IL-2 is a natural, T cell–derived cytokine that stimulates the cytotoxic functions of T and natural killer cells. IL-2 monotherapy has been evaluated in several randomized clinical trials (RCTs) for remission maintenance in patients with acute myeloid leukemia (AML) in first complete remission (CR1), and none demonstrated a significant benefit of IL-2 monotherapy. The objective of this meta-analysis was to reliably determine IL-2 efficacy by combining all available individual patient data (IPD) from 5 RCTs (N = 905) and summary data from a sixth RCT (N = 550). Hazard ratios (HRs) were estimated using Cox regression models stratified by trial, with HR < 1 indicating treatment benefit. Combined IPD showed no benefit of IL-2 over no treatment in terms of leukemia-free survival (HR = 0.97; P = .74) or overall survival (HR = 1.08; P = .39). Analyses including the sixth RCT yielded qualitatively identical results (leukemia-free survival HR = 0.96, P = .52; overall survival HR = 1.06; P = .46). No significant heterogeneity was found between the trials. Prespecified subset analyses showed no interaction between the lack of IL-2 effect and any factor, including age, sex, baseline performance status, karyotype, AML subtype, and time from achievement of CR1 to initiation of maintenance therapy. We conclude that IL-2 alone is not an effective remission maintenance therapy for AML patients in CR1.
Introduction
Patients with acute myeloid leukemia (AML) who achieve complete remission (CR) and subsequently relapse have very poor prospects for survival,1 and effective therapies have long been sought to maintain patients in first CR (CR1) and prevent relapse.2,3 One of the only interventions known to improve relapse rates in patients with AML is allogeneic hematopoietic stem cell transplantation (allo-HCT) used as consolidation or intensification therapy. In contrast, maintenance regimens used after consolidation that are aimed at preventing relapse have not been widely used in AML. Allo-HCT acts via a mechanism involving T- and natural killer (NK) cell–mediated destruction of the leukemic clone.4,–6 IL-2 is a potent immunoactivating cytokine that stimulates tumor-specific cytotoxic T lymphocytes and NK cells.7 Given that strong preclinical evidence suggests a cellular pathway for the antileukemic effects of IL-2,8,,–11 a logical question is whether IL-2, by virtue of its activity on T and NK cells, offers a pharmacotherapeutic approach to preventing relapse in AML. Clinical trials examining this therapeutic potential of IL-2 were initiated shortly after recombinant human IL-2 was introduced.12,13
Initially, nonrandomized trials of IL-2 in high doses (12-24 millions of international units [MIU]/m2/d) were reported to induce objective remissions in some patients with advanced leukemias.14,15 However, because toxicities of high-dose IL-2 were frequently severe or life-threatening, clinical development of IL-2 for AML was redirected to patients in CR with the goal of preventing relapse based on the premise that these patients might benefit from IL-2 immunotherapy given at lower doses for longer periods of time as maintenance therapy. Patients with AML in remission were enrolled in several small, nonrandomized trials of IL-2 (N = 9-39), the results of which at best suggested a modest benefit compared with historical controls.12,13,16,–18 The results of another randomized trial comparing a higher (9 MIU/m2) with a lower (0.9 MIU/m2) dose of postconsolidation IL-2 monotherapy in AML patients showed no difference between doses and were consistent with other trials demonstrating only a minimal benefit.19
Several randomized clinical trials (RCTs) of IL-2 monotherapy followed, each comparing the effect of a different dose of IL-2 with no treatment (ie, the standard-of-care [SOC]) on leukemia-free survival (LFS) and overall survival (OS) outcomes in AML patients in CR1.20,,,,–25 None of these RCTs found a benefit of IL-2 monotherapy as remission maintenance in AML patients, in contrast to a small RCT in relapsed AML patients, which showed a trend toward clinical benefit in favor of IL-2.26 Taken individually, the trials of maintenance therapy only had a modest statistical power to detect small, yet potentially worthwhile benefits of IL-2. The goal of the present meta-analysis was to achieve a higher statistical power to answer the question of whether IL-2 alone can be considered an effective remission maintenance therapy in this population. Individual patient data (IPD) were collected from all available RCTs with IL-2 monotherapy in AML patients in CR1. Subgroup analyses were performed to investigate the benefit, if any, of IL-2 in subsets of patients with different baseline prognostic factors.
Methods
Objectives
The primary objective of this study was to assess the efficacy of IL-2 monotherapy compared with no treatment in patients with AML who had achieved CR1. Efficacy was evaluated in terms of LFS by performing a meta-analysis on updated IPD from completed RCTs of IL-2 monotherapy as remission maintenance. Secondary objectives were to assess the efficacy of IL-2 compared with no treatment in AML patients in CR1 in terms of OS and to investigate the benefit of IL-2 alone in different subgroups differentiated by age, sex, performance status, karyotype, cytological AML subtype, and time elapsed between achievement of CR1 and initiation of remission maintenance therapy. Data on induction therapy were not available for all trials and showed too much heterogeneity for reliable analysis.
Trial search and selection strategy
Trials were identified by searching the following electronic databases for phase 2 or 3 trials of IL-2 as remission maintenance therapy in patients with AML in CR1: PubMed (Medline), Medscape, Google Scholar, Cochrane Library, Register of Clinical Trials (ClinicalTrials.gov), and conference proceedings of main congresses in hematology-oncology. In addition, investigators treating AML were contacted to confirm the status of trials. Only RCTs conducted in patients with AML in CR1 with at least 2 study arms consisting of IL-2 monotherapy versus no treatment were included. The RCTs also had to be closed to accrual and have a median follow-up of ≥ 3 years.
Fourteen trials of IL-2 in patients with AML in CR1 were identified, of which 6 met the criteria for inclusion (Table 1).20,,,,–25 The other 8 trials identified were excluded for the following reasons: 5 were nonrandomized12,13,16,–18 ; 1 was an ongoing RCT in children (age < 18 years) that was still recruiting patients (NCT00149162); 1 was an RCT comparing 2 doses of IL-2 with no control (SOC) arm19 ; and 1 was an RCT of histamine dihydrochloride (HDC) in conjunction with low-dose IL-2 versus SOC (no treatment).27
Data collection
The principal investigators of all eligible RCTs were asked to participate and requested to provide the most current IPD relating to remission maintenance with IL-2 versus no treatment. Specific data items used for the analysis included: trial, institution, and patient identifiers; randomization date; treatment assigned by randomization; demographic data at baseline (date of birth or age, sex, weight, body-mass index, and Eastern Cooperative Oncology Group [ECOG] performance status); karyotype (favorable, intermediate, or unfavorable as defined in each trial)20,,,–24,28 ; cytological AML subtype; months elapsed from achievement of CR; treatment dose and duration (first dose, last dose, and premature treatment discontinuation); reason for treatment discontinuation (disease progression, adverse event including toxic death, other reason, or unknown); date of last observation (or date of death if patient died); last observation censoring indicator (alive or death); cause of death (alive, nondisease/nontoxicity, disease-related/nontoxicity, toxicity-related, or unknown); date of progression (or date of last observation if patient did not progress); and progression indicator (no progression, progression).
Datasets containing IPD were obtained for 5 RCTs: Blaise et al,20 Acute Leukemia French Association (ALFA) 9801,21 Cancer and Leukemia Group B (CALGB) 9720,22 CALGB 19808,23 and Children's Cancer Group (CCG) 2961.24 ) The sample sizes, age group, IL-2 doses, and results of these studies are summarized in Table 1. To facilitate comparison across the different RCTs, the average planned monthly IL-2 doses in millions of international units of IL-2 per square meter of body surface area were derived from individual published RCTs. For 1 RCT (the European Organisation for Research and Treatment of Cancer (EORTC)–Gruppo Italiano Malattie e Matologiche dell'Adulto [Italian Group for Adult Hematologic Diseases or GIMEMA] 0699125 ), results have not yet been published in full, and the IPD could not yet be made available; however, the published abstract for this trial reported the LFS and OS hazard ratios (HRs), which could be incorporated into the meta-analysis (this trial, however, did not contribute to the subset analyses). None of the individual RCTs had stratified the randomization of patients to the IL-2 versus SOC arms by any of the factors known to affect prognosis (ie, age, ECOG performance status, karyotype, cytological AML subtype, or months from CR).
Meta-analysis
Standard meta-analysis methods were used, incorporating all updated IPD that were available (5 of 6 RCTs).29 A sensitivity analysis was also performed using data from all RCTs, including the trial for which the HRs were available but not the IPD.
All analyses were performed on an intent-to-treat basis, including all randomized patients in each trial, according to the treatment assigned by randomization regardless of treatment actually received. There were no “per-protocol” analyses.
The end points of interest were LFS, defined as the time from randomization to leukemic relapse or death from any cause, and OS, defined as the time from randomization to death from any cause. These time-related end points were analyzed according to Kaplan-Meier methods with stratified log-rank significance testing, using the study as the stratification factor. HRs were estimated using Cox regression models stratified by trial (“adjusted HR”) with HR < 1 indicating treatment benefit. Cox proportional hazards models were used to determine whether the estimates of treatment effects changed after adjustment for known prognostic factors.
Forest plots of HRs were produced for LFS and OS, both overall and within subsets according to meaningful trial and patient characteristics. Tests for heterogeneity were performed to assess the statistical significance of observed differences between the treatment effects in different RCTs. Subset analyses were performed using interaction tests to assess the statistical significance of observed differences between the treatment effects in different subsets.29
Statistical analyses were performed on SAS Version 9.1.3 software (SAS Institute). Graphs were produced using S-Plus Version 7.0 software (Insightful Corporation).
Results
IPD from 449 patients treated with IL-2 and 456 control patients were included in the meta-analysis, along with summary data from 550 patients randomized to IL-2 (n = 276) versus observation (n = 274) in the EORTC-GIMEMA 06991 trial (also known as AML-12).25 Patient demographics for the 5 RCTs for which IPD were available are shown in Table 2.20,,,–24 Patient characteristics varied slightly from trial to trial, and the most notable between-trial difference was age. CCG 2961 was conducted in children (mean age 8.2 years), whereas Blaise et al studied adults whose mean age was ∼ 40 years, similar to that of CALGB 19808 (∼ 43 years). The ALFA 9801 trial included an older population (∼ 60 years of age), and CALGB 9720 enrolled the oldest population (∼ 70 years of age). The mean age of patients enrolled in GIMEMA-EORTC 06991 has not been reported, although eligibility was restricted to patients < 61 years of age and the group is presumed to be similar in age to those enrolled in ALFA 9801 and CALGB 19808. Planned doses and schedules of IL-2 administration also differed across trials (Table 1). Standardized in units of millions of international units per square meter of body surface area per month, the average (intended) monthly IL-2 doses ranged from 12-24 MIU/m2 for 12 months25 to 120 MIU/m2 for 2 months.20
Effect on LFS
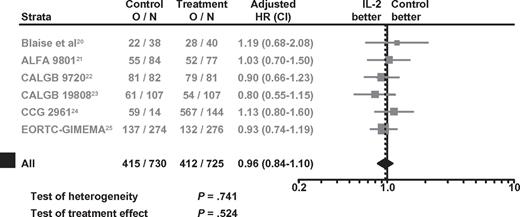
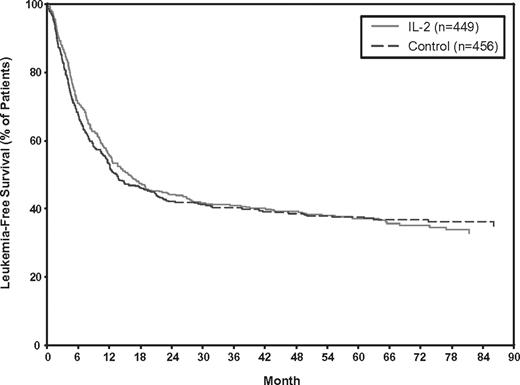
For IL-2 monotherapy, no significant LFS benefit was observed over controls, either when IPD from 5 RCTs were analyzed or when combining all 6 RCTs (Figure 1). The HRs were 0.97 (95% confidence interval [CI]: 0.82-1.15; P = .74) and 0.96 (95% CI: 0.84-1.10; P = .52), based on data from the 5 RCTs or 6 RCTs, respectively. Tests for heterogeneity were also not statistically significant (P = .62 and P = .74) for the 5 and 6 RCTs, respectively. The Kaplan-Meier LFS curves generated with IPD from the 5 RCTs showed no significant separation at any point in time (Figure 2).
Forest plots of HRs for the benefit of IL-2 monotherapy in terms of LFS in all 6 RCTs. O/N = event rate per arm where O is the number of observed events (relapse or death) and N is the sample size.
Forest plots of HRs for the benefit of IL-2 monotherapy in terms of LFS in all 6 RCTs. O/N = event rate per arm where O is the number of observed events (relapse or death) and N is the sample size.
Kaplan-Meier estimates of LFS using IPD from 5 RCTs of IL-2 monotherapy versus control (no treatment).
Kaplan-Meier estimates of LFS using IPD from 5 RCTs of IL-2 monotherapy versus control (no treatment).
Effect on OS
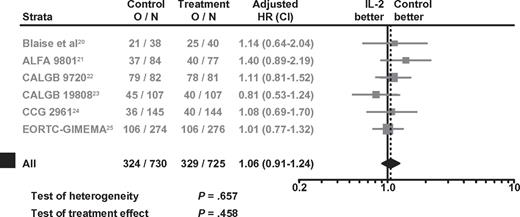
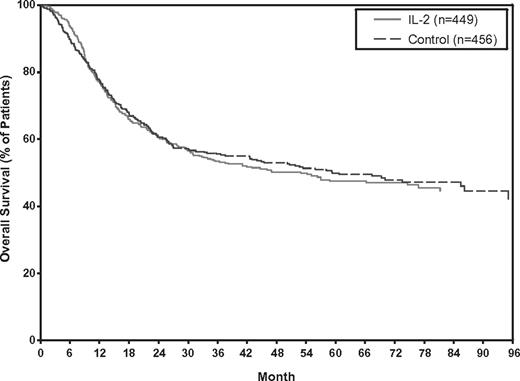
No significant OS benefit was observed with IL-2 monotherapy over controls, either using IPD from 5 RCTs or from all 6 RCTs (Figure 3). The HRs were 1.08 (95% CI: 0.90-1.31; P = .39) and 1.06 (95% CI: 0.91-1.24; P = .46), based on data from the 5 RCTs or 6 RCTs, respectively. Tests for heterogeneity were also not statistically significant (P = .54 and P = .66) for the 5 and 6 RCTs, respectively. The Kaplan-Meier OS curves generated from the 5 RCTs with IPD showed no significant separation at any point in time (Figure 4).
Forest plots of HRs for the benefit of IL-2 monotherapy in terms of OS in all 6 RCTs. O/N = event rate per arm where O is the number of observed events (relapse or death) and N is the sample size.
Forest plots of HRs for the benefit of IL-2 monotherapy in terms of OS in all 6 RCTs. O/N = event rate per arm where O is the number of observed events (relapse or death) and N is the sample size.
Kaplan-Meier estimates of OS using IPD from 5 RCTs of IL-2 monotherapy versus control (no treatment).
Kaplan-Meier estimates of OS using IPD from 5 RCTs of IL-2 monotherapy versus control (no treatment).
Subset analyses
Subset analyses are provided using available IPD from RCTs based on age group, sex, karyotype category, AML cytological subtype category, ECOG performance status (0-1 or other) and time from CR (≤ 4 months or > 4 months). Baseline performance status was not available for the Blaise trial nor for CCG 2961; therefore, these subset analyses are limited to IPD from 3 trials. A summary of the HRs, together with the significance and interaction testing conducted on all subset analyses, are provided in Table 3.
No statistically significant differences were found for the effect of IL-2 over controls on either LFS or OS according to age category (< 21 vs 21-60 vs > 60 years), sex (male vs female), ECOG performance status (0-1 vs other), karyotype category (favorable vs intermediate vs unfavorable vs unknown), AML cytological subtype (M0-M5-M6-M7 vs M1-M2-M4 vs other or unknown), or time from CR (≤ 4 months or > 4 months) to initiating maintenance therapy (Table 3). Forest plots of these subset analyses are shown in supplemental Figures 1-6 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The tests for interaction between the effect of IL-2 and these factors were in all cases far from statistically significant.
Discussion
After AML patients receive induction therapy and if they achieve CR, the duration of their CR1 is a primary prognostic indicator. Survival duration after relapse depends on various factors,30 but is generally short-lived, with a median of just 5-6 months.1 Therefore, the ability to maintain AML patients in CR1 remains one of the most significant challenges facing hematologists today.
Several multicenter and multinational clinical trials have shown the value of allo-HCT in preventing relapse.31,–33 However, a major limitation of allo-HCT is that recognition and elimination of leukemic cells by donor T cells (the GVL reaction) is also accompanied by destruction of normal host cells (GVHD), which leads to significant morbidity and mortality.34 Several trials aimed at controlling GVHD by removing donor T cells led to high leukemic relapse rates, which confirmed the major role of activated T cells in leukemic control after allo-HCT.35,–37 This explains the persistent interest on the part of clinical investigators in IL-2 as a pharmacoimmunotherapeutic means of replicating the beneficial effects of allo-HCT to prevent relapse in AML.
The fundamental premise linking IL-2, a cytokine known to stimulate T- and NK-cell function to destroy leukemic cells, and relapse prevention in AML is compelling from a scientific perspective.4,10,38,–40 Interest in the role of this cytokine did not subside even after an early RCT failed to demonstrate a significant benefit of IL-2 in survival outcomes in AML patients,20 and several more RCTs ensued.21,,,–25
Results of these trials are challenging to compare at face value, given the different age groups, remission induction regimens, lengths of follow-up, number of patients discontinuing due to toxicity, and doses of IL-2 that were used. For example, the ALFA 9801 trial21 randomized 161 AML patients in CR1 (50-70 years of age) to 12 months of intermediate-dose IL-2 versus observation. Of 77 patients allocated to IL-2, 22 completed 1 year of therapy and no difference in IL-2 versus observation was evident after 3 years of follow-up. CALGB 9720 was a trial in older patients (≥ 60 years of age) with AML in CR1.22 Eighty-one patients were randomized to low-dose subcutaneous IL-2 therapy, and 66% and 63% subsequently developed grade 4 thrombocytopenia and neutropenia, respectively. Median LFS and OS were similar for both IL-2–treated patients and controls, leading the investigators to conclude that low-dose IL-2 is not a successful strategy for prolonging disease-free survival and OS in older AML patients. CALGB 1980823 evaluated intermediate-dose IL-2 versus observation in 316 AML patients < 60 years of age in CR1 after consolidation chemotherapy. Ninety days of treatment were planned. Of 107 patients randomized to IL-2, only 47% completed therapy: 29% refused after randomization or were unable to start, 28% failed to complete, and 11% and 17% had grade 4 thrombocytopenia and neutropenia, respectively. The median 3-year follow-up revealed a trend in favor of IL-2 for LFS (P = .11) and OS (P = .09). CCG 2961 was a trial in 289 children with AML24 in which patients received a short course (18 days) of high-dose IL-2 versus no treatment after consolidation. After a median follow-up of 5 years, there was no difference in DFS or OS between the 2 regimens. The last trial of IL-2 monotherapy conducted by the EORTC-GIMEMA group began enrolling patients in 1999 and recently reported no difference in LFS or OS at 3 years of follow-up.25
We conducted these analyses after considering the possibility that the relatively small sample sizes of these trials may have prevented significant results from being detected. Moreover, no subset analyses were possible within the individual RCTs mainly due to sample size restrictions. Because all of these trials had a similar control group of SOC (no treatment) patients, it was possible to carry out this meta-analysis to increase the power of the data collected previously with the goal of reliably determining the existence of any clinical benefit of IL-2.
The present meta-analysis has some limitations, including the fact that patient populations and doses/schedules of IL-2 varied widely across the trials included. However, the inability to demonstrate significant effects of IL-2 on LFS and OS in any of the individual trials and in all subsets analyzed here makes it unlikely that this treatment, when used as monotherapy for remission maintenance, provides meaningful benefits. A lack of effect of IL-2 was consistently observed across all subsets of patients grouped by the most important prognostic factors known for AML: age, karyotype category, AML cytological subtype, ECOG performance status, and time from CR to initiation of maintenance therapy. The type of induction therapy and its potential impact on any benefit of IL-2 maintenance therapy could not be analyzed here. Sex also had no impact on the lack of difference between IL-2 and controls, which is consistent with the absence of benefit observed across any other subsets.
An important question remaining is why, given such strong preclinical evidence predicting IL-2 efficacy in AML patients in CR, does IL-2 fail to demonstrate clinical benefit? First, it can be argued that the long-term GVL effect, which is well documented after allo-HCT, results from a permanent donor chimerism including a constant activation of antileukemic subclones of donor origin.41 This situation is obviously not easy to mimic by the relatively short-term administration of IL-2 after remission after autologous HCT. Second, it has been proposed that the efficacy of IL-2 monotherapy could be hampered by the activity of other immune cells that prevent the activation and proliferation of cytotoxic lymphocytes (ie, the effector cells of IL-2).2 Reactive oxygen species (ROS) or “oxygen radicals” derived from adjacent phagocytic cells have been shown to inhibit the ability of IL-2 to effectively activate T and NK cells.42 Reactive oxygen species also reduce the expression of the CD3ζ antigen that is critical to signal transduction in lymphocytes43,–45 and trigger apoptosis of cytotoxic lymphocytes.46,–48 Several preclinical studies (reviewed in Romero et al2 ) have established a role for histamine dihydrochloride (HDC) in AML. When added to immunotherapy with IL-2, HDC inhibits ROS production, which is mediated by H2 receptors on myeloid cells, and may help to maintain viability and function of antileukemic lymphocytes. Clinical pharmacodynamic data from a phase 2 trial in AML patients demonstrated that HDC protected the effects of IL-2 on T and NK cells, with encouraging clinical results.39 In a subsequent randomized trial of AML patients in CR (N = 320),27 remission maintenance therapy with HDC in conjunction with low-dose IL-2 resulted in a significant prolongation of LFS (P < .01) and a trend toward improvement in OS in patients in CR1 (P = .12). Therefore, the suggestion that IL-2 has the potential to improve LFS and OS in AML may be valid, but for IL-2 to exert a significant clinical effect on relapse prevention in this disease, its activity on T and NK cells may need to be protected from interference by ROS.2
In conclusion, this meta-analysis confirms that IL-2, when given as monotherapy, is not effective as a remission maintenance therapy for AML patients in CR1, a conclusion also reached by others based on data extracted from the literature.49 However, the optimism that clinicians once held for immunotherapy to prevent relapse in AML should not be discounted, because it may be possible to protect the activity of IL-2 on T and NK cells from ROS–mediated destruction in the tumor microenvironment.27,39,50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Donald Fallon, MA, ELS, of MedVal Scientific Information Services LLC for editing the manuscript.
Funding to support these analyses and preparation of this manuscript was provided by EpiCept Corporation (to M.B., P.S., and K.J.L.). EpiCept was not involved in the collection or analyses of data nor in the review of this manuscript.
Authorship
Contribution: M.B. and T.B. provided the study concept and design; B.J.L., T.A.A., J.E.K., R.A.L., S.L.G., C.D.B., S. Castaigne, and D.B. provided study materials or patients; P.S. collected and assembled data; M.B., P.S., S.L.G., T.B., B.J.L., T.A.A., R.A.L., J.E.K., C.D.B., S. Castaigne, S. Chevret, D.B., and D.M. analyzed and interpreted data; K.J.L., M.B., P.S., and T.B. wrote the manuscript; and all authors reviewed and provided final approval of the manuscript.
Conflict-of-interest disclosure: M.B., P.S., and K.J.L. have a consultant or advisory role with EpiCept and M.B. and P.S. received funding for this study from EpiCept. The remaining authors declare no competing financial interests.
Correspondence: Marc Buyse, ScD, International Drug Development Institute, 30 Avenue Provinciale, Louvain-la-Neuve, Belgium, 1340; e-mail: marc.buyse@iddi.com.