With the success of the anti-CD20 mAb rituximab in treating B-cell disorders from lymphoma through rheumatoid arthritis, anti-CD20 mAbs are now big business with as many as 16 biosimilars in clinical development.1 Understanding what makes CD20 such an ideal target and how to select new anti-CD20 mAbs is the subject of intense research.
Functional differences among anti-CD20 mAbs have been known for decades (reviewed in Tedder and Engel2 ), but the molecular basis behind this has remained obscure until now. In this issue of Blood, Niederfellner and colleagues define the binding site for the next generation of anti-CD20 mAb, obinutuzumab (GA101).3 More than revealing a simple mAb:target structural relationship, this work may explain the decades-long conundrum of how mAbs recognising a common epitope can possess distinct functional activity and therapeutic potential.
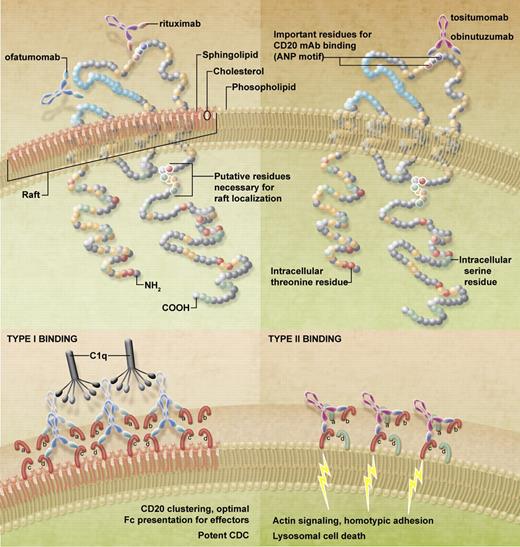
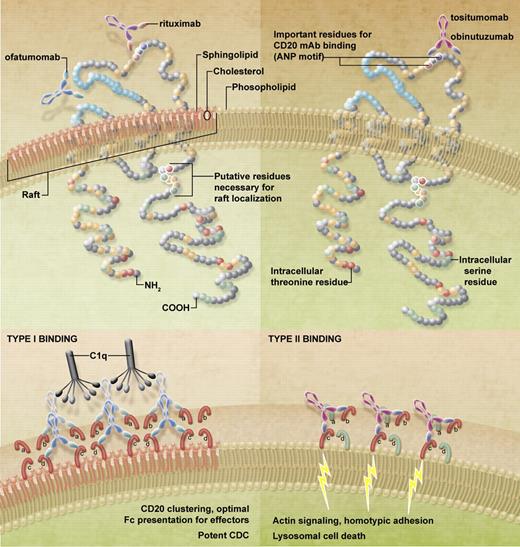
CD20 mAb come in 2 flavors, type I such as rituximab, IF5 (the first anti-CD20 mAb to be tested clinically), and the recently approved ofatumumab (arzerra), which redistributes CD20 into detergent-resistant lipid rafts; and type II such as tositumumab (B1) and obinutuzumab, which do not (see figure).4 The clustering by type I reagents promote association with other complexes such as the B-cell receptor (BCR), and binding to C1q, resulting in potent complement-dependent cytotoxicity (CDC). In contrast, type II mAbs trigger potent homotypic adhesion and lysosomal cell death.5,6 Importantly, both recruit FcR-expressing cells to mediate cellular effector functions such as antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. Therefore, proof of which of these delivers the most potent activity in patients is keenly awaited, especially as the type II mAbs have outperformed rituximab in a number of preclinical models.
Binding of type I and II anti-CD20 mAbs. In the top panels the binding sites of the clinically relevant type I (rituximab, ofatumumab) and type II (obinutuzumab, tositumomab) mAbs are shown. As can be seen, the fully human ofatumumab binds to a discontinuous region of CD20 apparently composed of both the smaller CD20 loop and the N-terminal region of the larger loop, unlike the other mAbs, which cluster around the ANP motif. At the bottom is a hypothesis of how these mAbs might bind to CD20 in the plasma membrane that may serve to explain their differing effects, such as the 2:1 binding ratio, raft accumulation, and subsequent effector functions. Accordingly, type I mAbs bind and interact with closed conformations of CD20 (red loops, labeled a-d) and are able to occupy all available binding sites. If these mAbs are favorably orientated to bind between tetramers, this would facilitate clustering and aggregation, suitable for raft accumulation, optimal C1q binding, and efficient CDC. In contrast, type II mAbs bind the first molecule of CD20 and are subsequently constrained for binding to an adjacent molecule, possibly within the same tetramer, potentially through the interaction with Asn 171 and opening of CD20 molecules (red and green loops). Intra-tetrameric binding will not facilitate large-scale aggregation and will preclude further binding to the same CD20 tetramer, thus causing half-maximal binding in comparison with type I mAbs. Both the reduced binding and lack of raft accumulation serve to limit C1q binding and impotent CDC. However, binding to the open CD20 may be the trigger for signaling to actin, resulting in homotypic adhesion and lysosomal cell death.
Binding of type I and II anti-CD20 mAbs. In the top panels the binding sites of the clinically relevant type I (rituximab, ofatumumab) and type II (obinutuzumab, tositumomab) mAbs are shown. As can be seen, the fully human ofatumumab binds to a discontinuous region of CD20 apparently composed of both the smaller CD20 loop and the N-terminal region of the larger loop, unlike the other mAbs, which cluster around the ANP motif. At the bottom is a hypothesis of how these mAbs might bind to CD20 in the plasma membrane that may serve to explain their differing effects, such as the 2:1 binding ratio, raft accumulation, and subsequent effector functions. Accordingly, type I mAbs bind and interact with closed conformations of CD20 (red loops, labeled a-d) and are able to occupy all available binding sites. If these mAbs are favorably orientated to bind between tetramers, this would facilitate clustering and aggregation, suitable for raft accumulation, optimal C1q binding, and efficient CDC. In contrast, type II mAbs bind the first molecule of CD20 and are subsequently constrained for binding to an adjacent molecule, possibly within the same tetramer, potentially through the interaction with Asn 171 and opening of CD20 molecules (red and green loops). Intra-tetrameric binding will not facilitate large-scale aggregation and will preclude further binding to the same CD20 tetramer, thus causing half-maximal binding in comparison with type I mAbs. Both the reduced binding and lack of raft accumulation serve to limit C1q binding and impotent CDC. However, binding to the open CD20 may be the trigger for signaling to actin, resulting in homotypic adhesion and lysosomal cell death.
Although the seminal work of Deans demonstrated that the majority of anti-CD20 mAbs (including rituximab and tositumomab) bind through the same alanine-N-proline (ANP) motif at residues 170-172 in the large extracellular loop,7 Niederfellner and colleagues demonstrate that obinutuzumab along with other type II mAbs bind a subtly different motif. Using pepscan analysis and mutation studies they demonstrate that type II mAbs bind predominantly to the carboxy-terminal side of the ANP motif and the type I bind more to amino-terminal. This subtle shift results in an entirely different mode of binding (admittedly to cyclic CD20 peptide, rather than native CD20) with a significantly altered orientation and elbow angle, probably through interaction with Asn171. With type I, Asn171 is central to the interaction interface but with obinutuzumab it is peripheral. In fact, it likely places a steric constraint on obinutuzumab binding that may elicit a preference for certain subpopulations/orientations of CD20 molecules, which was confirmed using 3-dimensional protein tomography and confocal microscopy. Moreover, these experiments also confirmed the well-known observation that type II mAbs bind approximately half as many CD20 molecules at the cell surface as type I mAbs.
How do we assimilate these new findings into a model of how type I and II mAbs bind, orientate, and induce their effector functions? The characteristic 2:1 ratio could, as the authors speculate, either reflect the relative abundance of different CD20 subpopulations recognized by each type of mAb or specific steric requirements that affect their binding. Given the almost universal 2:1 binding relationship on many different cell lines and cell types (which may well differ in their relative proportion of open and closed CD20 molecules), and the fact that type II mAbs apparently see both forms, it would seem that the latter possibility is more likely. However, to exclude the former hypothesis it would be helpful to assess the relative proportions of the different CD20 conformations in a wide variety of CD20-expressing cell types (both resting and after activation, say with IL-4 or BCR stimulation) and correlate this with type I:II binding and function.
In contrast, constrained binding through Asn171 may explain the majority of current observations. First, it may force CD20 into the open conformation only seen with type II mAbs. Second, it may preclude subsequent binding of mAbs to adjacent CD20 molecules, thereby potentially explaining the reduced binding with type II, perhaps by favoring intra- rather than intertetramer binding (see figure). Third, the constrained binding and altered orientation may also explain the associated effector functions of type I and II mAbs as the resulting angle of orientation of the Fab regions could restrict the accessibility for Fc-binding to effector molecules. For example, type II mAb efficacy in CDC may be further diminished, not only because of a lack of raft redistribution and clustering, but also because of an unfavorable orientation of the presented Fc region. Interestingly, we have preliminary evidence that type I mAbs interact with the inhibitory FcγRIIb at the cell surface (resulting in its activation) whereas type II mAbs do not engage FcγRIIb or elicit its activation (Lim et al, submitted) which may imply that engagement of FcR is also compromised by the type II binding mode (at least in this cis-interaction on the same cell surface). Furthermore, the open conformation CD20 binding evoked by type II mAbs may provides a clue toward their association with actin reorganization, homotypic adhesion and lysosomal cell death.
The next question that arises is what about ofatumumab? Ofatumumab is a fully human type I anti-CD20 mAb that binds a completely different, discontinuous epitope involving the smaller extracellular loop of CD20 and an amino-terminal region of the large loop at position 159-166.8 How then does it achieve its type I nature? Unfortunately, no mAb:CD20 structural information is available so we can only speculate that the type I nature of anti-CD20 mAbs is largely influenced by binding to the amino-terminal section of CD20. A secondary prediction is that it would bind only closed conformers of CD20, but this remains to be seen.
In summary, just like in the time warp, type I mAbs take a jump to the left and type II mAbs take a step to the right, and this small distinction in binding appears sufficient to markedly change the orientation of binding and underpin the functional diversity evoked by these different mAbs. Hopefully, we will not need to go through another decades-long time warp before opening the next chapter in our understanding of this intriguing target molecule CD20.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■