to fatal infections. The use of valganciclovir for prophylaxis of CMV reactivation at lower doses than those used for therapeutic intent as reported by O'Brien et al34 and advances in less-myelosuppressive CMV-directed therapy may ultimately permit safer administration of alemtuzumab to patients. Whereas not observed by us in a less-pretreated population of WM patients, disseminated Aspergillus and mycobacterial infections contributed to 2 deaths (of 7 patients) in the series by Owen et al26 in heavily pretreated WM patients receiving alemtuzumab.
In conclusion, alemtuzumab is an active agent in patients with LPL. However, short- and long-term toxicities need to be carefully weighed against other available treatment options. Late AITP is a newly recognized complication of alemtuzumab in this patient population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the clinicians who provided care to the participating patients while on this study: Harriet Bering, MD (Beverly, MA); Marvin Cooper, MD (New York, NY); Cynthia Chua, MD (Cincinnati, OH); Paul Donovan, MD (Kingston, NY); Larry Garbo, MD (Albany, NY); Judith Kleinerman, MD (Taunton, MA); Leslie Lockridge, MD (Pawtucket, RI); David Lovett, MD (Hyannis, MA); Robert Orlowski, MD, PhD (Chapel Hill, NC); Manuel Perry, MD (Middletown, NY); Peter Pickens, MD (Willow Grove, PA); Ronald Rohe, MD (Presque Isle, ME); Richard Shadduck, MD (Pittsburgh, PA); Joseph Songer, MD (Muncie, IN); Edward Stadtmauer, MD (Philadelphia, PA); Ronald Steis, MD (Atlanta, GE); Ronald Takvorian, MD (Boston, MA); and Robert Weinstein, MD (Boston, MA).
This work was supported by grants from the Research Fund for Waldenström's at the Dana-Farber Cancer Institute; by Berlex Pharmaceuticals Inc; by the Peter and Helen Bing Fund at the Dana-Farber Cancer Institute; and by a National Institutes of Health Career Development Award (K23CA087977-03 to S.P.T.).
National Institutes of Health
Authorship
Contribution: S.P.T. designed the trial, recruited and treated patients, and analyzed the data; J.D.S., Z.R.H., C.J.P., and L.I. served as clinical research coordinators for this trial and collected and analyzed data; B.K. and M.B. were site principal investigators and recruited patients, collected data, and reviewed study outcome; and S.P.T. and J.D.S. wrote the manuscript.
Conflict-of-interest disclosure: Berlex Pharmaceuticals provided research funding and drug replacement in support of these studies. The authors declare no competing financial interests.
Correspondence: Steven P. Treon, MD, MA, PhD, Bing Center for Waldenström's Macroglobulinemia, Dana-Farber Cancer Institute, Harvard Medical School, M547, 44 Binney St, Boston MA 02115; e-mail: steven_treon@dfci.harvard.edu.