Abstract
von Willebrand disease (VWD) is a common bleeding disorder, but diagnosis is sometimes challenging because of issues with the current von Willebrand factor (VWF) assays, VWF antigen (VWF:Ag) and VWF ristocetin cofactor activity (VWF:RCo), used for diagnosis. We evaluated 113 healthy controls and 164 VWD subjects enrolled in the T.S. Zimmerman Program for the Molecular and Clinical Biology of VWD for VWF:Ag, VWF:RCo, and a new enzyme-linked immunosorbent assay (ELISA)–based assay of VWF-glycoprotein Ib (GPIb) interactions using a gain-of-function GPIb construct (tGPIbα235Y;239V) as a receptor to bind its ligand VWF in an assay independent of ristocetin (VWF:IbCo ELISA). Healthy controls, type 1, 2A, 2M, and 2N subjects had VWF:RCo/VWF:Ag ratios similar to the ratio obtained with VWF:IbCo ELISA/VWF:Ag. Type 2B VWD subjects, however, had elevated VWF:IbCo ELISA/VWF:Ag ratios. Type 3 VWD subjects had undetectable (< 1.6 U/dL) VWF:IbCo ELISA values. As previously reported, VWF:RCo/VWF:Ag ratio was decreased with a common A1 domain polymorphism, D1472H, as was direct binding to ristocetin for a 1472H A1 loop construct. The VWF:IbCo ELISA, however, was not affected by D1472H. The VWF:IbCo ELISA may be useful in testing VWF binding to GPIb, discrimination of type 2 variants, and in the diagnosis of VWD as it avoids some of the pitfalls of VWF:RCo assays.
Introduction
von Willebrand disease (VWD) is a common bleeding disorder caused by defects in von Willebrand factor (VWF). Patients with VWD have a spectrum of bleeding symptoms, ranging from mild mucosal bleeding to severe menorrhagia and joint bleeding in the most severe forms. Diagnosis of VWD relies on a personal history of bleeding symptoms, family history of either bleeding symptoms or diagnosed VWD, and abnormal VWF laboratory tests.1 The latter category is sometimes challenging to fulfill, given the variability in current VWD diagnostic testing. Several laboratory tests are required to either confirm or exclude the diagnosis. VWF antigen (VWF:Ag) measures total protein but may be normal in type 2 VWD. VWF function is typically measured by the VWF ristocetin cofactor activity assay (VWF:RCo), which uses ristocetin as an in vitro agonist to stimulate VWF-platelet glycoprotein Ib (GPIb) interactions. This assay, however, has a relatively high coefficient of variation (CV), with values of up to 50% between laboratories.2,3 Additional tests include VWF multimer distribution to evaluate for loss of high molecular weight multimers, VWF collagen binding, VWF propeptide to evaluate for clearance defects, and factor VIII (FVIII) activity.4-7
Diagnosis of type 2 VWD, in particular types 2A, 2B, and 2M, relies on a discrepancy between VWF activity, as measured by the VWF:RCo (and/or VWF:CB), and VWF protein, as measured by the VWF:Ag. Although collagen binding is one aspect of VWF function in vivo and may provide valuable adjunct information in diagnosis of VWD variants, the VWF:CB cannot replace the VWF:RCo assay as a measure of VWF-platelet interactions.8 Current laboratory testing evaluates abnormal VWF-platelet interactions solely through the VWF:RCo assay. The VWF:RCo assay is only useful to screen for decreased VWF interactions with platelet GPIb and provides no assessment of gain-of-function variants, such as type 2B VWD. In addition, the high CV with this test raises the possibility of misdiagnosis, particularly involving the type 2 VWD variants.2,3 We have previously reported that a VWF A1 domain mutation, P1467S, leads to a decrease in VWF:RCo and absent ristocetin-induced platelet aggregation, but the index case with this mutation has no history of bleeding. The location of this mutation in a known ristocetin-binding region suggests that ristocetin-based assays may be affected without disrupting in vivo function of the P1467S variant.9 In addition, an A1 domain polymorphism, D1472H, is associated with lower VWF:RCo/VWF:Ag ratios in healthy controls.10 Alternate assays of VWF-platelet, or VWF-GPIb, interactions, particularly ones with a lower CV, could improve diagnosis of VWD and avoid potential errors associated with ristocetin-based assays.
We sought to evaluate VWF levels in healthy controls, type 1, type 2, and type 3 VWD subjects enrolled in the T.S. Zimmerman Program for the Molecular and Clinical Biology of von Willebrand Disease (ZPMCB-VWD). VWF:Ag and VWF:RCo were examined for all subjects. In addition, an alternate assay of VWF function was performed using a gain-of-function GPIbα construct, derived from mutations seen in platelet-type VWD, to enable spontaneous binding without the need for ristocetin. This assay demonstrates good reproducibility and correlates well with the current laboratory assays used in VWD diagnosis, the VWF:Ag and VWF:RCo. In addition, this assay does not display abnormal results with polymorphisms in the VWF ristocetin binding domain, suggesting more accurate assessment of VWF activity in the absence of ristocetin.
Methods
Patient population
Healthy control subjects were recruited from 8 primary centers in Milwaukee, Atlanta, Detroit, Houston, Indianapolis, Iowa City, New Orleans, and Pittsburgh. VWD subjects were recruited from those primary centers along with multiple secondary centers throughout the United States (listed in the acknowledgments). VWD subjects all had a preexisting diagnosis of VWD. Healthy controls were enrolled from local institutions and communities. This study was approved by each institution's Human Research Review Board, and all subjects gave informed consent in accordance with the Declaration of Helsinki. All enrolled subjects received a detailed bleeding questionnaire, including questions from the European Molecular and Clinical Markers for the Diagnosis and Management of type 1 VWD study.11
VWF testing
Plasma from each subject was assayed using standard methods by the Hemostasis Reference Laboratory at BloodCenter of Wisconsin for VWF:Ag, VWF:RCo, FVIII activity, VWF:CB, VWFpp, and multimer analysis as previously described.10 Platelet binding assays were performed on all suspected type 2B VWD index cases using a modification of the previously described “neutral” monoclonal antibody binding assay.12 In the 2B binding assay, ristocetin at a concentration of 0.3 mg/mL was added to formalin-fixed washed platelets to identify samples with markedly increased binding of plasma VWF. For evaluation of type 2N VWD subjects, FVIII-binding activity was measured using plasma VWF bound to a monoclonal antibody as previously described.13 DNA was collected for VWF genotyping on all healthy controls and VWD index cases. Gene sequencing of the VWF coding region, including intron-exon boundaries, was performed as previously described.10 Sequencing also included 3.5 kb upstream of exon 1 and 1 kb downstream of the C-terminal stop codon. Primer sequences are available on request.
VWF:IbCo ELISA
A gain-of-function GPIbα construct (tGPIbα235Y;239V) was synthesized containing 2 mutations, D235Y and M239V, with a histidine tag for purification and a C65A mutation to prevent dimerization. D235Y is a novel mutation,14 and M239V has been previously documented to lead to increased VWF binding.15 The construct extends from nucleotide 85 through 960 (amino acid 290) based on the sequence of Lopez et al.16 A similar construct with the wild-type GPIbα sequence was also synthesized. The tGPIbα235Y;239V construct was expressed in S2 insect cells, and supernatant was collected and purified over a nickel column (GE Healthcare). A monoclonal antibody against human GPIbα was bound to a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Immulon 4 HBX, ThermoScientific) and incubated at 4°C overnight. The tGPIbα235Y;239V construct was then added and incubated at room temperature for 1 hour. Diluted plasma was added as the VWF source and also incubated at room temperature for 1 hour. No ristocetin was used in this assay. A mixture of biotinylated monoclonal anti-VWF antibodies (AVW-1 and AVW-15) was used to detect the presence of VWF. Streptavidin-conjugated alkaline phosphatase and p-nitrophenyl phosphate was added and the optical density measured on an ELISA reader. Lyophilized, reconstituted normal control plasma calibrated to the World Health Organization standard was used to construct the reference curve. The stability of the VWF-GPIbα interaction was assessed by varying incubation times and by varying the number of washes used between each step. The VWF:IbCo ELISA results reported here use 3 washes unless otherwise specified. For assays using the wild-type GPIbα, 1 mg/mL ristocetin was added along with the VWF.
ELISA reproducibility studies
Repeatability testing was initially performed using a lyophilized, reconstituted normal control plasma sample. Aliquots were frozen, and thawed on the day of testing. The control sample was run 19 times, each on a freshly prepared plate. The same laboratory technician performed each assay. Further repeatability testing was performed on a set of normal control samples from the ZMPCB-VWD with varying VWF:Ag levels, including a sample with VWF:Ag of 20 IU/dL. Assays were performed across 7 days to examine day-to-day variability. Two different technicians performed a total of 13 assays to examine technician-to-technician variability.
Statistics
The statistical program Stata (StataCorp LP) was used to perform statistical analyses. Comparisons of mean VWF:Ag, VWF:RCo, and VWF:RCo/VWF:Ag ratios used Student t test. VWF:Ag and VWF:RCo values were log-transformed to achieve a normal distribution before analysis. Multiple linear regression analysis was used to compare VWF:Ag, VWF:RCo, and VWF:IbCo ELISA results. One-way and 2-way mixed-effect analyses of variance were used to estimate the magnitude of variability of day and technician for each data point with the VWF:IbCo ELISA. Because the variability depended on whether VWF:Ag was high, low, or normal, separate analyses of variance were run for each VWF:Ag level (from 14 IU/dL to 168 IU/dL). Technician-to-technician variability was determined after removing the effect of the day-to-day variability.
VWF:IbCo ELISA multimer distribution
To determine whether VWF binding to the tGPIbα235Y;239V construct was primarily with high molecular weight multimers, similar to the VWF:RCo assay, a modified VWF:IbCo ELISA was performed using a lyophilized, reconstituted normal control plasma sample. Aliquots of the control plasma were diluted in phosphate-buffered saline with 1% bovine serum albumin and applied to ELISA wells. The samples were incubated at room temperature for 1 hour, and the supernatant aspirated and applied to a subsequent well for a total of 5 applications. After removal of the supernatant, the wells were then washed. The VWF that was bound to the captured tGPIbα235Y;239V was then eluted from the well with 5% sodium dodecyl sulfate at 95°C. The supernatant was saved from the final well to determine multimer distribution of VWF still remaining in the supernatant. VWF multimer distribution was also examined using a wild-type GPIbα construct with the addition of ristocetin at 1 mg/mL to induce binding. All samples were electrophoresed in a 0.65% agarose gel containing 25% Tris-glycine and 0.1% lithium dodecyl sulfate and blotted onto an Immobilon-P membrane (Millipore). VWF was detected using a polyclonal rabbit antihuman VWF antibody (Dako North America) and horseradish peroxidase-conjugated goat antirabbit IgG (ThermoScientific). The membrane was developed with SuperSignal Chemiluminescent Substrate (ThermoScientific) and exposed to BioMax film (Kodak) for visualization.
ELISA to measure VWF A1 loop binding to ristocetin
VWF A1 domain constructs were synthesized to include the signal peptide sequence and the entire A1 domain from nucleotide 3724 (amino acid 1242) through 4434 (amino acid 1478) in a pcDNA3.1 vector with a myc epitope and a histidine tag (Invitrogen). Two mutant constructs, 1472H and 1467S, were also synthesized. Maleic anhydride plates (ThermoScientific) were used to capture ristocetin (American Biochemical and Pharmaceutical Ltd), plated at a concentration of 1 mg/mL in carbonate coating buffer (15mM sodium carbonate and 35mM sodium bicarbonate), and incubated overnight at 4°C. An ELISA assay was performed using these VWF A1 domain constructs, which were incubated at room temperature for 60 minutes. Detection was with a mouse anti-His antibody (AbD Serotec). The VWF A1 domain constructs were also compared using a VWF ELISA assay to determine the amount present with a mouse anti-myc capture antibody (9E10, produced from a cell line grown at the Blood Research Institute) and mouse anti-His detection antibody (AbD).
Results
VWF:IbCo ELISA in healthy control subjects
We first examined the relationship between VWF:Ag, VWF:RCo, and the VWF:IbCo ELISA in 113 healthy controls. The mean VWF:Ag was 109 IU/dL and the mean VWF:RCo was 95 IU/dL. As this group included 50 African American subjects and 63 white subjects, the increased proportion of African American subjects probably accounts for the elevated VWF:Ag, which has been observed in previous studies comparing African American and white VWF levels.10,17 Table 1 lists the VWF results for these and additional tests of VWF function, including VWF:CB and VWFpp. The VWF:IbCo ELISA, using a gain-of-function GPIbα construct, tGPIbα235Y;239V, resulted in a mean of 102 U/dL (Figure 1). There was no significant difference between VWF:Ag and the VWF:IbCo ELISA (P = not significant). The difference between VWF:Ag and VWF:RCo, however, was statistically significant (P < .005).
VWF assays in healthy control subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 113 healthy control subjects enrolled in the ZPMCB-VWD. The line indicates the geometric mean for each assay.
VWF assays in healthy control subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 113 healthy control subjects enrolled in the ZPMCB-VWD. The line indicates the geometric mean for each assay.
The VWF:IbCo ELISA demonstrated a low CV when a control plasma sample was analyzed on repeated occasions (n = 19). The mean VWF:IbCo value for this sample was 78, with an SD of 5 and CV of 7% (Table 2). To reproduce the conditions of a reference laboratory more closely, the VWF:IbCo ELISA was performed by 2 different technicians daily for a total of 13 measurements using 4 VWF samples encompassing both low and high VWF:Ag. The CV for those samples with normal VWF:Ag (60-100 IU/dL) was 8.6% to 10.4%. The highest variance was seen at the lowest and highest VWF levels (Table 2). Similar results were observed with an ELISA using a wild-type truncated GPIbα construct with 1 mg/mL ristocetin added along with the VWF, where CVs again varied from 6% to 21% with the highest CV obtained using the sample with the lowest VWF:Ag. The only difference observed between the technicians was in VWF:IbCo ELISA results at the lowest level of VWF tested, attributed to the low level of VWF in that sample. It should be noted that interlaboratory variability for the ELISA assays may be greater; however, the low intralaboratory variability suggests that the VWF:IbCo ELISA will yield reasonable reproducibility across different laboratory settings. The ELISA plates were each coated individually the day before use. Batch-produced precoated plates might be expected to remove or reduce the plate-to-plate variability from the coating step. There was no difference in day-to-day variability between technicians.
ELISA stability was first evaluated by increasing the number of washes from 3 to 6 and then by decreasing from 3 to 1 or 2 washes after each step. There was no difference in VWF binding detected regardless of the number of washes (P > .05 comparing 1, 2, or 6 washes to 3 washes). In addition, VWF incubation times were varied from 30 minutes to 120 minutes, again without detectable differences in final VWF level bound to GPIbα. The lower limit of detection of the VWF:IbCo ELISA was 1.6 U/dL of VWF. This contrasts favorably to the VWF:RCo, which in our clinical laboratory has a lower limit of detection of 10 IU/dL and in many clinical laboratories is 20 IU/dL.
VWF:IbCo ELISA preferentially binds high-molecular-weight VWF multimers
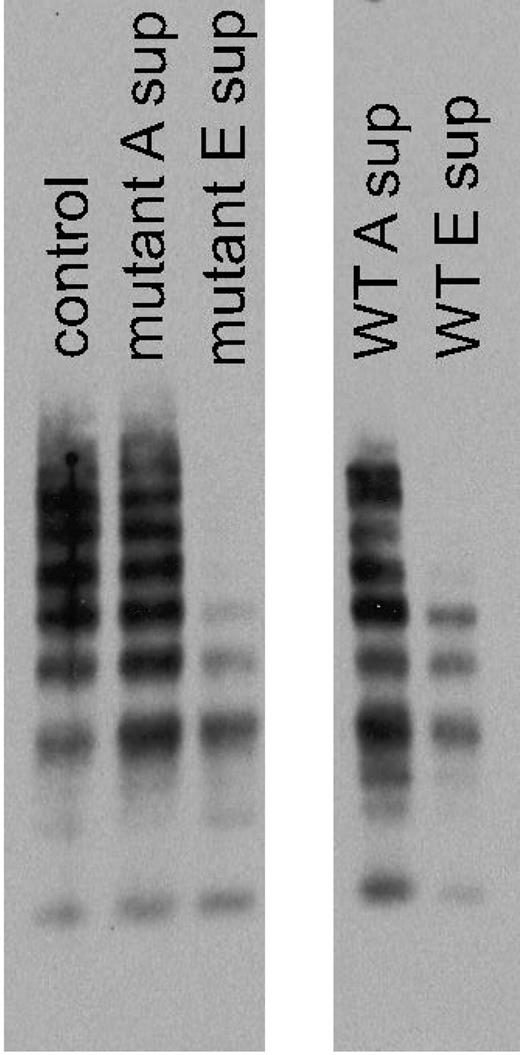
To determine what form of VWF was binding to the captured tGPIbα235Y;239V in the VWF:IbCo ELISA assay, serial aspirations of unbound supernatant were performed, adding each successive supernatant to a new well coated with tGPIbα235Y;239V. Multimer analysis was then performed to evaluate the VWF multimer distribution present in each supernatant. Figure 2 shows that the VWF:IbCo ELISA format preferentially binds high-molecular- weight multimers. The unbound supernatant shows loss of high- molecular-weight multimers, whereas the bound substrate consists primarily of the high-molecular-weight multimer forms. Compared with an ELISA using wild-type GPIbα and ristocetin, both have the same propensity for high-molecular-weight multimers. The wild-type construct did not demonstrate any VWF binding in the absence of ristocetin (data not shown).
VWF multimers from VWF:IbCo ELISA. Serial VWF:IbCo ELISA measurements were performed and the supernatants analyzed for multimer distribution. A control plasma sample is shown in the first lane, followed by the supernatant from the initial ELISA well for the tGPIbα235Y;239V construct (mutant A sup) and the supernatant after 5 serial incubations with tGPIbα235Y;239V (mutant E sup) in the absence of ristocetin. The last wells show the supernatant from the initial ELISA well for the wild-type GPIbα construct (WT A sup) and the supernatant after 5 serial incubations with wild-type GPIbα (WT E sup) in the presence of 1 mg/mL ristocetin.
VWF multimers from VWF:IbCo ELISA. Serial VWF:IbCo ELISA measurements were performed and the supernatants analyzed for multimer distribution. A control plasma sample is shown in the first lane, followed by the supernatant from the initial ELISA well for the tGPIbα235Y;239V construct (mutant A sup) and the supernatant after 5 serial incubations with tGPIbα235Y;239V (mutant E sup) in the absence of ristocetin. The last wells show the supernatant from the initial ELISA well for the wild-type GPIbα construct (WT A sup) and the supernatant after 5 serial incubations with wild-type GPIbα (WT E sup) in the presence of 1 mg/mL ristocetin.
D1472H and P1467S polymorphisms do not affect the VWF:IbCo ELISA
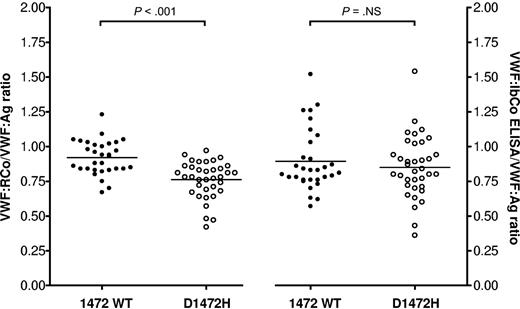
Polymorphisms in VWF have been shown to affect the VWF:RCo assay. We have previously shown that the D1472H polymorphism is associated with a decreased VWF:RCo/VWF:Ag ratio, and that a gain-of-function GPIb assay based on flow cytometry to measure VWF-GPIb interactions did not show any difference related to presence or absence of D1472H.10 The VWF:IbCo ELISA did not differ between the D1472H subjects and those without the single nucleotide polymorphism (Figure 3). A significant difference was again seen in VWF:RCo/VWF:Ag ratios, with a mean ratio of 0.76 for the subjects with the D1472H polymorphism and a mean ratio of 0.92 for those without the polymorphism (P < .001). However, the VWF:IbCo ELISA/VWF:Ag ratios were not significantly different, with a mean ratio of 0.85 for those with the D1472H polymorphism and a mean ratio of 0.89 for those with the wild-type VWF A1 domain sequence (P = not significant). Removal of ristocetin from VWF assays may be useful when mutations or polymorphisms affect the ability of VWF to interact with the ristocetin itself while preserving the VWF-platelet interaction. Of note, subjects with another A1 domain polymorphism, A1381T, were excluded from this analysis as previous work by Szanto et al has shown this mutation to be associated with an increase in VWF:RCo/VWF:Ag ratios.18 When 34 subjects with A1381T were examined separately, they did indeed have an elevated VWF:IbCo ELISA/VWF:Ag ratio, with a mean VWF:RCo/VWF:Ag ratio of 1.02 and a mean VWF:IbCo ELISA/VWF:Ag ratio of 1.12 (P < .001 compared with 31 subjects without either D1472H or A1381T).
Effect of D1472H polymorphism on VWF:RCo and VWF:IbCo ELISA assays. VWF:RCo/VWF:Ag and VWF:IbCo ELISA/VWF:Ag are shown for wild-type A1 domain subjects (●) compared with subjects with the D1472H polymorphism (○). The line indicates the mean for each assay.
Effect of D1472H polymorphism on VWF:RCo and VWF:IbCo ELISA assays. VWF:RCo/VWF:Ag and VWF:IbCo ELISA/VWF:Ag are shown for wild-type A1 domain subjects (●) compared with subjects with the D1472H polymorphism (○). The line indicates the mean for each assay.
D1472H polymorphism affects VWF binding to ristocetin
To evaluate the effect of the D1472H polymorphism on VWF-ristocetin interactions, a direct binding assay was developed to evaluate A1 loop binding to ristocetin rather than A1 loop binding to platelets. The wild-type A1 domain construct was compared with a 1472H construct and a 1467S construct previously described to have a significant reduction in ristocetin-based assays of VWF function.9 A1 loop constructs were used to avoid potential interactions of ristocetin with other binding sites present in the full-length VWF sequence. The 1472H construct had reduced binding to ristocetin, at 77% of that seen with the wild-type construct (P < .001). The 1467S construct had an even greater reduction in binding, at 51% of wild-type (P < .0005). These results suggest that the decrease in VWF:RCo may be directly attributable to a decrease in the ability of VWF containing the D1472H polymorphism to interact with ristocetin.
Type 1 VWD
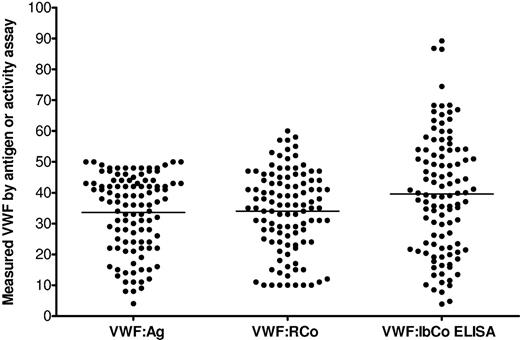
We then examined results for ZPMCB-VWD subjects with type 1 VWD. Analysis is restricted here to subjects whose VWF:Ag was less than or equal to 50 IU/dL on the sample obtained at enrollment. Figure 4 shows results comparing VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for the 107 type 1 subjects analyzed. As the lower limit of detection for the VWF:RCo in our clinical laboratory was 10 IU/dL, all subjects with VWF:RCo < 10 were analyzed using a value of 5 for statistical purposes. The mean VWF:Ag was 34 IU/dL, the mean VWF:RCo was 34 IU/dL, and the mean VWF:IbCo ELISA was 40 U/dL (Table 3). We thought that the increased mean VWF:IbCo ELISA might be the result of inclusion of subjects with type 1C VWD, but no significant difference was seen in VWF:IbCo ELISA/VWF:Ag ratio when those subjects with a VWFpp/VWF:Ag ratio more than or equal to 2 were compared with those with a ratio less than 2 (P = not significant).
VWF assays in type 1 VWD subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 107 type 1 VWD subjects (all with VWF:Ag ≤ 50) enrolled in the ZPMCB-VWD. The line indicates the mean for each assay. All VWF:RCo values reported as less than 10 IU/dL are graphed at 10 IU/dL for visualization purposes.
VWF assays in type 1 VWD subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 107 type 1 VWD subjects (all with VWF:Ag ≤ 50) enrolled in the ZPMCB-VWD. The line indicates the mean for each assay. All VWF:RCo values reported as less than 10 IU/dL are graphed at 10 IU/dL for visualization purposes.
Type 2 VWD
There were 18 type 2A and 11 type 2B subjects available for analysis. All type 2B subjects had known type 2B mutations on DNA sequencing as well as abnormal VWF platelet binding. All type 2A subjects included here had loss of high-molecular-weight multimers in our laboratory or were found to have a known type 2A mutation. The mean VWF:Ag for the type 2A subjects was 29 IU/dL, the mean VWF:RCo was 10 IU/dL, and the mean VWF:IbCo ELISA was 13 U/dL. The true mean VWF:RCo is difficult to calculate as many of the type 2A VWD subjects had VWF:RCo less than 10. For calculation purposes, a value of 5 was used for each sample where the VWF:RCo was less than 10, but the true mean may be even lower for this subgroup. For the type 2B subjects, the mean VWF:Ag was 31 IU/dL, the mean VWF:RCo was 19 IU/dL, and the mean VWF:IbCo ELISA was 81 U/dL (Table 3). The increase in VWF:IbCo ELISA compared with the traditional VWF:RCo may represent “activated VWF,” similar to that shown by Hulstein et al using the AU/VWFa-11 nanobody.19 Figure 5 compares the type 2A and type 2B VWD results. The type 2A VWD subjects appear to cluster in 2 groups, with one set having low VWF:IbCo ELISA/VWF:Ag ratio and one set with a higher ratio. Three of the type 2A subjects with low VWF:IbCo ELISA/VWF:Ag ratios have the R1597W mutation, a known type 2A group 2 mutation. As we have previously published, the group 2 mutations have decreased high-molecular- weight multimers.20 The lower VWF:IbCo ELISA/VWF:Ag ratios in this group may reflect multimer size. Further investigation into this phenomenon is ongoing.
VWF assays in type 2 VWD subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 18 type 2A (●) and 11 type 2B subjects (○) enrolled in the ZPMCB-VWD. The line indicates the mean for each assay. All VWF:RCo values reported as less than 10 IU/dL are graphed at 10 IU/dL for visualization purposes.
VWF assays in type 2 VWD subjects. Results for the VWF:Ag, VWF:RCo, and VWF:IbCo ELISA for 18 type 2A (●) and 11 type 2B subjects (○) enrolled in the ZPMCB-VWD. The line indicates the mean for each assay. All VWF:RCo values reported as less than 10 IU/dL are graphed at 10 IU/dL for visualization purposes.
There were 6 type 2M patients enrolled in the ZPMCB-VWD with VWF:RCo/VWF:Ag ratios less than 0.7 and with normal multimers. The mean VWF:Ag for the type 2M subjects was 47 IU/dL, the mean VWF:RCo was 19 IU/dL, and the mean VWF:IbCo ELISA was 14 U/dL. There were 5 type 2N patients enrolled in the ZPMCB-VWD with decreased VWF-factor VIII binding and/or homozygous type 2N mutations. The mean VWF:Ag for the type 2N subjects was 63 IU/dL, the mean VWF:RCo was 74 IU/dL, and the mean VWF:IbCo ELISA was 88 U/dL (Table 3).
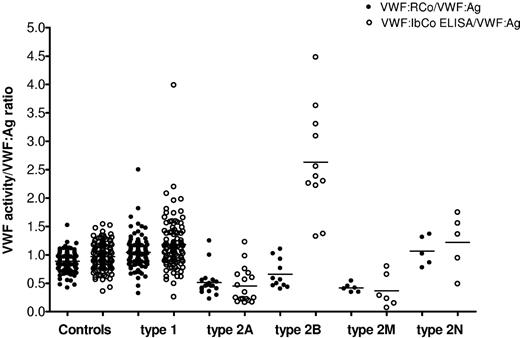
We next compared the VWF:RCo/VWF:Ag and VWF:IbCo ELISA/VWF:Ag ratios, depicted in Figure 6. The mean VWF:RCo/VWF:Ag ratio was 0.89 for the healthy controls and 1.05 for the type 1 VWD subjects. The type 2A VWD subjects had a significantly lower mean VWF:RCo/VWF:Ag ratio of 0.51 compared with the healthy controls (P < .001), as did the type 2B VWD subjects, with a mean VWF:RCo/VWF:Ag ratio of 0.66 (P < .005). However, no significant different exists between the 2A and 2B subjects using the classic VWF:RCo/VWF:Ag ratio (P = .065). For the VWF:IbCo/VWF:Ag ratio, the mean for the healthy controls was 0.97 and for the type 1 VWD subjects was 1.18. The mean ratio for the 2A subjects was low at 0.45 (P < .001 compared with controls). The 2B subjects, on the other hand, had a significantly elevated mean ratio at 2.63 (P < .001 compared with controls). Using the VWF:IbCo ELISA/VWF:Ag ratio, there was also now a significant difference between the 2A and the 2B subjects (P < .001). No control subject had a mean ratio higher than 1.54. Indeed, one subject initially identified in the ZPMCB-VWD study as type 2A VWD had a VWF:IbCo/VWF:Ag ratio of 2.26. Sequencing analysis subsequently confirmed this subject to be type 2B, with a R1308C mutation in exon 28.19,21 Not all the type 2B subjects, however, demonstrated a significant elevation in VWF:IbCo ELISA. Two subjects had VWF:IbCo ELISA/VWF:Ag ratios in the normal range despite the presence of confirmed type 2B mutations and abnormal platelet binding.21 Both had the V1316M mutation, for which variability within families has been previously reported.22
Comparison of VWF:RCo and the VWF:IbCo ELISA. VWF:RCo/VWF:Ag (●) and VWF:IbCo ELISA/VWF:Ag (○) are shown for healthy controls, type 1, type 2A, type 2B, type 2M, and type 2N VWD subjects. The line indicates the mean for each assay.
Comparison of VWF:RCo and the VWF:IbCo ELISA. VWF:RCo/VWF:Ag (●) and VWF:IbCo ELISA/VWF:Ag (○) are shown for healthy controls, type 1, type 2A, type 2B, type 2M, and type 2N VWD subjects. The line indicates the mean for each assay.
Type 2B VWD is the result of gain-of-function mutations in VWF leading to increased GPIb binding and subsequent clearance of VWF-platelet complexes.21,23 The decreased VWF:RCo/VWF:Ag ratio, however, may also be seen in platelet-type VWD. Plasma samples from 3 subjects with platelet-type VWD were tested with the VWF:IbCo ELISA. Results were similar to those seen with the type 2A subjects, with a mean VWF:RCo/VWF:Ag ratio of 0.38 and a mean VWF:IbCo ELISA/VWF:Ag of 0.41 (Table 3).
Type 3 VWD
All 17 type 3 subjects with VWF:Ag less than 1 IU/dL also had VWF:RCo less than 10, the lower limit of detection in our reference laboratory. All of these subjects also demonstrated undetectable binding when tested using the VWF:IbCo ELISA. The lower limit of detection with the VWF:IbCo ELISA was 1.6 U/dL. Therefore, the VWF:IbCo ELISA results correlate well with the VWF:Ag and VWF:RCo results in this population.
Discussion
VWF assays have been examined in several large patient populations, including studies from the European Union and Canada.24,25 We report here on a population of patients with VWD, as contrasted to a healthy control population derived from multiple centers across the United States. We observed that the VWF:IbCo ELISA correlated with the VWF:RCo results for most VWD subjects, including type 1, type 2A, type 2M, and type 2N VWD. The VWF:IbCo ELISA yielded undetectable levels for the type 3 subjects, similar to results obtained with VWF:Ag testing and more sensitive than most VWF:RCo assays. Elevated VWF:IbCo ELISA values in the majority of our type 2B subjects suggests that this assay may be of use in discrimination of type 2A and type 2B VWD, which demonstrate very similar VWF:Ag, VWF:RCo, and VWF multimer distribution.
The ELISA format has been used by other investigators to demonstrate VWF-GPIb interactions. Vanhoorelbeke et al demonstrated good correlation between VWF:RCo and an ELISA using recombinant GPIb and ristocetin.26 Federici et al showed an ELISA with recombinant GPIb, and ristocetin yielded results similar to the VWF:RCo in VWD subjects, but with better reproducibility.27 However, all these assays share the requirement for ristocetin. The VWF:IbCo ELISA allows the VWF-platelet relationship to be quantified in the absence of ristocetin.
Platelet adhesion in vivo requires GPIb-VWF interactions.28 The binding site for GPIb is located just outside the VWF A1 loop.29,30 Numerous mutations in the A1 domain have been reported to cause decreased VWF-GPIb interactions, resulting in type 2M VWD.31-34 The gain-of-function mutations that lead to type 2B VWD are all located within the A1 domain.1 The VWF binding site on GPIb is located in the N-terminal region of GPIbα.35 A crystal structure of the VWF A1 domain in complex with the amino-terminal domain of GPIb demonstrated that the GPIb loop from 227 to 241, known as the β switch, is responsible for VWF binding.36
Most of the reported platelet-type VWF mutations to date have been found in this region, including G233V and M239V.15,37 One deletion in the macroglycopeptide region has also been reported to lead to platelet-type VWD.38 Site-directed mutagenesis of this region identified D235V as a gain-of-function mutation with increased binding affinity to VWF.39 This correlates with our findings that D235Y, a naturally occurring mutation of this residue, also creates a gain-of-function phenotype. Analysis of the combined effects of the 2 gain-of-function mutants G233V and M239V showed higher affinity to VWF than either mutation alone.40 In our hands, use of 2 mutations together resulted in optimal binding compared with the use of either mutation alone. There was no difference in binding between constructs containing any 2 of the 3 mutations when analyzed for ability to bind VWF by flow cytometry (data not shown). We therefore elected to proceed with the tGPIbα235Y;239V construct for our ELISA studies with the gain-of-function GPIbα.
The VWF:IbCo ELISA has the theoretical advantage of measuring ristocetin-independent VWF binding to GPIbα, avoiding aberrant results that might ensue because of VWF polymorphisms in ristocetin-binding domains. We have previously reported that the P1467S mutation results in markedly decreased VWF:RCo but normal VWF function by other measures.9 In addition, a common polymorphism, D1472H, is associated with decreased VWF:RCo/VWF:Ag ratios in healthy persons, in some cases low enough to be considered consistent with type 2M VWD.10 There was no difference when VWF function was examined with a gain-of-function GPIb construct expressed in HEK293T cells and analyzed by flow cytometry. We now have data on an improved assay, the VWF:IbCo ELISA, which is easier to perform and provides good reproducibility, making it more amenable to clinical laboratory use than a flow cytometry–based version. An additional gain in reproducibility may be possible with precoated plates, eliminating the variability present when plates are coated individually just before use, as performed in this report.
Discrimination of VWD subtypes at present depends on VWF:Ag, VWF:RCo, and VWF multimers, particularly in determining the presence of type 2A or 2B. Whereas the VWF:Ag has excellent reproducibility, with a low CV, the VWF:RCo does not demonstrate the same interlaboratory reliability.2,3,41 Type 2A and type 2B VWD classically present with a decrease in high- molecular-weight multimers, in contrast to the relatively normal multimer distribution seen in type 2M VWD. Analysis of multimer distribution, however, is restricted to certain laboratories, and the sensitivity dependent on laboratory skill.4 Collagen binding has also been endorsed as an important assay of VWF function. The VWF:CB does provide important information about VWF-collagen interactions and also serves as a surrogate test of multimer presence because VWF:CB is sensitive to the presence or absence of high-molecular-weight multimers.5 Rare VWD mutations exclusively affect collagen binding.42-44 However, the VWF:CB does not distinguish between type 2A and type 2B VWD, unlike the VWF:IbCo ELISA. Confirmation of type 2B VWD requires platelet-binding assays that can demonstrate increased binding of VWF at lower doses of ristocetin than normally observed.12 Alternate assays of VWF function, particularly ones with a low CV that do not require special techniques, could facilitate faster, more precise, diagnosis of the VWF subtypes.
Type 2B VWD represents a gain-of-function in terms of the mutant VWF having an increased affinity for platelet GPIb. Classically, type 2B VWD mutants lead to both reduced high- molecular-weight multimers and thrombocytopenia, as hyperactive VWF-platelet binding results in clearance of both from the circulation. Originally described as type IIB, this subtype is characterized by the increased interaction between platelets and VWF, even at low doses of ristocetin.45 Recently, however, studies have shown that not all type 2B subjects experience significant baseline thrombocytopenia, with some demonstrating decreased platelet counts only when challenged.21 In addition, a wide range of VWF:RCo values were noted, even for subjects with the same genetic mutation.21 Previously, the V1316M mutation has also been reported with a variable phenotype within the same family.22 The presence of activated VWF in type 2B VWD has been demonstrated by the use of a nanobody derived from llamas.19 In a series of type 2B patients from Milan, Vicenza, and Utrecht, VWF in the active conformation was found in some, but not all, of the subjects.21 Our results demonstrate elevated VWF:IbCo ELISA results in all but 2 of the type 2B VWD subjects. Those subjects both have the V1316M mutation, as do 3 other subjects who did present with an elevated VWF:IbCo ELISA result. All the type 2B subjects in our study lack high-molecular-weight multimers, but it is possible that the minor difference in multimer distribution might affect the VWF:IbCo ELISA. Further investigation into this observation is in progress.
In our population of VWD subjects, the VWF:IbCo ELISA using our tGPIbα235Y;239V construct provided additional information in classification compared with the VWF:Ag and VWF:RCo, particularly in type 2 VWD subjects. Compared with classification using VWF:Ag and VWF:RCo, using VWF:Ag and VWF:IbCo led to the same classification of subjects as either normal (VWF:Ag > 50 IU/dL) or type 1 (VWF:Ag < 50 IU/dL and normal VWF:IbCo). A low VWF:Ag and corresponding low VWF:IbCo ELISA result (with a ratio < 1) could be either type 2A VWD (if abnormal multimer distribution present) or type 2M VWD (if multimer distribution normal). A low VWF:Ag with a high VWF:IbCo ELISA (with a ratio > 1) and abnormal multimer distribution was diagnostic of type 2B VWD. Type 3 VWD had undetectable results with both VWF:Ag and VWF:IbCo ELISA. In addition, one subject originally enrolled as a type 2A had an elevated VWF:IbCo ELISA result consistent with type 2B VWD, and was subsequently found to have a 2B mutation and type 2B VWD.
Exceptions to this classification included a type 2M subject with VWF:Ag 88, which might have been placed in the normal category except that the VWF:IbCo ELISA was low at 25, consistent with type 2 VWD. Therefore, combining VWF:Ag, VWF:IbCo, and multimer distribution provided a reasonable classification system for our subjects. It is important to note that these subjects were selected because they met definitive diagnostic criteria. This assay has yet to be tested on a population of subjects without a preexisting diagnosis. The VWF:IbCo ELISA is, however, highly reproducible, with a low CV, and has the added advantage of demonstrating normal activity in persons with the D1472H and P1467S polymorphisms. This assay may be useful in distinguishing type 2A from type 2B VWD, without sacrificing the decreased VWF activity to antigen ratio that distinguishes type 2 from type 1 VWD. Further characterization of this assay is required, and exploration of the VWF:IbCo ELISA in VWD subtypes is ongoing, but the studies presented here suggest that this ELISA may yield useful information as part of a laboratory workup for patients suspected of having VWD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the subjects, physicians, and staff involved in the ZPMCB-VWD study, especially the directors of the primary clinical centers for the Zimmerman Program: T.C. Abshire and A.L. Dunn, Atlanta, GA; J.M. Lusher, Detroit, MI; W.K. Hoots and D.L. Brown, Houston, TX; A.D. Shapiro, Indianapolis, IN; J.A. Di Paola and S.R. Lentz, Iowa City, IA; J.C. Gill, Milwaukee, WI; C. Leissinger, New Orleans, LA; M.V. Ragni, Pittsburgh, PA. In addition, numerous secondary centers contributed to subject recruitment: J. Hord, Akron Children's Hospital, Akron, OH; M. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO; A. Ma, University of North Carolina Chapel Hill, Chapel Hill, NC; L. Valentino, Rush University Medical Center, Chicago, IL; R. Gruppo, Cincinnati Children's Hospital, Cincinnati, OH; B. Kerlin, Nationwide Children's Hospital, Columbus, OH; R. Kulkarni, Michigan State University, East Lansing, MI; D. Green, Northwestern University, Evanston, IL; D. Mahoney, Baylor College of Medicine, Houston, TX; L. Mathias, Loma Linda University Medical Center, Loma Linda, CA; C. Diamond, University of Wisconsin Madison, Madison, WI; A. Neff, Vanderbilt University, Nashville, TN; D. DiMichele, Weill Cornell Medical College, New York, NY; A. Cohen, Newark Beth Israel Medical Center, Newark, NJ; E. Werner, Children's Hospital of the King's Daughters, Norfolk, VA; A. Matsunaga, Children's Hospital & Research Center Oakland, Oakland, CA; M. Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; F. Shafer, Drexel University College of Medicine, Philadelphia, PA; B. Konkle, University of Pennsylvania, Philadelphia, PA; P. Kouides, Rochester General Hospital, Rochester, NY; and D. Stein, Toledo Children's Hospital, Toledo, OH.
This work was supported by the National Institutes of Health (project grant HL081588). V.H.F. was supported by the National Hemophilia Foundation and Hemostasis and Thrombosis Research Society. R.R.M. was supported by the National Institutes of Health (grants HL33721 and HL044612).
National Institutes of Health
Authorship
Contribution: V.H.F., P.A.M., J.C.G., S.L.H., and R.R.M. designed the research; P.A.M., P.A.C., and K.D.F. collected data and performed experiments; R.G.H. performed the statistical analyses; V.H.F. and R.R.M. analyzed results and wrote the paper; and all authors edited the final paper.
Conflict-of-interest disclosure: V.H.F. has served as a consultant for CSL Behring. R.R.M. is a consultant for GTI Diagnostics, Baxter, CSL Behring, and AstraZeneca. J.C.G. is a consultant for Archemix, Baxter, Bayer, and CSL Behring. The other authors declare no competing financial interests.
Correspondence: Veronica H. Flood, Comprehensive Center for Bleeding Disorders, 8739 Watertown Plank Rd, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: vflood@mcw.edu.