To the editor:
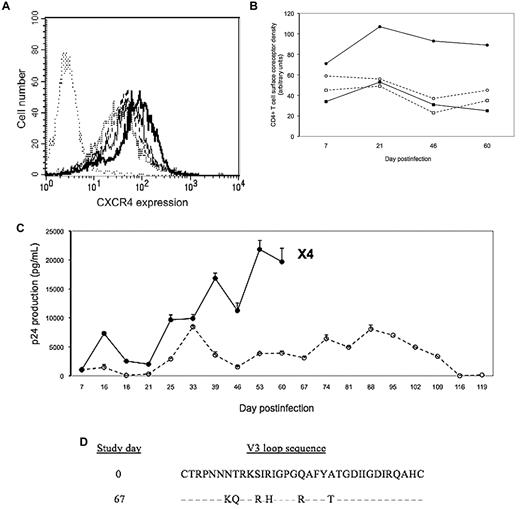
Virions using CXCR4 as a coreceptor (X4 strains) often emerge in addition to CCR5-using virions (R5 strains) in HIV type 1–infected subjects, particularly at late stages of the disease.1 This emergence of X4 virions is associated with faster disease progression.1 It is, therefore, of major importance to identify the factors determining this phenomenon. We have recently shown that CXCR4 overexpression at the surface of CD4+ T cells is one of these factors.2 Because interleukin-7 (IL-7) is overproduced at late stages of the disease3 and has been reported to increase CXCR4 expression at the surface of CD4+ T cells,4 we tested the hypothesis that it could induce an R5-to-X4 switch. To this purpose, we first analyzed the in vitro effect of various concentrations of IL-7 on peripheral blood CD4+ T-cell surface CXCR4 density. As shown in Figure 1A, we observed a dose-dependent rise in CD4+ T-cell surface expression of the coreceptor. Second, we cultured primary peripheral blood mononuclear cells (PBMCs) in the presence or absence of 10 ng/mL of IL-7, infected them with the R5 strain AD8, and monitored over time CCR5 and CXCR4 expression, HIV-1 production, and the emergence of X4 virions. CXCR4 density remained approximately 2-fold higher at the surface of IL-7–treated CD4+ T cells, whereas CCR5 density remained unchanged throughout the experiment, compared with nontreated cells (Figure 1B). IL-7 induced a progressive increase in viral production and the appearance of X4 virions at day 67 (Figure 1C). In contrast, HIV-1 production always remained low in the absence of IL-7, and no X4 strain was detected. The X4 phenotype of the virions isolated from the cell culture was confirmed by sequencing (Figure 1D).
(A) Effect of IL-7 on CXCR4 expression at the surface of primary CD4+ T cells. PBMCs were cultured for 7 days in the absence (line with bold dots) or presence of various concentrations (dashed line, 100 pg/mL; thin solid line, 500 pg/mL; bold solid line, 10 ng/mL) of IL-7 (R&D Systems) indirectly labeled with the anti-CXCR4 antibody 12G5 (BD PharMingen) and directly labeled with a fluorescent anti-CD4 antibody as described.2 CXCR4 expression was analyzed by flow cytometry after gating on CD4+ lymphocytes. An irrelevant isotype-matched antibody was used as a negative control (line with thin dots). (B) One million PBMCs were cultured in triplicate in RPMI supplemented with 10% fetal calf serum in presence (solid line) or absence (dashed line) of 10 ng/mL of IL-7. Five days later the cells were exposed for 4 hours to 10 ng of p24 equivalent of the R5 strain AD8, washed, and further cultured in presence or absence of IL-7. Once a week, fresh PBMCs were added to the culture. The mean expression over time of CCR5 (squares) and CXCR4 (circles) at the surface of CD4+ T cells treated (closed symbol) or not (open symbol) was monitored. (C) In the same experiment, p24 concentration was measured in the cell supernatant. Thrice a week, 200 μL of culture supernatant were added to CD4+CXCR4+CCR5− MT2 cells to detect the presence of X4 virions as previously described.2 The emergence of an X4 strain at day 67 in IL-7–treated PBMC culture is indicated. R5-to-X4 switch occurred at day 65 in a similar, independent experiment. (D) Alignment of V3 loop sequences from the input virus and the mutant clone obtained at day 67. Predicted amino acid sequences are shown, and similarities are indicated with dashes.
(A) Effect of IL-7 on CXCR4 expression at the surface of primary CD4+ T cells. PBMCs were cultured for 7 days in the absence (line with bold dots) or presence of various concentrations (dashed line, 100 pg/mL; thin solid line, 500 pg/mL; bold solid line, 10 ng/mL) of IL-7 (R&D Systems) indirectly labeled with the anti-CXCR4 antibody 12G5 (BD PharMingen) and directly labeled with a fluorescent anti-CD4 antibody as described.2 CXCR4 expression was analyzed by flow cytometry after gating on CD4+ lymphocytes. An irrelevant isotype-matched antibody was used as a negative control (line with thin dots). (B) One million PBMCs were cultured in triplicate in RPMI supplemented with 10% fetal calf serum in presence (solid line) or absence (dashed line) of 10 ng/mL of IL-7. Five days later the cells were exposed for 4 hours to 10 ng of p24 equivalent of the R5 strain AD8, washed, and further cultured in presence or absence of IL-7. Once a week, fresh PBMCs were added to the culture. The mean expression over time of CCR5 (squares) and CXCR4 (circles) at the surface of CD4+ T cells treated (closed symbol) or not (open symbol) was monitored. (C) In the same experiment, p24 concentration was measured in the cell supernatant. Thrice a week, 200 μL of culture supernatant were added to CD4+CXCR4+CCR5− MT2 cells to detect the presence of X4 virions as previously described.2 The emergence of an X4 strain at day 67 in IL-7–treated PBMC culture is indicated. R5-to-X4 switch occurred at day 65 in a similar, independent experiment. (D) Alignment of V3 loop sequences from the input virus and the mutant clone obtained at day 67. Predicted amino acid sequences are shown, and similarities are indicated with dashes.
In this study, we report evidence that IL-7 increases CD4+ T-cell surface CXCR4 density, HIV-1 production and the risk of emergence of X4 strain. These data reinforce the hypothesis that in advanced patients, lymphopenia-induced IL-7 production might favor R5 to X4 switch.5 Moreover, at a moment when IL-7 is being tested to improve immune recovery in patients undergoing antiretroviral therapy, these data draw attention to a risk of side effect of this strategy. Even though IL-7 will be administrated to aviremic patients, the facts that ongoing viral replication may persist at low levels in some of these patients and that IL-7 boosts viral production raise the concern of a detrimental effect of such a therapy that will have to be monitored.
Authorship
Contribution: N.B. performed research and analyzed data; P.P. and M.-J. C. analyzed data; and P.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: P.C. has served as consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme-Chibret, Pfizer, and ViiV Healthcare. The remaining authors declare no competing financial interests.
Correspondence: Pierre Corbeau, Laboratoire d'Immunologie, Hôpital Saint Eloi, 80 ave A. Fliche, 34295, Montpellier cedex 5, France; e-mail: p-corbeau@chu-montpellier.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal