Abstract
The present study focuses on a large family with an X-linked immunodeficiency in which there are variable clinical and laboratory phenotypes, including recurrent viral and bacterial infections, hypogammaglobulinemia, Epstein-Barr virus–driven lymphoproliferation, splenomegaly, colitis, and liver disease. Molecular and genetic analyses revealed that affected males were carriers of a hypomorphic hemizygous mutation in XIAP (XIAPG466X) that cosegregated with a rare polymorphism in CD40LG (CD40 ligandG219R). These genes are involved in the X-linked lymphoproliferative syndrome 2 and the X-linked hyper-IgM syndrome, respectively. Single expression of XIAPG466X or CD40LG219R had no or minimal effect in vivo, although in vitro, they lead to altered functional activities of their gene products, which suggests that the combination of XIAP and CD40LG mutations contributed to the expression of clinical manifestations observed in affected individuals. Our report of a primary X-linked immunodeficiency of oligogenic origin emphasizes that primary immunodeficiencies are not caused by a single defective gene, which leads to restricted manifestations, but are likely to be the result of an interplay between several genetic determinants, which leads to more variable clinical phenotypes.
Introduction
The X-linked lymphoproliferative (XLP) syndrome is a rare inherited immunodeficiency characterized by an exquisite susceptibility to Epstein-Barr virus (EBV) infection. In 60%-80% of cases, patients develop severe infectious mononucleosis characterized by hemophagocytic lymphohistiocytosis.1-3 Another hallmark of XLP is the hypogammaglobulinemia that is found in 40% of patients. On a molecular basis, XLP is now divided into 2 distinct diseases, XLP-1 and XLP-2, which are caused by hemizygous mutations in SH2D1A and XIAP (also denoted as BIRC4), respectively. SH2D1A encodes the small adaptor protein SAP (SLAM-associated protein) that couples the SLAM family of immunoreceptors to intracellular signaling pathways. XIAP encodes the X-linked inhibitor of apoptosis, which acts mainly as a potent inhibitor of caspases 3, 7, and 9 but is also involved in a variety of other signaling pathways, including nuclear factor-κB and c-Jun N-terminal kinase activation and copper metabolism.4,5 Although SAP and XIAP deficiencies are both characterized by EBV-driven hemophagocytic lymphohistiocytosis, each displays specific manifestations: Lymphoproliferative disorders such as lymphomas thus far have only been described in SAP deficiency, whereas XIAP patients often exhibit recurrent splenomegaly and may develop chronic hemorrhagic colitis.1,3,6
The XLP syndromes are usually seen in infants or children and have a severe outcome, although several adult and pediatric patients have been misdiagnosed on the basis of their variable clinical manifestations as having common variable immunodeficiency disorders (CVIDs), the most frequent antibody deficiencies in humans.7-10 These patients carried mutations in SH2D1A and developed progressive hypogammaglobulinemia or lymphoma; however, a search for XIAP mutations in a cohort of 28 male patients with CVIDs did not reveal any abnormalities.11 In SAP deficiency, the hypogammaglobulinemia is associated with a decreased number of memory B cells and a lack of germinal center formation that results from an impaired help of T cells to B cells.12 In XIAP deficiency, the hypogammaglobulinemia may be late in onset and is sometimes transient; its origin is currently unknown.3 The spectrum of clinical phenotypes in both subtypes of XLP is broad and the severity variable, even between patients from the same family. To date, there is no clear evidence for a correlation between phenotypes and mutations, which indicates that other genetic or environmental factors participate in the expression and severity of the different phenotypes.
CD40L/CD154 deficiency is responsible for the X-linked hyper-IgM syndrome (XHIGM), which is characterized by impaired T-helper function that causes defects in B-cell function.13,14 CD40L is transiently expressed on activated helper T cells and interacts with its cognate receptor, CD40, which is constitutively expressed on B cells and antigen-presenting cells (APCs). Cooperation between T follicular helper cells and B cells through CD40L/CD40 in secondary lymphoid organs is required for B-cell proliferation and antibody maturation. XHIGM is characterized by defective immunoglobulin class-switch recombination (CSR) from IgM to IgG, IgA, or IgE, as shown by low serum levels of IgG, IgA, and IgE. Defective production of IgG and IgA is responsible for susceptibility to recurrent infections, as observed in other severe B-cell primary immunodeficiencies. The defect in CD40L also accounts for the impaired cooperation between T lymphocytes and APCs that results in altered T-cell responses that cause susceptibility to opportunistic infections. However, the severity of the CD40L deficiency varies, and some mutations that allow residual expression of CD40L molecule have been shown to lead to a milder immunodeficiency that resembles CVIDs.14-16
Here, we report the identification of an X-linked syndrome in 6 males from a large family presenting with various clinical manifestations. In this family, the disease was found to be associated with 2 hypomorphic mutations in XIAP and in CD40LG, respectively, which reveals that primary immunodeficiencies could have an oligogenic origin.
Methods
Detailed procedures of the Methods are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Sequencing
CD40LG, XIAP, and SH2D1A genes were sequenced from genomic DNA.
Complementary DNA constructs
Human CD40L, CD40LG219R, XIAP, and XIAPG466X cDNAs were cloned by RT-PCR according to standard procedures.
In vitro ubiquitin ligase assay
Assays were performed as described previously.17
Caspase-binding assay
Preparation of apoptotic extracts and protein-binding assays was performed as described previously.18
Western blot
Immunoblots were performed according to standard protocols.19
Apoptosis
Assays were performed as described previously.1
Flow cytometric analysis
Staining of cells was performed and analyzed by standard procedures.
Cells, transduction, and transfection
Viral infections of cells were performed according to standard procedures.
In vitro B-cell proliferation and CSR toward IgE production
Assays were performed as described previously.20
Clinical histories and genotypes
We examined 7 males from a large family with suspicion of an immunodeficiency and 1 unrelated control patient (patient C). All patients who were alive in 2003 at the start of the study and subject II-5 were found to be EBV IgG+ (anti–Viral Capsid Antigen) except patient C, who was not tested because he was already undergoing intravenous immunoglobulin treatment.
Subject II-5 has the XIAP G466X and CD40L wild-type genotype. He is the older brother of subject II-7. He is now 42 years old and healthy with normal serum immunoglobulin levels, has not had infections or autoimmune diseases, and is not taking any medication.
Patient II-7 had the XIAP G466X and CD40L G219R genotype. The first symptoms of the disease appeared at the age of 5 years as an infective illness associated with idiopathic thrombocytopenic purpura (inosine 5′-triphosphate). He then developed acute hepatitis (negative for hepatitis C virus and hepatitis B virus by PCR), which evolved into idiopathic chronic liver disease with hepatosplenomegaly. He also had recurrent bacterial infections (pneumonia and meningitis) that were associated with progressive hypogammaglobulinemia. He developed bronchiectasis and required therapeutic intravenous immunoglobulin (IVIG) substitution. His clinical picture was complicated by a polyclonal lymphoproliferation, which consisted of persistent lymphadenopathy, suspected lymphocytic infiltrative pneumonitis, and major gastrointestinal bleeding from gastric varices as a result of cirrhosis of unknown cause (on biopsy). He died at the age of 28 years because of progressive liver failure.
Patient III-1 has the XIAP G466X and CD40L G219R genotype. After an eventless childhood, he developed a persistent splenomegaly and mild hypogammaglobulinemia associated with intermittent mild pulmonary infections and diarrhea at 21 years of age.
Patient III-2 has the XIAP G466X and CD40L G219R genotype. At the age of 7 years, he presented with fever, mild hepatitis, and splenomegaly because of infectious mononucleosis associated with primary infection (EBV IgM+). He had pancytopenia (iron deficiency anemia, neutropenia, and mild thrombocytopenia) but no erythrophagocytosis on bone marrow examination. This episode resolved over 4 months, but the symptoms recurred 36 months later. He recovered clinically. Now he is 29 years old and still has persistent splenomegaly and increasing hypogammaglobulinemia with mild but recurrent pulmonary infections.
Patient III-6 has the XIAP G466X and CD40L G219R genotype. He presented as an infant with febrile viral illnesses with recurrent episodes of pneumonia. Five years later, he developed iron deficiency anemia, intermittent episodes of facial herpes with cold sores, impetigo, shingles, and transient lymphadenopathy and was found to have raised serum immunoglobulin levels that have now normalized. He then developed splenomegaly and most recently, at the age of 12 years, developed colitis with extensive bleeding consistent with a Crohn disease–type pathology.
Patient I-3's genotype was not determined. He developed a severe hemorrhagic colitis consistent with a Crohn's type of disease, which he died of when he was 27 years old (53 years ago); there were no further investigations.
Patient I-5's genotype was not determined. He had a long-standing history of recurrent bacterial and viral infections (4 episodes of measles infection), lymphadenopathy, hepatosplenomegaly, and pernicious anemia. He died at 52 years of age (20 years ago) of pneumonia associated with smoking.
Patient C has the XIAP wild-type and CD40L G219R genotype. He was born 10 weeks prematurely and developed bronchodysplasia associated with severe recurrent pulmonary infections between the age of 6 months and 1 year. At the age of 3 months, he had hypogammaglobulinemia (IgG, 0.9 g/L; IgA, 0.11 g/L; IgM, 0.56 g/L), probably secondary to his premature birth, that persisted at the age of 6 months. He had received IVIG substitution since the age of 6 months. On the basis of these clinical features and hypogammaglobulinemia, a defect in CD40L was suspected, and sequencing of CD40LG was undertaken.
The proportions of the different B-cell populations were determined when patient C was 6 years old, and he had rather low proportions of memory and switched B cells [7% CD19+, 4.9% CD27+(/CD19+), 1% IgD−IgM−(/CD19+)]. In the 9 years since that time, he has been well and has not had liver disease or opportunistic infections, which indicates a normal cellular immunity. He has normal values of serum IgA and IgM. His switched B-cell subpopulations increased to a normal range (Table 2). He has not taken prophylactic medication but is still receiving IVIG substitution.
Two patients with XIAP-null mutations were used as control subjects in this study in functional experiments. One carried a deletion of exon 2 in XIAP, the other a deletion of 3 nucleotides in exon 1 leading to XIAPD130Gfs140X. Both mutations lead to the absence of XIAP expression.
Informed consent was obtained from each patient, and the study protocols conformed to the 1975 Declaration of Helsinki and the local ethics regulations (Necker-Enfants Malades ethics board committee).
Results
A family of Caucasian European origin with several affected male individuals was identified in a cohort of patients analyzed for XIAP defects.3 This family was first investigated for suspected immunodeficiency of undefined origin in the context of increased susceptibility to and complicated courses of bacterial and viral infections. Three generations of male individuals were affected, which strongly suggests an X-linked transmission of the disease (Figure 1A). The clinical manifestations and the severity of the disease varied within the separate members of the family (Table 1; detailed case reports in “Clinical histories and genotypes”). Several of these signs evoked XLP syndrome type 1 and type 2, whereas others, such as recurrent bacterial and viral infections, hepatitis, and decreased IgG and IgA serum, were reminiscent of the XHIGM/CD40L deficiency. For these reasons, the SH2D1A, XIAP, and CD40LG genes (respectively defective in XLP-1, XLP-2, and XHIGM) were sequenced.
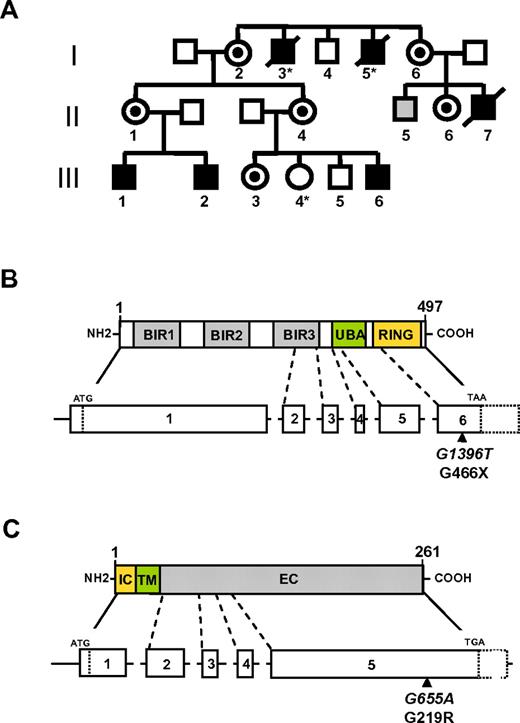
XIAPG466X and CD40LG219R mutations. (A) Pedigree of the family presenting the X-linked condition that cosegregates with XIAPG466X and CD40LG219R mutations. All numbered individuals have been sequenced with the exception of patients I-3, I-5, and III-4 (*). Black boxes represent affected hemizygous carriers of both XIAP and CD40LG mutations. Circles with a black dot represent heterozygous carrier of both XIAP and CD40LG mutations. The gray box represents asymptomatic hemizygous carriers of the XIAP mutation. (B and C) Schematic of XIAP (B) and CD40L (C). The XIAP-encoding domains from exons 1 to 6 and CD40L-encoding domains from exons 1 to 5 are shown. Localization of the CD40LG219R mutation and the XIAPG466X mutation is shown by a black arrow. The nucleotide position of each mutation is indicated in italics. BIR indicates baculovirus inhibitor of apoptosis repeats; UBA, ubiquitin-binding domain; IC, intracellular; TM, transmembrane; and EC, extracellular.
XIAPG466X and CD40LG219R mutations. (A) Pedigree of the family presenting the X-linked condition that cosegregates with XIAPG466X and CD40LG219R mutations. All numbered individuals have been sequenced with the exception of patients I-3, I-5, and III-4 (*). Black boxes represent affected hemizygous carriers of both XIAP and CD40LG mutations. Circles with a black dot represent heterozygous carrier of both XIAP and CD40LG mutations. The gray box represents asymptomatic hemizygous carriers of the XIAP mutation. (B and C) Schematic of XIAP (B) and CD40L (C). The XIAP-encoding domains from exons 1 to 6 and CD40L-encoding domains from exons 1 to 5 are shown. Localization of the CD40LG219R mutation and the XIAPG466X mutation is shown by a black arrow. The nucleotide position of each mutation is indicated in italics. BIR indicates baculovirus inhibitor of apoptosis repeats; UBA, ubiquitin-binding domain; IC, intracellular; TM, transmembrane; and EC, extracellular.
XIAP and CD40LG mutations
We first sequenced affected individuals III-2 and III-6. The SH2D1A sequences were found to be normal, but sequencing of XIAP revealed a hemizygous G-to-T transversion at position 1396 in exon 6 that led to a stop codon at position 466 (G466X) in XIAP in both patients (Figure 1B). We also identified a G-to-A transition at position 655 in the exon 5 of the CD40LG that led to a Gly-to-Arg substitution at the codon position 219 in CD40L (G219R; Figure 1C). We then sequenced the other members of the family, except for individuals I-3, I-5, and III-4, for whom DNA samples were not available. Affected individuals II-7 and III-1 were carriers of both XIAP and CD40LG mutations. The healthy male individual II-5 was a carrier of the XIAP G1396T mutation alone, whereas the 2 healthy male individuals I-4 and III-5 had neither XIAP nor CD40LG mutations. The 6 females tested (mothers and sisters of patients; I-2, I-6, II-1, II-4, II-6, and III-3) were heterozygous carriers of both XIAP and CD40LG mutations. XIAP and CD40LG are located in near proximity on the X chromosome, physically separated only by 12 Mb. This explains the cosegregation of these 2 mutations in all carriers with the exception of 1 healthy male individual (II-5) in whom analysis of polymorphic markers surrounding and between XIAP and CD40LG revealed a recombination event between the 2 genes (data not shown). Therefore, all affected individuals in this family express both mutations in XIAP and CD40LG, whereas the male carrier of the single XIAP mutation is asymptomatic (II-5). This 42-year-old individual has been infected with EBV, as shown by his positive EBV serology.
The CD40LG219R substitution was initially reported to be expressed at a low frequency of 1.5% (2/129) in the white population.21,22 A very similar incidence (1.4%) was recently observed in 208 unrelated males (3/208), which confirms that CD40LG219R is a rare recurrent polymorphism.23 By contrast, the XIAPG466X mutant was not expressed in different cohorts of white individuals examined that included patients with CVIDs (data not shown and Salzer et al11 ). Altogether, these observations suggest that both mutations could cooperate for the expression of variable clinical phenotypes in this family.
Confirming that the single expression of CD40LG219R protein has no or only minor effects in vivo, a control individual carrier of the CD40LG219R mutation alone was identified from an unrelated family (referred to hereafter as patient C). The CD40LG219R variant was detected in this individual after an episode of hypogammaglobulinemia with recurrent infections at the age of 6 months; he was treated with IVIG infusion. Since then, he has remained asymptomatic and has normal values of serum IgA and IgM and normal B-cell subpopulations (Table 2). He has wild-type SH2D1A and XIAP gene sequences.
XIAPG466X and CD40LG219R proteins
To further investigate whether the XIAPG466X and CD40LG219R variants can cause the clinical phenotypes observed in this family, we performed functional and biochemical studies to characterize the 2 mutant proteins. XIAP contains 3 BIRs (baculovirus inhibitor of apoptosis repeats), 1 ubiquitin-binding domain, and 1 carboxyl-terminal RING finger domain4 (Figure 1B). The BIR domains of XIAP directly interact with the cleaved active forms of caspases 3, 7, and 9, thereby inhibiting their proteolytic activity. The carboxyl-terminal RING domain of XIAP possesses an E3 ubiquitin ligase activity, which has been involved in the signaling functions of XIAP.4 Analysis of XIAP expression in lysates of T-cell blasts from individuals III-1, III-2, and II-5 revealed weak expression of a truncated protein that corresponded to the expected size of the XIAPG466X protein in which a premature stop codon removed the last 31 carboxyl-terminal amino acids (Figures 1B and 2A). The amount of XIAPG466X protein detected in cells of patients corresponded to approximately 10%-20% of the wild-type XIAP found in cells of healthy individuals. Expression of both wild-type XIAP and XIAPG466X proteins was detected within the cell lysate of the heterozygous female carrier II-6 (sister of II-5). Ectopic expression of V5-, GST-, or Myc-tagged versions of wild-type XIAP and XIAPG466X into 293T cells or bacteria, respectively, showed decreased expression of the XIAPG466X mutant in contrast to wild-type XIAP (see Figures 2B and 5C-D). Similar results were obtained with nontagged forms (see Figure 5A). These data suggest that XIAPG466X is less stable than the wild-type protein.
XIAPG466X protein expression. (A) Western blot analysis of XIAP and XIAPG466X protein expression. Lysates from PHA-expanded T-cell blasts derived from healthy control (Ctr.), male patients (III-2, III-1, and II-5), and a female carrier (II-6, sister of II-5) were immunoblotted with anti-XIAP antibodies and with anti-Ku70 antibodies as loading control. II-6 is a heterozygous carrier expressing both XIAP and XIAPG466X proteins. (B) Expression of V5-tagged forms of XIAP and XIAPG466X proteins in 293T cells. Total lysates were prepared 48 hours after transfection and immunoblotted with anti-V5 antibodies.
XIAPG466X protein expression. (A) Western blot analysis of XIAP and XIAPG466X protein expression. Lysates from PHA-expanded T-cell blasts derived from healthy control (Ctr.), male patients (III-2, III-1, and II-5), and a female carrier (II-6, sister of II-5) were immunoblotted with anti-XIAP antibodies and with anti-Ku70 antibodies as loading control. II-6 is a heterozygous carrier expressing both XIAP and XIAPG466X proteins. (B) Expression of V5-tagged forms of XIAP and XIAPG466X proteins in 293T cells. Total lysates were prepared 48 hours after transfection and immunoblotted with anti-V5 antibodies.
CD40L is a type II transmembrane molecule that belongs to the TNF superfamily. Like other members of the TNF family of ligands, the trimerization of CD40L is required for its interaction with CD40.24 Expression of CD40L was analyzed on T-cell blasts from the control individual who expressed CD40LG219R alone (patient C) and compared with wild-type CD40L on T-cell blasts from a healthy individual (Figure 3A; supplemental Figure 1a). After activation by phorbol 12-myristate 13-acetate and ionomycin, the level of CD40LG219R on cells was comparable to that of wild-type CD40L when detected with the anti-CD40L monoclonal antibody TRAP-1 (TNF-receptor–associated protein 1). By contrast, detection of CD40LG219R with the anti-CD40L monoclonal antibody 24.31 was significantly lower than that of wild-type CD40L, which suggests an alteration in the epitope recognized by the antibody, as described previously.22 Similar data were obtained with primary T cells of patients III-1 and III-2 (supplemental Figure 1b). These results were confirmed in 3T3 cells that expressed recombinant CD40LG219R or wild-type CD40L in which the 24.31 monoclonal antibody detected decreased levels of CD40LG219R but not of CD40L (Figure 3B). In the different cells and conditions tested, the binding of CD40LG219R to a CD40 recombinant fusion protein (CD40-Ig) was systematically decreased compared with wild-type CD40L, thus revealing a weaker capacity of CD40LG219R to bind CD40 (Figure 3C; supplemental Figure 1b). Collectively, these data show that the G219R substitution likely results in an abnormal conformation of the CD40L that decreases its ability to bind to CD40; this could affect CD40L-mediated cellular interactions and functions.22 The G219R substitution may also affect CD40L trimerization, because the G219 residue is located in the extracellular domain of CD40L, between amino acids 218 and 220, which are involved in CD40L trimerization.24
CD40LG219R protein expression. (A) CD40L expression on Con A–expanded T-cell blasts from a healthy control (CD40L) and an individual expressing the CD40LG219R mutant (CD40LG219R). Cells were stimulated or not (No stim.) with phorbol 12-myristate 13-acetate and ionomycin (PMA/Iono) for 6 hours and analyzed by flow cytometry with anti-CD40L TRAP-1 or anti-CD40L 24.31 antibodies. Gray histograms correspond to staining with control immunoglobulin. (B) CD40L expression on wild-type 3T3 cells (3T3) and 3T3 expressing green fluorescent protein (GFP)-CD40L (CD40L) or GFP-CD40LG219R (CD40LG219R). After infection with retroviral plasmids that contained CD40LG219R or wild-type CD40L with a GFP reporter gene, cells were analyzed by flow cytometry for their GFP content (GFP) or for their expression of CD40L with TRAP-1 or 24.31 mAbs. Shaded histograms as in (A). (C) CD40L expression on ConA–, PHA-expanded T-cell blasts and 3T3 cells expressing CD40L or CD40LG219R was detected with CD40-Ig (histograms on right). Histograms on left correspond to staining with control immunoglobulin. The profile of cells expressing CD40LG219R (blue line) was overlaid on the profile of cells expressing CD40L (red line). Max indicates maximum.
CD40LG219R protein expression. (A) CD40L expression on Con A–expanded T-cell blasts from a healthy control (CD40L) and an individual expressing the CD40LG219R mutant (CD40LG219R). Cells were stimulated or not (No stim.) with phorbol 12-myristate 13-acetate and ionomycin (PMA/Iono) for 6 hours and analyzed by flow cytometry with anti-CD40L TRAP-1 or anti-CD40L 24.31 antibodies. Gray histograms correspond to staining with control immunoglobulin. (B) CD40L expression on wild-type 3T3 cells (3T3) and 3T3 expressing green fluorescent protein (GFP)-CD40L (CD40L) or GFP-CD40LG219R (CD40LG219R). After infection with retroviral plasmids that contained CD40LG219R or wild-type CD40L with a GFP reporter gene, cells were analyzed by flow cytometry for their GFP content (GFP) or for their expression of CD40L with TRAP-1 or 24.31 mAbs. Shaded histograms as in (A). (C) CD40L expression on ConA–, PHA-expanded T-cell blasts and 3T3 cells expressing CD40L or CD40LG219R was detected with CD40-Ig (histograms on right). Histograms on left correspond to staining with control immunoglobulin. The profile of cells expressing CD40LG219R (blue line) was overlaid on the profile of cells expressing CD40L (red line). Max indicates maximum.
Functions of XIAPG466X
XIAP deficiency in humans results in increased lymphocyte apoptosis by activation of the extrinsic pathways of apoptosis that involve TNF receptors, such as TRAIL receptor (TRAIL-R), Fas (CD95), or the T-cell receptor–CD3 complex that triggers activation-induced cell death (AICD).1 These stimuli were analyzed in T-cell blasts from patients and compared with patients with null hemizygous XIAP mutations (deletion of exon 2 in XIAP or XIAPD130Gfs140X) or healthy donors (Figure 4; supplemental Figure 2). Stimulation of TRAIL-R in T-cell blasts from patients expressing XIAPG466X resulted in a low induction of apoptosis, comparable to that observed in cells from healthy donors expressing wild-type XIAP (Figure 4A). By contrast, in XIAP-deficient cells from a patient expressing an XIAP-null mutation (deletion of exon 2 in XIAP), TRAIL-R stimulation induced a marked apoptosis, as expected (Figure 4A). Thus, weak expression of XIAPG466X appears to be sufficient to protect T-cell blasts from TRAIL-R–induced apoptosis. On the other hand, apoptosis induced by anti-CD3 antibody stimulation (AICD), which depends on Fas-FasL interactions, was significantly enhanced in T-cell blasts from patients expressing XIAPG466X compared with wild-type blasts from healthy donors (Figure 4B). Confirming these data, direct activation of apoptosis by anti-Fas (CD95) antibodies showed increased apoptosis of XIAPG466X-expressing T-cell blasts compared with wild-type cells (supplemental Figure 2). The XIAPG466X protein also failed to protect XIAP-deficient T-cell blasts from AICD in contrast to wild-type XIAP (supplemental Figure 3). Interestingly, we noticed that the level of AICD in T-cell blasts expressing both XIAPG466X and CD40LG219R was significantly higher than that of XIAP-deficient blasts from patients carrying null XIAP mutations with wild-type CD40L (deletion of exon 2 in XIAP or XIAPD130Gfs140X; Figure 4B). This effect appears to be the result of coexpression of the CD40LG219R molecule, because AICD in blasts expressing XIAPG466X and wild-type CD40L (individual II-5) was not enhanced and was comparable to that of XIAP-deficient cells. Taken together, these results demonstrate that the XIAPG466X mutant failed to protect cells from AICD and Fas-mediated apoptosis, although its ability to inhibit TRAIL-R–mediated apoptosis was preserved.
Increased AICD but not TRAIL-R–mediated-apoptosis of XIAPG466X-expressing T lymphocytes. (A) TRAIL-R–mediated apoptosis of T-cell blasts from healthy controls (Ctr.), patients expressing null mutations of XIAP (XIAP−), patients III-1 and III-2 expressing both XIAPG466X and CD40LG219R, and a female (II-1) heterozygous carrier of both XIAPG466X and CD40LG219R. Cells were stimulated with trimeric TRAIL. One experiment that is representative of 3 independent experiments is shown. (B) AICD of T-cell blasts expressing both XIAPG466X and CD40LG219R (patients III-1, III-2, and II-7), XIAPG466X alone (individual II-5), or CD40LG219R alone (individual IV) compared with that of cells from 4 healthy donors (Ctr.) and 2 XIAP-null patients (XIAP−). The graph was compiled from 7 independent experiments performed in duplicate. Each point represents the mean of duplicates in which the raw values differed by < 5%. Cells were stimulated with an anti-CD3 antibody. Bars correspond to mean values of each group. **P < .001 and ***P < .0001.
Increased AICD but not TRAIL-R–mediated-apoptosis of XIAPG466X-expressing T lymphocytes. (A) TRAIL-R–mediated apoptosis of T-cell blasts from healthy controls (Ctr.), patients expressing null mutations of XIAP (XIAP−), patients III-1 and III-2 expressing both XIAPG466X and CD40LG219R, and a female (II-1) heterozygous carrier of both XIAPG466X and CD40LG219R. Cells were stimulated with trimeric TRAIL. One experiment that is representative of 3 independent experiments is shown. (B) AICD of T-cell blasts expressing both XIAPG466X and CD40LG219R (patients III-1, III-2, and II-7), XIAPG466X alone (individual II-5), or CD40LG219R alone (individual IV) compared with that of cells from 4 healthy donors (Ctr.) and 2 XIAP-null patients (XIAP−). The graph was compiled from 7 independent experiments performed in duplicate. Each point represents the mean of duplicates in which the raw values differed by < 5%. Cells were stimulated with an anti-CD3 antibody. Bars correspond to mean values of each group. **P < .001 and ***P < .0001.
The signaling functions of XIAP depend on its ability to autoubiquitinate and ubiquitinate substrates.4 The XIAPG466X mutant lacks the 31 C-terminus amino acids of the RING domain, but the autoubiquitination sites Lys 322 and 328 are preserved.25 The capacity of XIAPG466X to autoubiquitinate was analyzed in 293T cells in the presence or absence of ubiquitin and compared with that of wild-type XIAP (Figure 5A). High-molecular weight forms of XIAP that corresponded to polyubiquitinated XIAP were only detected when XIAP was expressed in the presence of ubiquitin. These high-molecular species were almost completely absent when XIAPG466X was expressed, which suggests that the E3 ubiquitin ligase activity of the XIAPG466X mutant is altered. To confirm these data, the E3 ubiquitin ligase activity of XIAPG466X was tested in vitro in the presence of E1 and E2 ubiquitin ligases, which promote E3 autoubiquitination.17 In these conditions, wild-type XIAP induced a strong autoubiquitination that was comparable to that obtained with the E3 ubiquitin ligase TRAF6 (TNF-receptor–associated factor 6; Figure 5B). This effect was dependent on the RING domain of XIAP, because the XIAPG437X mutant protein, in which the entire RING domain was removed, failed to induce ubiquitination. Similarly, the XIAPG466X mutant was not able to induce detectable autoubiquitination. The ability of XIAPG466X to interact with active caspases was further analyzed in pull-down assays with either 293T cell extracts treated with cytochrome C and dATP to activate caspase 3 (Figure 5C) or synthetic peptides that corresponded to the IAP-binding motif of active caspase 9 (Figure 5D). The capacity of XIAPG466X to bind cleaved caspase 3 or the IAP-binding motif of caspase 9 was preserved and comparable to that of the wild-type XIAP, even though XIAPG466X expression was weaker than of wild-type XIAP. Thus, these observations indicate that the global ability of the XIAPG466X mutant to block caspases is diminished because of its instability (or low expression), since its intrinsic capacity to bind active caspases remains intact. However, the XIAPG466X protein has no more E3 ubiquitin ligase activity but is still able to interact with active caspases. Overall, XIAPG466X behaves as a hypomorphic mutant that retains some protein expression and has sufficient antiapoptotic activity to protect cells from TRAIL-R–induced but not AICD- and Fas-mediated apoptosis.
Ubiquitin E3 ligase activity and binding to caspases of the XIAPG466X mutant. (A) 293T cells transfected with XIAP or XIAPG466X in the presence of ubiquitin (Ubi) or not (−) were analyzed by immunoblotting with anti-XIAP antibodies. Higher-mass products correspond to ubiquitinated XIAP species (XIAP-Ubin). (B) In vitro XIAP autoubiquitination assay. GST recombinant E3 ubiquitin ligase proteins (E3) were tested in the presence of ubiquitin (Ubi) and E2 and E1 ubiquitin ligases and then submitted to Western blot with anti-ubiquitin antibodies (top panels). The amount of GST proteins was controlled by Coomassie blue staining (bottom panels). (Left) Test with GST-XIAP, -XIAPG437X, and -TRAF6. (Right) Test with GST-XIAP, -XIAPG437X, and -XIAPG466X. (C and D) Binding assays of XIAP and XIAPG466X to caspases 3 and 9. (C) Glutathione-sepharose–immobilized GST-XIAP, GST-XIAPG466X, and GST alone were incubated with different amounts of protein from dATP and cytochrome C–treated 293T cell extracts. Bound cleaved caspase 3 was detected by immunoblotting (top panel). The amount of GST proteins was controlled by Coomassie blue staining (bottom panel). (D) Different amounts of proteins extracted from 293T cells transfected with Myc-XIAP or -XIAPG466X were immunoprecipitated (intraperitoneal) with a biotinylated peptide that corresponded to caspase 9 (CASP9) or a control peptide (Ctr.). Because the expression level of Myc-XIAPG466X was 4- to 5-fold less expressed than Myc-XIAP (lysates, right panel), 4-fold greater amounts of proteins were immunoprecipitated from extracts that expressed Myc-XIAPG466X.
Ubiquitin E3 ligase activity and binding to caspases of the XIAPG466X mutant. (A) 293T cells transfected with XIAP or XIAPG466X in the presence of ubiquitin (Ubi) or not (−) were analyzed by immunoblotting with anti-XIAP antibodies. Higher-mass products correspond to ubiquitinated XIAP species (XIAP-Ubin). (B) In vitro XIAP autoubiquitination assay. GST recombinant E3 ubiquitin ligase proteins (E3) were tested in the presence of ubiquitin (Ubi) and E2 and E1 ubiquitin ligases and then submitted to Western blot with anti-ubiquitin antibodies (top panels). The amount of GST proteins was controlled by Coomassie blue staining (bottom panels). (Left) Test with GST-XIAP, -XIAPG437X, and -TRAF6. (Right) Test with GST-XIAP, -XIAPG437X, and -XIAPG466X. (C and D) Binding assays of XIAP and XIAPG466X to caspases 3 and 9. (C) Glutathione-sepharose–immobilized GST-XIAP, GST-XIAPG466X, and GST alone were incubated with different amounts of protein from dATP and cytochrome C–treated 293T cell extracts. Bound cleaved caspase 3 was detected by immunoblotting (top panel). The amount of GST proteins was controlled by Coomassie blue staining (bottom panel). (D) Different amounts of proteins extracted from 293T cells transfected with Myc-XIAP or -XIAPG466X were immunoprecipitated (intraperitoneal) with a biotinylated peptide that corresponded to caspase 9 (CASP9) or a control peptide (Ctr.). Because the expression level of Myc-XIAPG466X was 4- to 5-fold less expressed than Myc-XIAP (lysates, right panel), 4-fold greater amounts of proteins were immunoprecipitated from extracts that expressed Myc-XIAPG466X.
Functions of CD40LG219R
Several clinical manifestations that are not commonly found in other patients with an XIAP deficiency, including recurrent bacterial infections and non-EBV viral infections, were observed in affected individuals of this family.3 Furthermore, 3 patients (II-7, III-1, and III-2) have developed progressive hypogammaglobulinemia (Table 2). These manifestations could be related in part to the expression of CD40LG219R, because individual II-5, who does not express CD40LG219R, has normal immunoglobulin levels, is free of infections, and has normal B-cell subsets. CD40L deficiency is characterized by low numbers of memory B cells (CD19+CD27+) with decreased proportions of immunoglobulin-switched B cells (CD19+IgM−IgD−), which is not a hallmark of XIAP deficiency. Analysis of B-lymphocyte subpopulations in the individuals harboring both CD40LG219R and XIAPG466X mutants (III-1, III-2, and III-6) showed reduced proportions of memory B cells and immunoglobulin-switched memory B cells in peripheral blood compared with individuals expressing either CD40LG219R (patient C) or XIAPG466X (II-5; Table 2). These observations suggest that the expression of CD40LG219R impairs the differentiation of memory B cells in patients who expressed XIAPG466X but not in the patient with wild-type XIAP.
To prove that the CD40LG219R protein alone could impair immune responses, we examined the capacity of CD40LG219R to provide direct help to B cells in vitro. The ability of CD40L- or CD40LG219R-expressing 3T3 cells to induce proliferation and immunoglobulin CSR of B lymphocytes was analyzed in the presence of interleukin-4 (Figure 6). 3T3-CD40L cells induced proliferation of B cells from healthy donors at day 5 of culture (Figure 6A). With 3T3-CD40LG219R cells, B-cell proliferation was significantly reduced. Significant IgE production by B lymphocytes was triggered by 3T3-CD40L cells compared with wild-type 3T3 cells that expressed no CD40L (Figure 6B). In contrast, 3T3-CD40LG219R cells only induced weak IgE production, not significantly different from wild-type 3T3 cells. The reduced ability of CD40LG219R to trigger CSR was also observed with the B-cell line 2E2 (supplemental Figure 4). Taken together, these data indicate that CD40LG219R has a decreased capacity to activate B cells. Thus, the G219R substitution in CD40L results in a significantly reduced function of the protein in vitro.
Impaired B-cell proliferation and IgE production by the CD40LG219R mutant. (A) In vitro proliferation of B cells from PBMCs of healthy donors was assessed by measuring [3H]thymidine incorporation after a 5-day stimulation in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. *P < .02. (B) Production of IgE by B cells. PBMCs were stimulated to activate CSR to IgE production for 12 days in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. At day 12, production of IgE was measured by enzyme-linked immunosorbent assay in the supernatants of activated B cells. ***P < .001.
Impaired B-cell proliferation and IgE production by the CD40LG219R mutant. (A) In vitro proliferation of B cells from PBMCs of healthy donors was assessed by measuring [3H]thymidine incorporation after a 5-day stimulation in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. *P < .02. (B) Production of IgE by B cells. PBMCs were stimulated to activate CSR to IgE production for 12 days in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. At day 12, production of IgE was measured by enzyme-linked immunosorbent assay in the supernatants of activated B cells. ***P < .001.
Discussion
The clinical phenotypes of affected individuals in the family in the present study are varied and may represent a mix of features associated with CD40L and XIAP deficiencies. Many of the severe manifestations appear rather characteristic of the XIAP deficiency, including splenomegaly associated with cytopenia, severe infectious mononucleosis, and colitis.1,6 However, patient II-7 developed fatal idiopathic liver disease that led to death. Liver disease is commonly observed in patients with CD40L deficiency but generally in the context of Cryptosporidium infections, which was not the case in this patient.13,14 The relative predominance of XLP-2 clinical phenotypes strongly suggests that the mutation in XIAP is the primary cause of the immunodeficiency in these patients. Nevertheless, only 1 patient clearly developed EBV-driven disease with limited signs of hemophagocytic lymphohistiocytosis, which is the first clinical trait in XIAP-deficient patients with null mutations. This may be explained by the hypomorphic nature of the XIAPG466X mutation. Most of the other clinical manifestations were reminiscent of those observed in CD40L deficiency, including recurrent bacterial infections and recurrent non-EBV viral infections, which are not found in XIAP deficiency. In all likelihood, these phenotypes are the result of the expression of the CD40LG219R variant, although they appear to be less severe than those classically observed in patients with CD40L deficiency. However, cells from patients with CD40L mutations that result in diminished expression of CD40L are still able to bind CD40, and these patients have milder clinical manifestations.14 This is in accordance with the nature of the G219R substitution, which partially decreased the binding of CD40L to CD40.
The XIAPG466X mutation alone does not appear to be sufficient to reveal a disease phenotype. Similarly, the CD40LG219R mutation, which was previously identified as a rare polymorphism, appears to have no persistent effect in vivo when expressed alone.21,22 Nevertheless, the CD40LG219R carrier reported in the present study, even though he is well now, had an episode of hypogammaglobulinemia with recurrent infections in infancy, which suggests that CD40LG219R by itself could have a transient effect. However, in the context of XIAPG466X expression, CD40LG219R appears to play a crucial role in the expression of clinical manifestations in this family, because it is only expressed in affected individuals. We propose that in the context of such hypomorphic hemizygous mutation of XIAP, CD40LG219R acts as a second genetic factor required for expression of disease. Conversely, in the context of CD40LG219R expression, XIAPG466X is an additional genetic factor necessary for expression of the clinical manifestations related to CD40L deficiency.
The XIAPG466X mutation results in a truncated protein that lacks the C-terminus portion of the RING domain and exhibits decreased stability. Critical residues for the conformation and the ubiquitin ligase activity of the RING domain, including histidine 467 and cysteines 471, 474, 481, and 484, are missing in the XIAPG466X protein.26 As expected, XIAPG466X has lost its E3 ubiquitin ligase activity but retains the capacity to interact with caspases in vitro. Accordingly, even the weakly expressed XIAPG466X was capable of inhibiting TRAIL-mediated apoptosis but did not block AICD (which depends on Fas). Apoptosis triggered by TRAIL-R in T-cell blasts is always weaker than Fas-mediated apoptosis (or AICD). In this context, the level of activated caspases would be sufficiently low to be blocked by the reduced level of XIAPG466X. The observation that the only individual in the family (II-5) who expressed XIAPG466X (in association with wild-type CD40L) is asymptomatic at 45 years of age indicates that the low expression level of XIAPG466X is sufficient to protect him from clinical disease for the moment and suggests that the E3 ubiquitin ligase activity of XIAP may be dispensable in vivo. However, we cannot exclude that reduced ubiquitination of XIAP targets might also contribute to the disease. Several of these targets, such as TAK-1, MEKK2, and COMMD1, are involved in the capacity of XIAP to activate the NF-κB pathway.4 Although the exact role of XIAP in T and B lymphocytes is not known, it might be required for full NF-κB activation in these cells.
Reduced serum levels of IgG, IgA, and IgM associated with decreased proportions of circulating CD19+CD27+ and switched B cells occurred in 3 of 4 patients with XIAPG466X and CD40LG219R mutations. The youngest patient (III-6) had reduced memory B cells but high serum levels of IgG and IgA for his age; he went on to develop colitis. These immunological defects were not observed in the carriers of single XIAPG466X or CD40LG219R mutations or in patients with XIAP-null mutations.1 Moreover, low IgM levels are not a feature of XHIGM/CD40L deficiency, which is characterized by normal or elevated IgM levels. Taken together, these observations support the hypothesis that low numbers of CD19+CD27+ B cells and hypogammaglobulinemia result from the concomitant expression of XIAPG466X and CD40LG219R in these patients. One hypothesis is that both mutations could cooperate to diminish the survival of CD19+CD27+ B cells, because they are both involved in antiapoptotic and survival functions. We previously reported that XIAP-deficient B/EBV cell lines were more prone to Fas-induced apoptosis, which suggests that XIAP acts as an antiapoptotic molecule in B cells as it does in T cells.1
The fact that defects in the CD40LG and XIAP genes appear to cooperate for disease expression might indicate a functional proximity between these 2 genes. Interestingly, signaling cascades initiated by the interaction of CD40L with CD40 in B cells involved TRAF-2 and TRAF-3, which are regulated by cIAP-1 and cIAP-2, 2 XIAP-related proteins.27-29 These interactions are required for activation of the noncanonical NF-κB pathway. Moreover, interaction of XIAP with cIAP-1 has been documented.30,31 Therefore, it is tempting to speculate that XIAP could be implicated in CD40 signaling in B cells and in other cells that express CD40 (such as APCs). Further experiments will be required to test this possibility. Similarly, cross talk between XIAP and the CD40-CD40L pathway might also occur in T cells. There are some data indicating that CD40-CD40L interactions in T cells could provide active costimulation signals via CD40 or CD40L32,33 ; however, the CD40LG and XIAP mutations could also act independently without being involved in closely related pathways.
The variability of clinical phenotypes in apparently monogenic immunodeficiencies is supposed to reflect the influence of multiple environmental and genetics factors that differ between individuals. The present study provides good evidence that 2 genes normally involved in monogenic diseases as null mutants (with complete penetrance of the disease) can have interplay when partially altered to confer an oligogenic disease. Our results suggest that both the XIAP and CD40L variants are needed for the expression of the various clinical phenotypes in this family, although there must be interplay with other genes or environmental factors to result in this clinical variability. Thus, other diseases in which there is considerable genotype/phenotype variability may also be caused by oligogenic mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and their families for their cooperation. We thank Siraj Misbah, Richard Moxon, and Andrew Pollard for their interest and clinical care of patients II-3, III-2, and III-6, respectively. We also thank Monique Forveille for technical assistance and Robert Weil and Emmanuel Laplantine for technical advice with regard to the ubiquitin ligase assays.
This work was supported by grants from Inserm, Association pour la Recherche sur le Cancer (ARC), and the Agence Nationale de la Recherche (ANR-08-MIEN-012-01) (France); the National Institute for Health Research–Oxford Biomedical Research Center, the Primary Immunodeficiency Association, Baxter Healthcare, and the XLP Research Trust (United Kingdom); and EUROPOLICY (SP23-CT-2005-006411) and EUROPAD (No. 201549) (European Union).
S.L. is a senior scientist of the Centre National de la Recherche Scientifique (CNRS; France), and G.G. is a postdoctoral researcher of the Fond National de la Recherche Scientifique (FNRS; Belgium).
Authorship
Contribution: S.R. performed experiments, analyzed the data, and participated in writing of the report; E.L-G. contributed to the data analysis, patients' care, experiments, and the writing of the report; S.S., G.G., N.L., C.S., C.L., and M.S. performed experiments; C.P., L.F., A.F., J.-L.S., and A.D. contributed to study design, data analysis, and writing of the report; H.C. identified the families, provided clinical information on patients' status, contributed to study design and to the writing of the manuscript, and took care of the patients; and S.L. coordinated the study, designed research, performed experiments, analyzed the data, and wrote the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.R. is Department of Immunology and Microbial Science, The Scripps Research Institute, La Jolla, CA. The current affiliation for E.L.-G. is Servicio de Inmunología, Hospital Universitario La Paz, Madrid, Spain. The current affiliation for S.S. is Inserm U945, Hôpital Pitié Salpêtrière, Paris, France. The current affiliation for M.S. is Department of Infection, Immunity and Biochemistry, Cardiff University, Cardiff, United Kingdom.
Correspondence: Sylvain Latour, Laboratoire du Développement Normal et Pathologique du Système Immunitaire, Inserm Unité 768, Hôpital Necker Enfants-Malades, 149 rue de Sèvres, F-75015 Paris, France; e-mail: sylvain.latour@inserm.fr.
References
Author notes
S.R. and E.L.-G. contributed equally to this study.






![Figure 6. Impaired B-cell proliferation and IgE production by the CD40LG219R mutant. (A) In vitro proliferation of B cells from PBMCs of healthy donors was assessed by measuring [3H]thymidine incorporation after a 5-day stimulation in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. *P < .02. (B) Production of IgE by B cells. PBMCs were stimulated to activate CSR to IgE production for 12 days in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. At day 12, production of IgE was measured by enzyme-linked immunosorbent assay in the supernatants of activated B cells. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/2/10.1182_blood-2011-01-328849/4/m_zh89991173530006.jpeg?Expires=1761824523&Signature=0kWXwYKdSK7l-LqXhVZiyZig~kI-7C55zZ7m68YIc-4BBZWLJDFHbWKNF7yEyFodHwq2vvh8yX-MCW-n2wF7fGLICtEZwLayPLCN74Ts3NIOvbConQKxtpr6coc55VTMTiRJ9RNkkIdspaGcKNnfE32JEI-KQNTPhP-4scWvmhFaQ3rtSvWVXrI6OkzeJUCuD0tNtYttiy2kVtlxcJXu-xOXYVD4QusPpp66XplMSb4El3PxBX7~ly2H~bbc8ZrG1uG02j6HLTnExXLPcrmfGcY1wzQKjXdQurJ3ylXGrvqpT-xDlDDJiJlN-cvNUJmEZabr1-nSXJTzwuZ28GQCTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 6. Impaired B-cell proliferation and IgE production by the CD40LG219R mutant. (A) In vitro proliferation of B cells from PBMCs of healthy donors was assessed by measuring [3H]thymidine incorporation after a 5-day stimulation in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. *P < .02. (B) Production of IgE by B cells. PBMCs were stimulated to activate CSR to IgE production for 12 days in the presence of interleukin-4 and irradiated 3T3 cells (3T3), 3T3-expressing CD40L, or 3T3-expressing CD40LG219R. At day 12, production of IgE was measured by enzyme-linked immunosorbent assay in the supernatants of activated B cells. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/2/10.1182_blood-2011-01-328849/4/m_zh89991173530006.jpeg?Expires=1762670811&Signature=3BuvtRfiHMlfqUI7un6odJEXpGQ2bWxUXzB5Mv7j~PbQpdOsRir02k38Sa9Mg92mMmT0oPCTUdf9bdc9CyqPAohIxq4LxuMuiG3vZEJUUxcIAsQ--bR-U~q6pe5p~AeLCXRlZesBL7wQJXrs-nHkml02ta2dvP0IIZrfbzlaT96tTp6d4nEI7t8y9fvyCZBz9TDL7u~4oYui5bLyqYE-5pM798EGilIMpNyqy618qgfASh0BRBqks7draGNiwhfIXvzBGmU49rPr~jWywUBsaRzRNnQMiliFlMJTXJNKojcP0WEGgqXrAkJHiosjzFzkm3HjOV7Z7OlgLKeULvnU2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)