Abstract
The ability of hematopoietic stem cells (HSCs) to undergo self-renewal is partly regulated by external signals originating from the stem cell niche. Our previous studies with HSCs obtained from fetal liver of mice deficient for the calcium-sensing receptor (CaR) have shown the crucial role of this receptor in HSC lodgment and engraftment in the bone marrow (BM) endosteal niche. Using a CaR agonist, Cinacalcet, we assessed the effects of stimulating the CaR on the function of murine HSCs. Our results show that CaR stimulation increases primitive hematopoietic cell activity in vitro, including growth in stromal cell cocultures, adhesion to extracellular matrix molecules such as collagen I and fibronectin, and migration toward the chemotactic stimulus, stromal cell-derived factor 1α. Receptor stimulation also led to augmented in vivo homing, CXCR4-mediated lodgment at the endosteal niche, and engraftment capabilities. These mechanisms by which stimulating the CaR dictates preferential localization of HSCs in the BM endosteal niche provide additional insights into the fundamental interrelationship between the stem cell and its niche. These studies also have implications in the area of clinical stem cell transplantation, where ex vivo modulation of the CaR may be envisioned as a strategy to enhance HSC engraftment in the BM.
Introduction
In the adult hematopoietic system, hematopoietic stem cells (HSCs) reside in a specific anatomic location in the bone marrow (BM) known as the stem cell niche.1 The signaling cues originating from the stem cell niche serve as instructions for the HSCs to undergo either self-renewal or differentiation for the maintenance of the hematopoietic system in the person. In the clinical transplantation setting, interactions between the transplanted HSCs and the stem cell niche are essential in determining the clinical outcome of the transplantation.2 For successful engraftment and reestablishment of hematopoiesis to occur in the recipient, the transplanted HSCs must first migrate from the peripheral circulation to the BM, a process known as homing,3 then adhere and be retained in the specialized niches within the BM, a process known as lodgment.4 After lodgment, the specific fate of the transplanted HSCs is determined through complex, bidirectional interactions with various extrinsic components in the niche.5
The process of HSC lodgment after transplantation is believed to be regulated in part by cell adhesion molecules (CAMs) expressed on the cellular surface. Previous studies have shown the importance of cell surface molecules such as α4 integrins,6 hyaluronic acid,7 or stem cell factor8 and osteopontin9 in retaining HSCs in the BM endosteal region through interactions with stem cell niche components. However, studies on the identification of the molecules that dictate lodgment are complicated by the vast array of CAMs that are expressed on HSCs and the broad range of potential ligands that are expressed on the stromal cells with possible overlapping functions.10 Because the endosteal surface of the bone is known to have a high concentration of Ca2+ ions that reaches as high as 40mM underneath resorbing osteoclasts,11 we hypothesized that lodgment of HSCs in the endosteal region of the BM is specifically dictated by this unique feature. Our previous work has shown the role of the calcium-sensing receptor (CaR), a G protein–coupled receptor that plays a key role in the regulation of extracellular calcium homeostasis,12,13 in HSC lodgment and engraftment in the BM, where HSCs deficient for the CaR lose their ability to lodge at the endosteal surface of bone, leading to defective engraftment.14
In this study we wanted to examine the precise cellular and molecular mechanisms that dictate CaR-mediated HSC lodgment in the adult BM stem cell niche. By stimulating the activity of CaR with the use of a CaR agonist, underlying mechanisms of HSC lodgment in the BM endosteal region after transplantation can be elucidated, providing additional insights for the fundamental interrelationship between the stem cell niche and stem cell fate. Cinacalcet, a calcimimetic compound clinically approved to be used as a treatment for secondary hyperparathyroidism, acts as a positive allosteric modulator of CaR to increase the sensitivity of the receptor to activation by extracellular Ca2+ ions. This allows modulation of the functional CaR without altering the level of extracellular Ca2+ concentration.15 Using in vitro and in vivo model systems, we assessed the effects of Cinacalcet treatment on the function of primitive hematopoietic cells. Specifically, we tested the hypothesis that ex vivo stimulation of the CaR on HSCs can lead to enhanced BM homing, lodgment, and engraftment in vivo, thereby also providing therapeutic implications pertinent to clinical stem cell transplantation.
Methods
Animals
Six- to 8-week-old male C57Bl/6 and B6.SJL mice (Taconic Farms Inc) were obtained and used in accordance with the University of Southern California Institutional Animal Care and Use Committee guidelines. Mice were housed in sterilized microisolator cages and received autoclaved food and water ad libitum.
Cinacalcet preparation and cell treatment
Thirty micrograms of Cinacalcet (Amgen) was dissolved in 95% ethanol (EMD Chemicals Inc), filtered through a 0.2-μm Acrodisc syringe filter (Pall Corporation), and diluted to a 1-mM stock. Cells were treated with either 2.5μM Cinacalcet or ethanol control in α–minimum essential medium (MEM) supplemented with 10% fetal bovine serum and penicillin/streptomycin (all from Mediatech Inc) in a 37°C water bath for 90 minutes protected from light.
Culture colony forming-unit assay
BM mononuclear cells (MNCs) were obtained from the hind limbs of C57Bl/6 mice and treated with Cinacalcet or ethanol control. The cells were then resuspended in MethoCult GF M3434 (StemCell Technologies) and cultured at 37°C/5% CO2 in a humidified atmosphere. The number of culture colony-forming units was scored according to standard criteria.
Cell sorting
BM MNCs were incubated in phosphate-buffered saline (PBS; Mediatech Inc) with fluorescent labeled (allophycocyanin [APC], fluorescein isothiocyanate, or phycoerythrin [PE], respectively) anti–mouse c-Kit, anti–mouse Sca-1, and anti–mouse Flk-2 antibodies (all from BD Biosciences). Concurrently, cells were incubated with a biotin lineage cocktail (anti–mouse CD3e, anti–mouse CD11b, anti–mouse B220, anti–mouse Gr-1, and anti–mouse TER-119; BD Biosciences). After primary antibody incubation, cells were stained with PE–cyanine 5 streptavidin (BD Biosciences). Hematopoietic stem and progenitor cells were purified with the use of a FACSAria flow cytometer (BD) on the basis of established cell surface phenotype.
Cobblestone area–forming cell assay
The Lin−c-Kit+ (LK) subpopulation was purified from BM MNCs as described in “Cell sorting” and treated with 2.5μM Cinacalcet or ethanol control. After treatment, Lin−c-Kit+Sca-1+Flk-2− (LKS+F−) cells, Lin−c-Kit+Sca-1+Flk-2+ (LKS+F+) cells, and Lin−c-Kit+Sca-1−Flk-2+ (LKS−F+) cells were purified and seeded in serial dilutions on a confluent layer of OP9 stromal cells that were previously irradiated at 35 Gy. The cells were maintained in α-MEM supplemented with 10% fetal bovine serum and penicillin/streptomycin medium (Mediatech Inc) in a humidified atmosphere at 33°C/5% CO2. The presence of cobblestone areas were scored on week 5, and the frequency of cobblestone area–forming cells (CAFCs) were calculated with the use of the L-Calc software (StemCell Technologies).
Calcium flux assay
LK cells were sorted from BM MNCs and treated with Cinacalcet at the following dosages: 0.625μM, 1.25μM, and 2.5μM. Cells were then incubated with 2 μg/mL indo-1 (Molecular Probes) in PBS for 30 minutes in a 37°C water bath in the dark. Calcium flux was measured by a ratio of 390:30 (short) to 530:30 (long) wavelengths with UV light with the use of an LSR II cytometer (BD) after the addition of 1.5mM CaCl2 (Sigma-Aldrich) or 100 ng/mL stromal cell-derived factor 1 α (SDF-1α; PeproTech Inc) as the stimulus. FlowJo software (TreeStar) was then used to examine the calcium flux response in the LKS+F−, LKS+F+, and LKS−F+ subpopulations after treatment.
Chemotaxis assay
Chemotaxis assays were performed with the use of Transwells (5-μm pore size; Corning Inc) by adding 1 × 105 Cinacalcet or control-treated LK cells in fully supplemented α-MEM to the upper well. Chemotaxis toward 100 ng/mL murine SDF-1α (PeproTech Inc) was allowed to continue for 3 hours at 37°C/5% CO2 in a humidified atmosphere. Nonspecific migration was quantified through the analysis of chemokinesis of the cells in response to medium or SDF-1α. Cells were harvested from the lower well and counted on a hemacytometer, and the relative numbers of the different stem and progenitor cell subpopulations were subsequently analyzed by flow cytometry.
Cell adhesion assay
LKS+F−, LKS+F+, and LKS−F+ cells were sorted from BM MNCs and treated with Cinacalcet or ethanol control. Cells (5 × 102) were added to wells coated with fibronectin or collagen I (both from BD Biosciences) in cell culture–treated 96-well plates (BD) and incubated for 3 hours at 37°C/5% CO2 in a humidified atmosphere. To control for nonspecific binding, adhesion to 1% bovine serum albumin (Sigma-Aldrich) was quantified. Nonadherent cells were washed off with PBS, and adherent cells were visually counted microscopically.
Cell-cycle analysis
To stain for DNA content, cells were incubated with 10 μg/mL Hoechst 33342 (Sigma-Aldrich) at 37°C for 45 minutes, then stained with lineage and stem cell antibodies as described in “Cell sorting,” except rat anti–mouse Flk-2 (eBioscience) was used as the primary antibody and goat anti–rat IgG F(ab′)2-APC-Cy7 (Santa Cruz Biotechnology) was used as the secondary antibody. The stained cells were resuspended and fixed in 10% buffered formalin and incubated at 4°C overnight. To stain for RNA content, pyronin Y (Polysciences Inc) was added to the cells at a final concentration of 0.75 μg/mL and incubated at 4°C for 30 minutes. Cell-cycle status was examined with the use of an LSR II flow cytometer (BD).
Apoptosis assay
BM MNCs were stained with lineage and stem cell antibodies. Treated cells were then stained with 7-amino-actinomycin and PE Annexin V (both from BD Biosciences) according to manufacturer's instructions. With the use of an LSR II flow cytometer (BD), the percentage of apoptotic cells after treatment was determined as 7-amino-actinomycin negative and PE Annexin V positive.
Quantitative reverse transcription–polymerase chain reaction
Total RNA was extracted with the use of the RNA Miniprep Kit (Stratagene) and was reverse-transcribed into cDNA with the use of the SuperScript VILO cDNA synthesis kit (Invitrogen) in accordance with the manufacturer's instructions. To quantify the expression of car, cxcr4, and hprt1, Taqman Gene Expression Assay primers and probe sets (Applied Biosystems and Roche Diagnostics) were used. Levels of gene expression were quantified with the use of the 7900HT real-time polymerase chain reaction system (Applied Biosystems). Standard curves in these experiments were created with the use of total mouse kidney RNA obtained from C57Bl/6 mice.
Adhesion molecule expression
BM MNCs were treated with Cinacalcet or ethanol control, then stained with lineage and stem cell markers along with PE anti–mouse CXCR4 (BD Biosciences), CD49d, and CD62L antibodies (both from eBioscience) for CXCR4, α4β1 integrins, and L-selectins, respectively. The expression levels of adhesion molecules were measured on an LSR II flow cytometer (BD).
Immunohistochemistry
Tibias were dissected from C57Bl/6 mice and fixed in 10% formalin overnight at 4°C. The bones were then decalcified with 20% EDTA (ethylenediaminetetraacetic acid) over a 2-week period and were processed and embedded in paraffin with the use of standard histologic procedures. Whole bone sections were cut at 5 μm and incubated with anti–collagen I goal polyclonal immunoglobulin G (IgG; Abcam) overnight at 4°C. Slides were then blotted with donkey anti–goat secondary antibody (Santa Cruz Biotechnology) for 30 minutes at room temperature. The Vectastain ABC kit (Vector Laboratories) was applied to the slides and incubated for 30 minutes at room temperature. The slides were then rinsed with PBS-Tween, and Alkaline Phosphatase Vector Substrate was added to the blots. The slides were then incubated in the dark for 10 minutes. On color formation, the slides were rinsed with distilled water and then counterstained with Methyl Green. Then a cover-slip was applied to the slides with the use of the Cytoseal XYL mounting medium (Richard-Allan Scientific) and examined with a Nikon Eclipse 50i upright microscope.
In vivo homing
LK cells were sorted with a FACSAria flow cytometer (BD). After treatment, to track the cells in vivo after coinjection into the same recipient, LK cells (∼ 5 × 105 to 1 × 106) were labeled with a green fluorescent dye, either carboxyfluorescein diacetate succinimidyl ester (CFSE) or DiO (3,3′-dihexadecyloxacarbocyanine perchlorate), and a red fluorescent dye, either seminaphtorhodafluor-1-carboxylic acid acetate succinimidyl ester (SNARF-1) or DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; all from Invitrogen), according to the manufacturer's instructions. Labeled cells were coinjected into the tail vein of a nonirradiated C57Bl/6 mouse. Mice were then killed after 16 hours, and the number of labeled cells was measured in the BM and spleen through the detection of CFSE+ and SNARF+ cells or DiO+ and DiI+ cells by flow cytometry.
In vivo lodgment
LK cells were labeled with 5μM CFSE or 5μM SNARF cell-labeling solutions and injected into the tail vein of nonirradiated C57Bl/6 mice, as described above. To block CXCR4 function during lodgment, cells were treated with both 2.5μM Cinacalcet and 10μM AMD3100 (Sigma-Aldrich) or ethanol control in the same fashion as described for cell treatment. Tibias and femurs were dissected from the recipient mice 16 hours after injection, decalcified for 3 days in Immunocal (Decal Chemical Corporation), and embedded in paraffin blocks after processing. Bone sections of 5-μm thickness were cut and mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (Vector Laboratories). To assess the lodgment of injected cells to the endosteal niche, the number of CFSE+ (green) or SNARF+ (red) cells within 2 cell diameters from the endosteal surface were counted on a total of 40–60 femoral and tibial sections per mouse. Each experiment was performed with 1–3 mouse recipients.
Competitive repopulation assay
LKS+F− cells were sorted with the use of a FACSAria flow cytometer (BD) and then treated with 2.5μM Cinacalcet or ethanol control. After treatment, 450 LKS+F− cells (CD45.1) and 225 000 BM MNCs (CD45.2) were injected into the tail vein of C57Bl/6 mice that were lethally irradiated at 9 Gy approximately 24 hours before transplantation. Engraftment levels and multilineage reconstitution were measured in peripheral blood samples obtained from the tail of recipients starting at week 4. PE anti–mouse CD45.1, fluorescein isothiocyanate anti–mouse CD45.2, APC anti–mouse CD3e, PE-Cy7 anti–mouse CD11b, and biotin anti–mouse B220 antibodies (all from eBioscience) were used to stain the peripheral blood samples.
Statistical analysis
Comparison of experimental groups was performed with the paired or unpaired 2-tailed Student t test as appropriate for the dataset. P < .05 was considered significant.
Results
Cinacalcet treatment of primitive hematopoietic cells augments signaling through the CaR
We first wanted to confirm that primitive murine hematopoietic cells express functional CaR and that signaling through the receptor could be augmented by Cinacalcet treatment. Cinacalcet has high selectivity for CaR and does not interact with several other G protein–coupled receptors and mGluRs that share significant homology with CaR.16 In addition, because Cinacalcet is an allosteric modulator, it possesses another level of specificity by being able to selectively propagate responses only in the tissues where the physiologic, endogenous agonist is active.17 In these experiments, primitive hematopoietic cells treated with Cinacalcet were subjected to calcium flux analysis after stimulation with extracellular Ca2+ ions. Analysis of the more primitive LKS+F− subset or the more differentiated LKS+F+ or LKS−F+ subsets of cells showed maximal enhanced calcium flux on stimulation of the CaR with 2.5μM Cinacalcet (Figure 1A), but not at lower concentrations (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These effects were not attributable to augmentation of receptor expression, because quantitative reverse transcription–polymerase chain reaction showed that the treatment with Cinacalcet did not alter mRNA levels (supplemental Figure 2). Altogether, these data showed that Cinacalcet treatment enhances CaR signaling on primitive hematopoietic cells without affecting receptor mRNA expression.
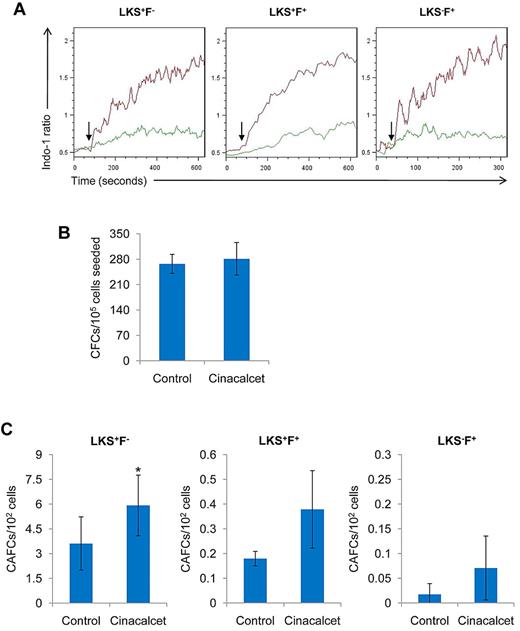
Effects of CaR stimulation on primitive hematopoietic cell activity in vitro. (A) LK cells were loaded with indo-1 dye (2 μg/mL) as a fluorescent probe to mark intracellular Ca2+ concentrations. CaCl2 (1.5mM) was added as an exogenous source of Ca2+ ions to induce a response. The response in LKS+F−, LKS+F+, and LKS−F+ subpopulations was then analyzed with FlowJo software. Arrow indicates the addition of CaCl2 stimulus. Green indicates control; red, Cinacalcet (n = 4 from 4 independent experiments). (B) BM MNCs treated with Cinacalcet or ethanol control were assessed for in vitro growth potential with the use of the culture colony-forming unit assay (n = 10 from 5 independent experiments). (C) LKS+F−, LKS+F+, and LKS−F+ cells were seeded on a confluent stromal layer of supportive OP9 cells in serial dilutions and cultured at 33°C and 5% CO2. Cobblestone areas were scored on week 5 (*P < .05; n = 9 for LKS+F−; n = 7 for LKS+F+; n = 3 for LKS−F+ from 5 independent experiments; error bars represent SEMs). CFC indicates colony-forming cell.
Effects of CaR stimulation on primitive hematopoietic cell activity in vitro. (A) LK cells were loaded with indo-1 dye (2 μg/mL) as a fluorescent probe to mark intracellular Ca2+ concentrations. CaCl2 (1.5mM) was added as an exogenous source of Ca2+ ions to induce a response. The response in LKS+F−, LKS+F+, and LKS−F+ subpopulations was then analyzed with FlowJo software. Arrow indicates the addition of CaCl2 stimulus. Green indicates control; red, Cinacalcet (n = 4 from 4 independent experiments). (B) BM MNCs treated with Cinacalcet or ethanol control were assessed for in vitro growth potential with the use of the culture colony-forming unit assay (n = 10 from 5 independent experiments). (C) LKS+F−, LKS+F+, and LKS−F+ cells were seeded on a confluent stromal layer of supportive OP9 cells in serial dilutions and cultured at 33°C and 5% CO2. Cobblestone areas were scored on week 5 (*P < .05; n = 9 for LKS+F−; n = 7 for LKS+F+; n = 3 for LKS−F+ from 5 independent experiments; error bars represent SEMs). CFC indicates colony-forming cell.
Cinacalcet treatment enhances in vitro primitive cell growth on stromal cell layers without alteration of differentiation potential
To address the question of whether Cinacalcet treatment alters the differentiation potential of hematopoietic progenitor cells, the functional culture colony-forming unit assay was performed. These data showed that Cinacalcet treatment did not cause cellular toxicity or inhibit differentiation potential of hematopoietic progenitor cells, because these cells were able to form colonies comparable to the control group (Figure 1B). To assess whether more primitive hematopoietic cell activity was altered by Cinacalcet treatment, the functional CAFC assay was performed. Compared with the control group, CAFC activity was significantly higher in the Cinacalcet treatment group, as evident in the LKS+F− subpopulation (P < .05; Figure 1C). However, Cinacalcet treatment did not appear to have any significant effects on CAFC activity in the LKS+F+ or LKS−F+ subpopulation (Figure 1C). These data indicate that with CaR stimulation, primitive hematopoietic cells are more capable of being maintained in coculture, resulting in a higher frequency of CAFCs after the 5-week coculture.
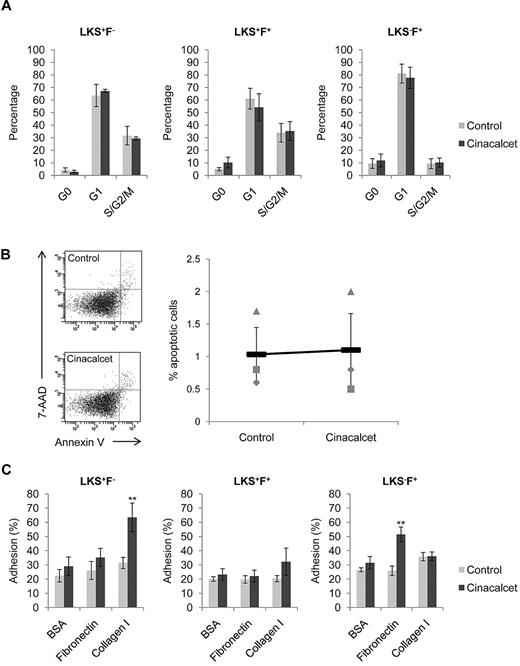
Cinacalcet treatment augments adhesion of hematopoietic stem and progenitor cells to extracellular matrix molecules
Multiple different mechanisms may explain the increase in CAFC frequencies with CaR stimulation, including altered cell cycle status, enhanced cell survival, or augmented cell adhesion to extracellular matrix (ECM) molecules. Specific analysis of each of these indicated that there was no significant alteration in the number of cells residing in G0, G1, or S/G2/M phase of the cell cycle immediately after ex vivo Cinacalcet treatment (Figure 2A). In addition, there was no change in the percentage of apoptotic cells after Cinacalcet treatment (Figure 2B), suggesting that CaR stimulation does not alter cell survival. Previous immunofluorescence studies on the distribution of ECM molecules in the BM have shown that 2 prominent molecules located in the BM to be collagen I and fibronectin, with collagen I present in the endosteal region and fibronectin in the central marrow region.18 Testing the ability of primitive hematopoietic cells to adhere specifically to these ECM molecules, we found that, although control primitive hematopoietic cells bound to the cell culture plates, the addition of collagen or fibronectin did not enhance the binding capabilities of the cells. CaR stimulation, however, significantly enhanced LKS+F− cell adhesion to collagen I but not to fibronectin (P < .01; Figure 2C). Furthermore, CaR stimulation slightly enhanced LKS+F+ cell adhesion to collagen I (Figure 2C). In contrast, CaR stimulation significantly enhanced LKS−F+ cell adhesion to fibronectin but not to collagen I (P < .01; Figure 2C). These results show that the effect of CaR stimulation was to augment cell adhesion specifically to collagen I or fibronectin, which was distinctive according to both the cell population and the ECM components expressed in the adult BM. However, note that adhesion to collagen I alone does not provide specificity in the localization of primitive hematopoietic cells in the endosteal region of the BM, because histologic assessment showed that collagen I is expressed ubiquitously along the surface of the bone (supplemental Figure 3). Therefore, collagen I may only play a part in the specific localization of primitive hematopoietic cells in the endosteal niche.
CaR stimulation does not alter cell cycle status, or cell survival, but significantly increases HSC adhesion to collagen I. (A) Cell cycle profiles for LKS+F−, LKS+F+, and LKS−F+ subpopulations (n = 4 from 2 independent experiments). (B) Percentage of LKS cells undergoing apoptosis after treatment. The percentage of apoptotic cells was determined as 7-amino-actinomycin (7-AAD) negative and PE Annexin V positive (n = 3 from 3 independent experiments). (C) Adhesion of LKS+F−, LKS+F+, and LKS−F+ cells to fibronectin and collagen I. Cells were allowed to adhere to wells coated with fibronectin and collagen I for 3 hours at 37°C and 5% CO2. Bovine serum albumin (BSA; 1%) was used as a control for nonspecific binding (**P < .01; n = 8 from 3 independent experiments; error bars represent SEMs).
CaR stimulation does not alter cell cycle status, or cell survival, but significantly increases HSC adhesion to collagen I. (A) Cell cycle profiles for LKS+F−, LKS+F+, and LKS−F+ subpopulations (n = 4 from 2 independent experiments). (B) Percentage of LKS cells undergoing apoptosis after treatment. The percentage of apoptotic cells was determined as 7-amino-actinomycin (7-AAD) negative and PE Annexin V positive (n = 3 from 3 independent experiments). (C) Adhesion of LKS+F−, LKS+F+, and LKS−F+ cells to fibronectin and collagen I. Cells were allowed to adhere to wells coated with fibronectin and collagen I for 3 hours at 37°C and 5% CO2. Bovine serum albumin (BSA; 1%) was used as a control for nonspecific binding (**P < .01; n = 8 from 3 independent experiments; error bars represent SEMs).
Cinacalcet treatment augments primitive hematopoietic cell activity in vivo
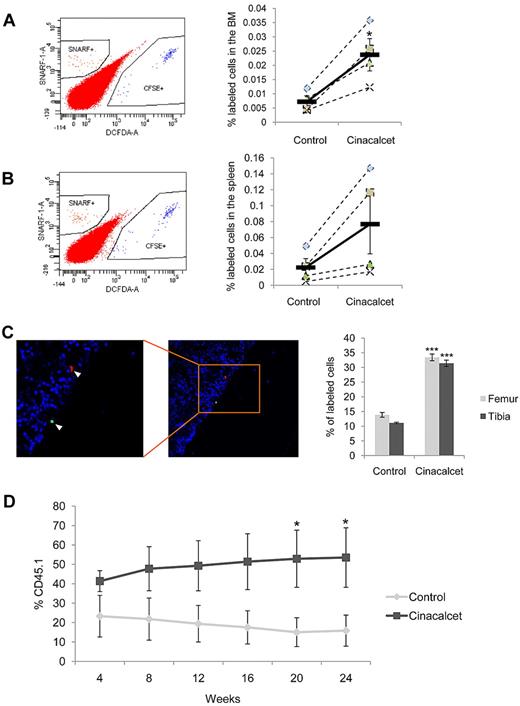
We next wanted to investigate the effects of CaR stimulation on hematopoietic stem and progenitor cell function in vivo with the use of models of homing, lodgment, and engraftment. For our homing studies, purified LK cells were differentially labeled with fluorescent dyes to track the cells in vivo and were then treated with Cinacalcet or ethanol control. These cells were then coinjected into the same nonirradiated mouse recipient, and the frequency of cells homing to the BM and spleen was calculated with the use of flow cytometric analysis. Our results showed that CaR stimulation by Cinacalcet treatment led to a significant improvement in the ability of the injected cells to home to the BM (P < .05; Figure 3A) and a slight improvement to the spleen (Figure 3B). To test for the effect of different dyes on homing efficiency, we labeled the cells with either DiI or SNARF (red dyes) and DiO or CFSE (green dyes) and analyzed the number of homed cells in the recipient. We found that there was no effect on homing efficiency from the use of different dyes to label the cells, because the same enhancement in homing was observed after Cinacalcet treatment (supplemental Figure 4). With respect to lodgment, histologic assessment of the femur and tibia showed that CaR stimulation resulted in an approximate 3-fold increase in the preferential anatomical localization of primitive hematopoietic cells at the endosteal niche after homing (P < .002; Figure 3C). To assess whether the increases in homing and lodgment led to increased engraftment of primitive hematopoietic cells, we performed a competitive repopulation analysis with the use of purified LKS+F− cells. These data demonstrated increased engraftment of the primitive hematopoietic cells after ex vivo Cinacalcet treatment over a 24-week period, with significant increase in engraftment levels starting on week 20 (P < .05; Figure 3D).
Cinacalcet treatment enhances in vivo homing, lodgment, and engraftment. (A) Cinacalcet- or control-treated LK cells were labeled with green or red membrane fluorescent dyes, respectively. The percentage of labeled cells present in the BM after transplantation was determined by flow cytometric analysis. Representative flow plots (left) and percentage of labeled cells present in the BM (right) are shown (*P < .05; n = 4 from 4 independent experiments). (B) Spleen cells from each recipient mouse were obtained, and homing was analyzed as for BM cells. (C) Quantification of the percentage of LK cells labeled with CFSE (Cinacalcet) or SNARF (control) present in the BM within 2 cell diameters of the endosteal surface in femoral and tibial sections. A representative picture of the anatomical localization of a CFSE+ LK cell (green) at the endosteal region and a SNARF+ LK cell (red) away from the endosteal region is shown. Cells were also stained with 4′,6-diamidino-2-phenylindole (blue) present in Vectashield (***P < .002; n = 180 sections from 3 independent experiments.). (D) Competitive repopulation of CD45.1 donor LKS+F− cells (Cinacalcet or control) and CD45.2 BM MNCs was analyzed by flow cytometric analysis on peripheral blood samples obtained from the recipient mice (*P < .05; n = 9 from 2 independent experiments; error bars represent SEMs).
Cinacalcet treatment enhances in vivo homing, lodgment, and engraftment. (A) Cinacalcet- or control-treated LK cells were labeled with green or red membrane fluorescent dyes, respectively. The percentage of labeled cells present in the BM after transplantation was determined by flow cytometric analysis. Representative flow plots (left) and percentage of labeled cells present in the BM (right) are shown (*P < .05; n = 4 from 4 independent experiments). (B) Spleen cells from each recipient mouse were obtained, and homing was analyzed as for BM cells. (C) Quantification of the percentage of LK cells labeled with CFSE (Cinacalcet) or SNARF (control) present in the BM within 2 cell diameters of the endosteal surface in femoral and tibial sections. A representative picture of the anatomical localization of a CFSE+ LK cell (green) at the endosteal region and a SNARF+ LK cell (red) away from the endosteal region is shown. Cells were also stained with 4′,6-diamidino-2-phenylindole (blue) present in Vectashield (***P < .002; n = 180 sections from 3 independent experiments.). (D) Competitive repopulation of CD45.1 donor LKS+F− cells (Cinacalcet or control) and CD45.2 BM MNCs was analyzed by flow cytometric analysis on peripheral blood samples obtained from the recipient mice (*P < .05; n = 9 from 2 independent experiments; error bars represent SEMs).
Effects of CaR stimulation on primitive hematopoietic cells are mediated through the CXCR4 signaling pathway
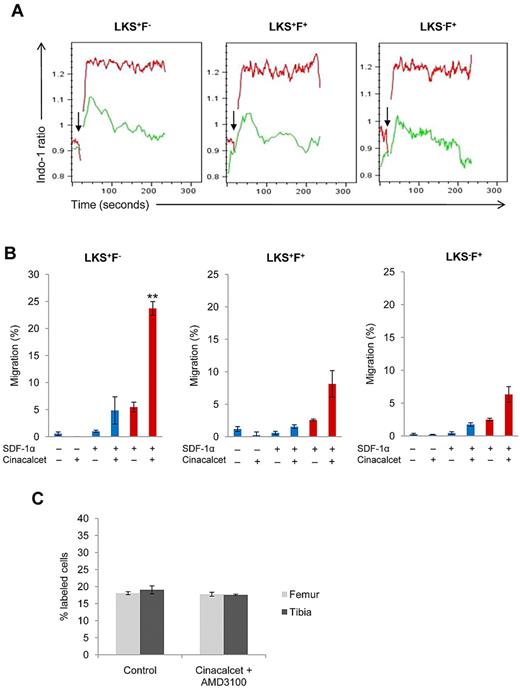
We reasoned that the enhancement in BM homing and lodgment after Cinacalcet treatment may be an effect of an altered response to chemotactic agents produced by the BM stroma. Previous studies on the migratory response of murine HSCs have shown that these cells migrate only toward the chemokine, SDF-1α, in vitro.19 Therefore, calcium flux assays in response to SDF-1α stimulus were conducted. Flow cytometric analysis showed that all hematopoietic stem and progenitor cells treated with Cinacalcet had an enhanced, sustained calcium flux response to SDF-1α compared with the control (Figure 4A). Chemotaxis assays were then used to determine whether migration to SDF-1α was also enhanced after Cinacalcet treatment. With CaR stimulation by Cinacalcet treatment, there were significant increases in the chemotactic response to SDF-1α in all 3 subpopulations of cells, especially in the LKS+F− subset (P < .002) (Figure 4B). CXCR4 is the chemokine receptor for SDF-1α, and its role in mediating the retention of HSCs in the BM has been documented previously with the use of transgenic CXCR4−/− chimeric mice.20 To investigate whether the increase in chemotactic response to SDF-1α was because of increased CXCR4 expression after Cinacalcet treatment, CXCR4 mRNA and cell surface expression levels were examined. Our results showed that CaR stimulation by Cinacalcet treatment did not induce CXCR4 mRNA or cell surface expression levels (supplemental Figures 5 and 6A). To further investigate the effect of Cinacalcet treatment on the expression of adhesion molecules important in interactions between HSCs and their niche, the expression levels of α4β1 integrins and L-selectins on the cell surface were also examined. We found no alteration in the expression levels of these adhesion molecules on the cell surface after Cinacalcet treatment (supplemental Figure 6B-C). Altogether, these results suggest that with CaR stimulation, CXCR4 signaling is enhanced without any alterations in the expression levels of CXCR4, α4β1 integrins, and L-selectins. Finally, to examine whether the effects of CaR stimulation could be blocked by the CXCR4 antagonist, AMD3100, LK cells were treated with both Cinacalcet and AMD3100 and coinjected with control treated cells into the same recipients. Our results showed that, when we stimulated CaR but blocked CXCR4, the enhancement in cell lodgment was eliminated (Figure 4C). This suggests that the effects of CaR stimulation on hematopoietic stem and progenitor cells are mediated in part through the CXCR4 signaling pathway.
CaR stimulation enhances CXCR4 signaling and cell migration toward SDF-1α. (A) Calcium flux assays in response to 100 ng/mL SDF-1α were performed with the use of purified LK cells. The response in LKS+F−, LKS+F+, and LKS−F+ subpopulations was then analyzed with FlowJo software. Arrow indicates the addition of SDF-1α stimulus; green, control; red, Cinacalcet treated (n = 3 from 3 independent experiments). (B) In vitro chemotaxis assay of LKS+F−, LKS+F+, and LKS−F+ cells to 100 ng/mL SDF-1α after a 3-hour incubation. Blue columns represent chemokinesis controls, red columns represent chemotaxis (***P < .002, *P < .05; n = 3). (C) In vivo lodgment showing the percentages of injected LK cells localized at the endosteal region after ex vivo Cinacalcet and AMD3100 treatment (n = 160 sections from 2 independent experiments; error bars represent SEMs).
CaR stimulation enhances CXCR4 signaling and cell migration toward SDF-1α. (A) Calcium flux assays in response to 100 ng/mL SDF-1α were performed with the use of purified LK cells. The response in LKS+F−, LKS+F+, and LKS−F+ subpopulations was then analyzed with FlowJo software. Arrow indicates the addition of SDF-1α stimulus; green, control; red, Cinacalcet treated (n = 3 from 3 independent experiments). (B) In vitro chemotaxis assay of LKS+F−, LKS+F+, and LKS−F+ cells to 100 ng/mL SDF-1α after a 3-hour incubation. Blue columns represent chemokinesis controls, red columns represent chemotaxis (***P < .002, *P < .05; n = 3). (C) In vivo lodgment showing the percentages of injected LK cells localized at the endosteal region after ex vivo Cinacalcet and AMD3100 treatment (n = 160 sections from 2 independent experiments; error bars represent SEMs).
Discussion
Here, we demonstrated that enhancements in cell adhesion and CXCR4 signaling associated with CaR stimulation promote homing, lodgment, and engraftment of transplanted hematopoietic stem and progenitor cells. Specifically, the results indicated that with CaR stimulation, HSC adhesion to collagen I, one of the main ECM molecules released by cells of the osteoblastic lineage, was increased. Furthermore, with CaR stimulation, HSCs became more responsive to SDF-1α, resulting in enhanced migration toward this chemotactic agent present in the BM. A model of these coordinated interactions is shown in Figure 5. However, Cinacalcet treatment did not affect cell proliferation or the number of cells undergoing apoptosis, suggesting that CaR stimulation does not affect the cell cycle status of HSCs or their survival. In addition, CaR stimulation by Cinacalcet treatment did not alter the expression of the chemokine receptor CXCR4 and CAMs such as α4β1 integrins and L-selectins. Interestingly, CaR stimulation appeared to have the most significant effect on the LKS+F− subpopulation compared with its more mature counterparts in hematopoietic lineage commitment.
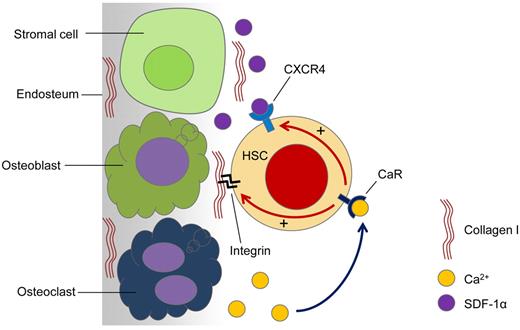
Proposed model for the role of CaR in HSC lodgment in vivo. Active bone remodeling releases Ca2+ ions into the endosteal region of the BM. HSCs arriving at the endosteal region are able to sense the released Ca2+ ions through the CaR. When the CaR is activated by Ca2+ ions, CXCR4 signaling is activated, which is known to be involved in cell survival and the retention of HSCs in the BM. Furthermore, activation of the CaR also enhances HSC adhesion to major ECM components in the adult BM, such as collagen I. This complex interplay of intracellular signaling dictates the fate of the transplanted HSCs.
Proposed model for the role of CaR in HSC lodgment in vivo. Active bone remodeling releases Ca2+ ions into the endosteal region of the BM. HSCs arriving at the endosteal region are able to sense the released Ca2+ ions through the CaR. When the CaR is activated by Ca2+ ions, CXCR4 signaling is activated, which is known to be involved in cell survival and the retention of HSCs in the BM. Furthermore, activation of the CaR also enhances HSC adhesion to major ECM components in the adult BM, such as collagen I. This complex interplay of intracellular signaling dictates the fate of the transplanted HSCs.
CaR was previously shown to be an important player in the regulation of HSC lodgment and engraftment after transplantation, whereby CaR−/− HSCs were found to have impaired ability to lodge in the endosteal surface of the bone, leading to defective engraftment.14 This defect in CaR−/− HSCs may have been attributable to a reduction in their ability to adhere to collagen I in the endosteal region. Collagen I is mainly secreted by osteoblasts in the endosteal region, and several studies have shown osteoblasts to be a key cellular component of the HSC niche.21-23 In addition, a previous immunofluorescence study has shown collagen I to be distributed nonuniformly throughout the endosteal region of the murine BM, with some areas of the region exhibiting high levels of expression, whereas others have much lower levels, such as the central marrow region.18 Because collagen I is mainly secreted by osteoblasts, the effect of CaR stimulation in enhancing adhesive interactions between LKS+F− cells and collagen I further confirms the importance of osteoblasts in supporting the endosteal HSC niche. Furthermore, by modulating the activity of CaR such that the receptor becomes more sensitive to activation by Ca2+ ions present in the endosteal region of the adult BM, this study provides new observations on the specific adhesive interaction between collagen I and LKS+F− cells in retaining these cells in the endosteal niche after transplantation. Altogether, these results seem to indicate that both Ca2+ ions released by active bone remodeling and collagen I secreted by osteoblasts in the endosteal region of the BM play important roles in HSC lodgment after transplantation and that the CaR may be a crucial player in this process that ultimately dictates the clinical outcome of the transplantation. Because these molecules are specifically expressed in the adult bone, this would further support our hypothesis that CaR allows for the preferential localization of the HSCs in the adult BM.14
Regulation of homing and lodgment of transplanted HSCs may involve crosstalk between CaR and CXCR4 signaling pathways. In adult hematopoiesis, CXCR4, the receptor for the CXC chemokine ligand 12 or SDF-1α, is known to play important roles in cell migration, proliferation, and survival.24,25 In relevance to transplantation, when CXCR4 is absent, retention of hematopoietic progenitors within the BM is impaired, resulting in defective hematopoietic reconstitution as shown by a study on CXCR4−/− chimeric mice.20 Results from this study have indicated that CXCR4 signaling is elevated with CaR stimulation, leading to enhanced retention of HSCs in the BM after transplantation. In fact, when CXCR4 is blocked by its antagonist AMD3100, the enhancement in cell lodgment after CaR stimulation is eliminated. This suggests that the CXCR4 signaling pathway may be an important mediator in the regulation of hematopoietic stem and progenitor cell function by the CaR. In addition, with CaR stimulation, the ability of LKS+F− cells to repopulate the BM and contribute to hematopoiesis in the recipient mice is enhanced. However, the underlying mediator that serves as the link between the CaR and CXCR4 signaling pathways is unknown.
Under homeostasis, HSCs reside close to the endosteal surface of the bone,26-28 where active bone remodeling occurs through tightly regulated interactions between osteoblasts and osteoclasts, resulting in the release of Ca2+ ions from the bone into the endosteal region. Localization of HSCs in the BM after transplantation has been one of the main focuses in the study of HSC niche biology. Because HSCs are able to sense and respond to fluctuations in Ca2+ concentrations through the CaR, the receptor might have a crucial role in specifying the localization of HSCs in the BM. By stimulating the CaR expressed on HSCs with the clinically approved allosteric agonist, Cinacalcet, we have identified some of the underlying mechanisms dictating the preferential localization of transplanted HSCs in the endosteal region of the BM, thereby providing additional insights into HSC niche biology. These mechanisms include activation of CXCR4 signaling and enhancement in adhesion to key ECM components, including collagen I and fibronectin that are expressed in the adult BM, after CaR activation by extracellular Ca2+ ions. Furthermore, clinical implications of this study include the use of pharmacologic agents such as calcimimetics to modulate the CaR activity ex vivo, thus enhancing HSC activity and improving lodgment and engraftment of transplanted HSCs in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr David T. Scadden for critical review of the manuscript and helpful suggestions; Dr Amir Goldkorn for assistance with obtaining reagents; and Lora Barsky, Dilani Rosa, and Xiaoying Zhou for technical assistance.
This work was supported by the American Society of Hematology (G.B.A.) and by the NIH Training Program in Cellular, Biochemical and Molecular Sciences (T32 GM067587) (B.S.L.).
National Institutes of Health
Authorship
Contribution: B.S.L. and C.C. performed experiments; B.S.L., C.C., and G.B.A. analyzed results and made the figures; and B.S.L. and G.B.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregor B. Adams, Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, Keck School of Medicine, University of Southern California, 1425 San Pablo St (BCC 410), Los Angeles, CA 90033; e-mail: gregor.adams@med.usc.edu.