Abstract
Previous randomized graft-versus-host disease (GVHD)-prophylaxis trials have failed to demonstrate reduced incidence and severity of chronic GVHD (cGVHD). Here we reanalyzed and updated a randomized phase 3 trial comparing standard GVHD prophylaxis with or without pretransplantation ATG-Fresenius (ATG-F) in 201 adult patients receiving myeloablative conditioning before transplantation from unrelated donors. The cumulative incidence of extensive cGVHD after 3 years was 12.2% in the ATG-F group versus 45.0% in the control group (P < .0001). The 3-year cumulative incidence of relapse and of nonrelapse mortality was 32.6% and 19.4% in the ATG-F group and 28.2% and 33.5% in the control group (hazard ratio [HR] = 1.21, P = .47, and HR = 0.68, P = .18), respectively. This nonsignificant reduction in nonrelapse mortality without increased relapse risk led to an overall survival rate after 3 years of 55.2% in the ATG-F group and 43.3% in the control group (HR = 0.84, P = .39, nonsignificant). The HR for receiving immunosuppressive therapy (IST) was 0.31 after ATG-F (P < .0001), and the 3-year probability of survival free of IST was 52.9% and 16.9% in the ATG-F versus control, respectively. The addition of ATG-F to standard cyclosporine, methotrexate GVHD prophylaxis lowers the incidence and severity of cGVHD, and the risk of receiving IST without raising the relapse rate. ATG-F prophylaxis reduces cGVHD morbidity.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is increasingly used worldwide as a curative therapy for malignant and nonmalignant hematologic disorders. Chronic graft-versus-host disease (cGVHD) is the leading cause of nontransplantation mortality and morbidity after allogeneic HSCT.1-3 cGVHD is a multiorgan disease resembling autoimmune disorders, such as scleroderma or systemic lupus.4,5 Its incidence and prevalence are rising because of transplantation practices known to be associated with increased risk of cGVHD.4,6 Indeed, older patients now undergo HSCT, and more transplantations are being performed from unrelated donors and/or with peripheral blood stem cells instead of bone marrow. Furthermore, the reduced-intensity conditioning (RIC) regimens developed during recent years have also led to higher numbers of transplantations performed worldwide.7,8 However, although the acute GVHD (aGVHD) rate appears lower after RIC, the incidence of cGVHD seems to be unaffected.9 Altogether, cGVHD thus remains the most challenging complication after allogeneic HSCT.10
The main risk factor for developing cGVHD is the previous occurrence of aGVHD.11 Thus, transplantation physicians have focused on decreasing the rate of aGVHD to lower nonrelapse mortality (NRM) associated with both aGVHD and cGVHD. However, although calcineurin inhibitors (cyclosporine or tacrolimus) in association with methotrexate have proven to decrease the aGVHD rate in randomized studies and although new regimens, such as the association of rapamycin with tacrolimus, seem to lower the aGVHD rate in phase 2 trials, none of those prophylactic regimens has reduced the incidence rate and severity of cGVHD.12-14
A key pathophysiologic role of donor T cells in the initiation and development of cGVHD is recognized.4 Donor T cells are also main effectors of aGVHD.4 Based on these experimental data, T-cell depletion has been proposed as a tool to lower GVHD incidence and severity. However, whereas numerous phase 2 trials have been conducted,15 few randomized trials using T-cell depletion versus drug prophylaxis have been performed.16-20 Lessons from all but one21 of these randomized trials indicated that, although aGVHD might be reduced and the relapse rate increased, cGVHD incidence rate and severity remained basically unchanged after T-cell depletion.
Different types of anti–T-cell globulin (ATG) preparations have been tested as part of conditioning regimens to achieve in vivo T-cell depletion so as to prevent GVHD.22 Anti-Jurkat ATG-Fresenius (ATG-F), in addition to cyclosporine and methotrexate, has shown promising results in several phase 2 trials for transplantation from matched or mismatched unrelated donors.23-25 We previously reported that, adding ATG-F to standard cyclosporine, methotrexate GVHD prophylaxis significantly reduced severe acute in a randomized phase 3 trial.26 The cumulative incidence of aGVHD grade 3 and 4 was 11.7% in the ATG-F group versus 25.5% in the control group (adjusted hazard ratio [HR] = 0.48, 95% confidence interval [CI], 0.24-0.96, P = .039), and cumulative incidence of grade 2 and 4 was 33.0% in the ATG-F group versus 52.0% in the control group (adjusted HR = 0.55, 95% CI, 0.35-0.85, P = .008). These results slightly differ from results published by Finke et al26 because one patient in the control group originally classified as having aGVHD grade 1 was later reviewed again and classified as grade 3. Here we present final data and unpublished results on cGVHD with extended follow-up.
Methods
Study design
We previously reported this randomized, multicenter, open-label, phase 3 trial to compare standard GVHD prophylaxis plus pretransplantation ATG-F to standard GVHD prophylaxis alone (control) in adult patients receiving myeloablative conditioning before hematopoietic cell transplantation from matched unrelated donors.26 All patients received myeloablative conditioning regimens containing total body irradiation (8-12 Gy), or busulfan (14-16 mg/kg orally or equivalent for intravenous administration) plus cyclophosphamide (2 × 60 mg/kg) or etoposide; or regimens containing thiotepa ≥ 15 mg/kg or carmustine ≥ 300 mg/m2. All patients received cyclosporine starting on day −1 with target trough levels ≥ 200 ng/mL in combination with methotrexate 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. Cyclosporine was recommended to be tapered after day 100. Patients in the ATG-F group received additional ATG-F at a dose of 20 mg/kg on day −3, day −2, and day −1 (total dose, 60 mg/kg) before transplantation. Concerning cGVHD, primary physicians were asked to stage organ involvement by severity (none, mild, moderate, and severe) and then to grade patients according to Shulman et al as none, limited, or severe.27 Other details on regimen and patients population are summarized in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
All patients gave written informed consent in accordance with the Declaration of the Helsinki, and approval was given by the University Medical Center Freiburg Institutional Review Board. Project management, statistical planning and analysis, randomization, data management, and clinical monitoring were conducted by the Clinical Trials Center, University Medical Center Freiburg, Freiburg, Germany. Patients were followed until 2 years after recruitment of the last patient, as specified in the study protocol.
Prespecified endpoints included incidence and severity of aGVHD, incidence and severity of cGVHD (cGVHD, limited/extensive and extensive), incidence of relapse, incidence of NRM (defined as death without preceding relapse), disease-free survival, overall survival, infections, and adverse events. Extensive versus limited cGVHD was defined according to Seattle's group criteria.27 However, aGVHD beyond day 100 in the absence of cGVHD was categorized as late aGVHD in accordance with the guidelines of the National Institutes of Health.28 In case acute and chronic symptoms of GVHD were present simultaneously, the GVHD was classified as cGVHD.26
Statistical analysis
Treatment groups were compared with respect to time to cGVHD (limited/extensive and extensive), time to cGVHD in different target organs (skin, eyes, mouth, lung, liver), time to relapse, time to NRM, overall survival time, time under immunosuppressive therapy (IST), and time to late bacterial infections (after day 100). Additional definitions of parameters are provided in the supplemental Methods.
Treatment groups with respect to time-to-event variables were compared with Cox regression models for the event-specific hazard functions using 2-sided Wald tests. Disease status (early vs advanced) and stem-cell source (bone marrow vs peripheral blood) were included for adjustment. To estimate the effect size, the HR of ATG-F versus control was calculated with 95% CI. In addition to the cumulative incidence rates29 as estimators of probability of event over time, we calculated Cox model-based rates adjusted for the covariates disease status and stem-cell source.30,31
The effect of cGVHD (limited/extensive and extensive) on relapse and NRM was investigated with a Cox regression model with cGVHD as time-dependent covariate. The HRs of cGVHD versus no cGVHD were estimated with 95% CI, and cumulative hazard functions were displayed for illustration.
Treatment groups were compared with respect to the transition hazards between the states “alive and free of IST” and “alive under IST” using a Cox regression model, including disease status and stem-cell source for adjustment. Transition HRs were estimated with 95% CI using robust estimators of SEs.32 The probabilities of survival under IST and of survival free of IST (adding up to the overall survival probability) over time were estimated with the Aalen-Johansen estimator33 using the R package “etm.”34
Statistical analysis was performed using the Statistical Analysis System, Version 9.2, and R, Version 2.11.1. The study is registered with WHO primary registers at www.clinicaltrials.gov as #DRKS00000002 and at https://drksneu.uniklinite-freiburg.del/drks_web as #NCT00655343.
Results
Study patients
The patient population has already been described in detail.26 A total of 202 patients were randomized in 31 centers. One patient was not transplanted. A total of 201 patients (103 ATG-F, 98 control patients) with median age of 40 years (range, 18-60 years), transplanted between 2003 and 2007, with acute myeloid leukemia (n = 101), myelodysplastic syndrome (n = 10), acute lymphoid leukemia (n = 70), chronic myeloid leukemia (n = 17), osteomyelofibrosis (n = 3) in early (first complete response or myelodysplastic syndrome-refractory anemia, n = 107), or advanced status of disease (all other, n = 94) were observed for a median follow-up time of 3 years (25% quartile, 2.5; 75% quartile, 3.9 years). Patient, disease, and transplantation characteristics are summarized in Table 1.
cGVHD incidence and severity
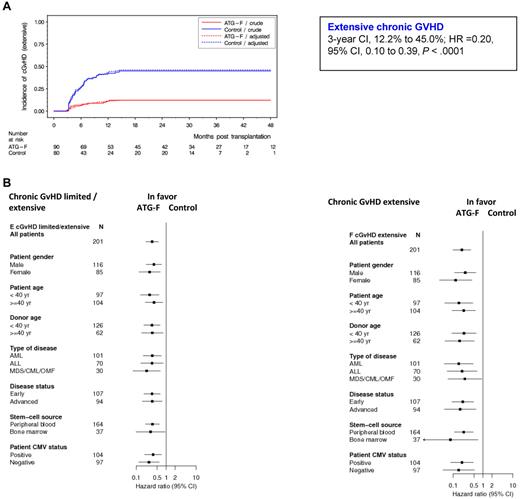
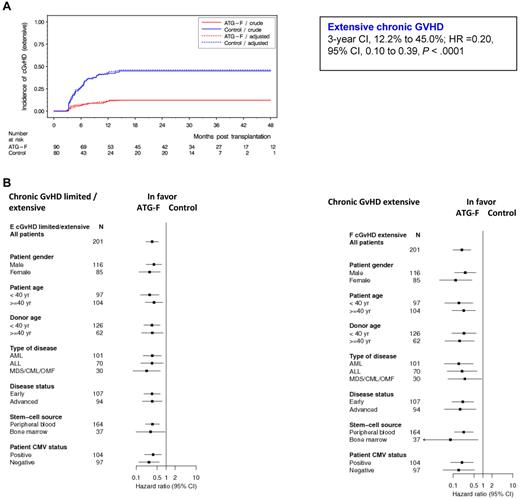
Of the 170 patients alive without second transplantation at day 100 (ATG-F, n = 90; control, n = 80), 75 patients experienced cGVHD (limited or extensive) (ATG-F, n = 27; control, n = 48), with 47 patients presenting extensive cGVHD (ATG-F, n = 11; control, n = 36). Detailed patient presentation is provided as supplemental Table 1. The cumulative incidence of extensive cGVHD after 3 years was 12.2% in the ATG-F group versus 45.0% in the control group (HR = 0.20, 95% CI, 0.10-0.39, P < .0001, Figure 1A). Cumulative incidence of limited/extensive cGVHD was 30.0% and 60.0% in the ATG-F versus control, respectively (HR = 0.34, 95% CI, 0.21-0.55, P < .0001). Treatment effect was also assessed in different subgroups of patients defined by prognostic factors (Figure 1B). ATG-F significantly reduced cGVHD (defined as limited/extensive or extensive alone) whatever the patient sex, patient or donor age, type of disease, disease status, stem cell source, or patient cytomegalovirus status was. Compared with the results shown in Finke et al26 (supplemental Methods), Figure 1B now shows slightly different, but similar, results after a median follow-up time of 3 years. The cumulative incidence of late aGVHD after 3 years was 4.4% in the ATG-F group versus 11.3% in the control group.
Cumulative incidence of extensive chronic GVHD by treatment groups overall and by prognostic subgroups. (A) Effect of treatment on extensive cGVHD. (B) Treatment effects within prognostic subgroups with regard to cGVHD, analyzed with Cox regression models adjusted for disease status and stem-cell source.
Cumulative incidence of extensive chronic GVHD by treatment groups overall and by prognostic subgroups. (A) Effect of treatment on extensive cGVHD. (B) Treatment effects within prognostic subgroups with regard to cGVHD, analyzed with Cox regression models adjusted for disease status and stem-cell source.
We then analyzed how ATG-F prophylaxis affected cGVHD in different target organs. As shown in supplemental Table 1 and supplemental Figure 2, cumulative incidences were reduced in all main cGVHD target organs: skin (3-year cumulative incidence, 5.6% vs 27.0%; ATG-F vs control, HR = 0.18, 95% CI, 0.07-0.48, P = .0006), eyes (2.2% vs 20.7%; HR = 0.10, 95% CI, 0.02-0.45, P = .0025), mouth (4.4% vs 18.8%; HR = 0.24, 95% CI, 0.08-0.74, P = .013), lung (3.3% vs 16.3%; HR = 0.17, 95% CI, 0.05-0.61, P = .006), and liver (1.1% vs 21.2%; HR = 0.05, 95% CI, 0.01-0.39, P = .004). The incidences in other, less involved target organs were not calculated because of few events, but crude numbers are described in a footnote to supplemental Table 1.
Impact of cGVHD on relapse rate and NRM
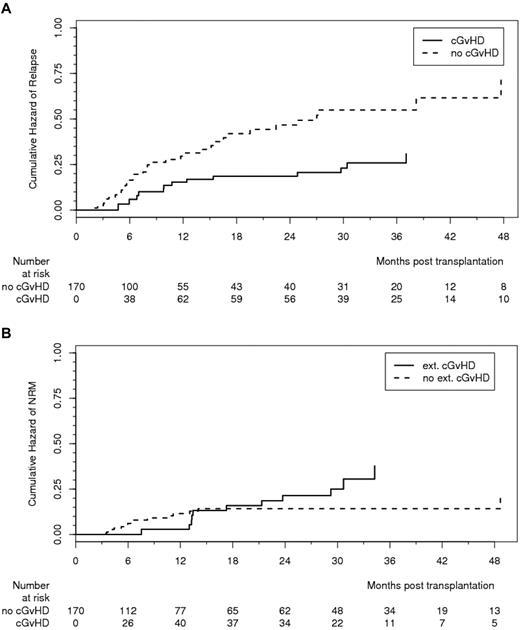
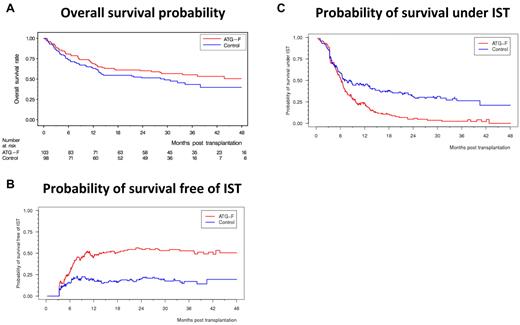
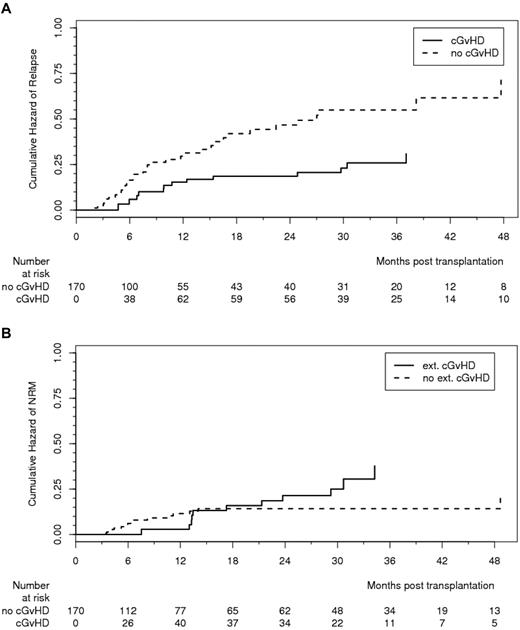
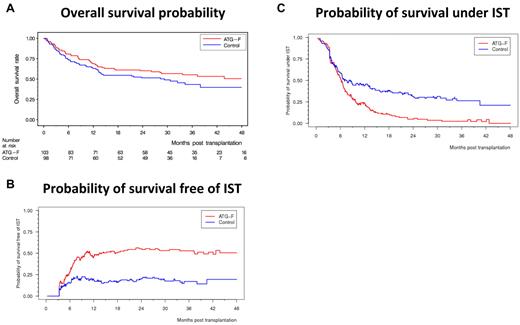
cGVHD lowered the relapse rate, resulting in an HR of 0.50 (95% CI, 0.26-0.96, P = .037; Figure 2A). Of note, GVHD prophylaxis with ATG-F was not associated with increased risk of relapse because the 3-year cumulative incidence of relapse was 32.6% in the ATG-F group and 28.2% in the control group (shown in supplemental Figure 3B; HR = 1.21, 95% CI, 0.72-2.02, P = .47). There was a trend to an increase in NRM by extensive cGVHD, resulting in an HR of 2.06 (95% CI, 0.93-4.58, P = .075; Figure 2B); the 3-year cumulative incidence of NRM was 19.4% in the ATG-F group and 33.5% in the control group (shown as supplemental Figure 3C; HR = 0.68, 95% CI, 0.38-1.20, P = .18). This still nonsignificant reduction in NRM without increased relapse risk led to an overall survival after 3 years of 55.2% in the ATG-F group and 43.3% in the control group (HR = 0.84, 95% CI, 0.56-1.25, P = .39; Figure 3A), and similar disease-free survival in both groups (supplemental Figure 3A).
Effect of chronic GVHD on relapse and nonrelapse mortality. (A) Effect of cGVHD (limited and extensive) on relapse effect estimated from Cox model with time-dependent covariate (HR = 0.49, 95% CI, 0.26-0.96, P = .037). (B) Effect of cGVHD (extensive) on NRM. Effect estimated from Cox model with time-dependent covariate (HR = 2.06, 95% CI, 0.93-4.58, P = .075).
Effect of chronic GVHD on relapse and nonrelapse mortality. (A) Effect of cGVHD (limited and extensive) on relapse effect estimated from Cox model with time-dependent covariate (HR = 0.49, 95% CI, 0.26-0.96, P = .037). (B) Effect of cGVHD (extensive) on NRM. Effect estimated from Cox model with time-dependent covariate (HR = 2.06, 95% CI, 0.93-4.58, P = .075).
Split of overall survival probability into probability of survival free of IST and probability of survival under IST. (A) Overall survival probability. (B) Probability of survival free of IST. (C) Probability of survival under IST.
Split of overall survival probability into probability of survival free of IST and probability of survival under IST. (A) Overall survival probability. (B) Probability of survival free of IST. (C) Probability of survival under IST.
Effect of ATG-F on treatment duration and time to stop IST
The HR for receiving IST was 0.31 (95% CI, 0.18-0.55, P < .0001), and that for being able to stop IST was 2.02 (95% CI, 1.41-2.91, P = .0001, ATG-F vs control, respectively). At 3 years, the probability of being alive and free of IST was 52.9% and 16.9%, and that of being alive and still under IST was 2.4% and 26.3% in the ATG-F versus control, respectively (Figure 3B-C). As can be seen in Figure 3, the probabilities of being alive and free of IST and of being alive under IST add up to the probability of overall survival. Although we see only a slight survival advantage of ATG-F versus control, our results show that patients in the ATG-F group predominantly live free of IST whereas patients in the control group predominantly live under IST.
Effect of ATG-F on late bacterial infection and cause of late nonrelapse-related death
We previously reported the occurrence of 4 fatal cases of post-transplantation lymphoproliferative disorders in patients treated in the ATG-F arm. In the present paper aiming to study cGVHD, we focus our attention to late bacterial infections, which are the main cause of mortality and lead to significant morbidity in patients with cGVHD.4 We analyzed here the distribution of these infectious complications. Main bacterial species were Streptococcus, Staphylococcus, Klebsiella, and Pseudomonas species (Table 2). The main infectious locations were bloodstream (n = 38, 17 vs 21), urinary tract (n = 19, 15 vs 4), oral/gastrointestinal (n = 18, 10 vs 8), and pulmonary (n = 18, 6 vs 12; Table 2) in the ATG-F group versus control group, respectively. The 3-year cumulative incidence of late bacterial infection (after day 100) was 26.3% versus 38.8% (Figure 4) in the ATG-F group versus control group, respectively (HR = 0.68, 95% CI, 0.39-1.17, P = .16). Main causes of non–relapse-related deaths over the long-term (> 100 days) included infection (5 and 4 patients) and cGVHD (0 and 4 patients), in the ATG-F group versus control group, respectively (Table 2). Overall updated causes of death are summarized in supplemental Table 2.
Effect of treatment on late bacterial infection. Treatment effect estimated from Cox model adjusted for disease status and stem-cell source (HR [ATG-F vs control]= 0.68, 95% CI, 0.39-1.17, P = .16).
Effect of treatment on late bacterial infection. Treatment effect estimated from Cox model adjusted for disease status and stem-cell source (HR [ATG-F vs control]= 0.68, 95% CI, 0.39-1.17, P = .16).
Prognostic factors for developing cGVHD
We finally aimed to study prognostic factors for developing cGVHD in our randomized trial (supplemental Table 4). Cox regression analyses of prognostic factors for developing extensive cGVHD adjusted for treatment arm and aGVHD (as a time-dependent factor) revealed 2 factors associated with increased extensive cGVHD risk: donor age of 40 years or more (HR = 2.01, 95% CI, 1.08-3.72, P = .027) and type of disease (HRs = 3.88, 95% CI, 1.25-12.0, 1.56, 0.76-3.20, and 2.63, 1.12-6.16 for patients with myelodysplastic syndrome, acute lymphoid leukemia, and chronic myeloid leukemia/osteomyelofibrosis compared with acute myeloid leukemia, respectively; P = .039; supplemental Table 4A). Factors found to be associated with increased cGVHD risk (limited or extensive) were type of disease (HRs = 1.88, 95% CI, 0.75-4.73, 1.60, 0.88-2.90, and 2.78, 1.38-5.58, for patients with myelodysplastic syndrome, acute lymphoid leukemia, and chronic myeloid leukemia/osteomyelofibrosis compared with acute myeloid leukemia, respectively; P = .023) and a non–irradiation-based conditioning regimen (HR = 2.58, 95% CI, 1.49-4.46, P = .0007; supplemental Table 4B).
Discussion
We present here data on cGVHD with extended follow-up (median, 3 years) on the largest randomized trial comparing standard GVHD prophylaxis versus standard prophylaxis plus ATG. The addition of ATG-F to cyclosporine, methotrexate GVHD prophylaxis significantly reduces the incidence and severity of cGVHD and the time to treat patients with IST without increasing relapse rate. In addition, our data showed that not only incidence, but disease severity and organ involvement, strongly favors the use of ATG-F. These data demonstrate that ATG-F prophylaxis decreases cGVHD morbidity.
Although ex vivo T-cell depletion has been used for decades with the aim to reduce aGVHD, there have been very few randomized trials.15-20 Indeed, one randomized study of hematopoietic cell transplantation from human leukocyte antigen-identical sibling donors already in the late 1980s demonstrated a decrease in the incidence of aGVHD, no difference in cGVHD, and an increased risk of relapse and rejection.16,17 Furthermore, T-cell depletion has been associated with increased risk of post-transplantation lymphoproliferative disorder.15-20 This risk of post-transplantation lymphoproliferative disorder was also found in our trial with 4 fatal cases in the ATG-F arm compared with no case in the control group. Only 2 randomized trials have been conducted on the use of ATG or T-cell depletion in hematopoietic cell transplantation from unrelated donors.18,19 An American trial17 compared 405 patients receiving bone marrow from unrelated donors, 2 different in vitro T-cell depletion methods (by anti–T-cell monoclonal antibody T10B9 [MEDI-500, Medimmune] or counter flow elutriation in combination with in vivo use of equine antithymocyte globulin [Pharmacia/Pfizer] and cyclosporine to a control group receiving cyclosporine and short-course methotrexate alone). The experimental group presented less severe aGVHD, but T-cell depletion had no influence on the incidence of cGVHD or survival.17 In the Italian sequential trial19,21 testing in vivo T-cell depletion, a total of 109 patients received bone marrow from unrelated donors and standard GVHD prophylaxis with or without antithymocyte globulin (Thymoglobulin, Genzyme). The reduction in GVHD was accompanied by a higher risk for lethal infections resulting in no improvement in survival. Extensive cGVHD developed more frequently in patients not given ATG.20 In a retrospective, nonrandomized analysis, the French registry35 most recently assessed the impact of rabbit ATG, incorporated within a standard myeloablative conditioning regimen before allogeneic stem cell transplantation using unrelated donors. In their retrospective series of 120 patients with leukemia, 69 did not receive ATG, whereas 51 patients did. With a median follow-up of 30.3 months, the cumulative incidence of extensive cGVHD was significantly lower in the ATG group compared with the “no-ATG” group (4% vs 32%, respectively; P = .0017). In multivariate analysis, the absence of ATG use was the strongest parameter associated with increased risk of extensive cGVHD (RR = 7.14; 95% CI, 1.7-33.3, P = .008). At 2 years, the probability of NRM, relapse, and overall and leukemia-free survivals did not significantly differ between the “no-ATG” and “ATG” groups. Thus, our trial definitively proves results suggested by the French retrospective analysis, as well as data suggested by the Italian sequential trials on a large patient population mainly receiving peripheral blood stem cells.
cGVHD is associated with a strong antileukemic effect,4-6 as confirmed here because cGVHD was associated with a nearly 50% decrease in relapse risk. However, in contrast with other reports using T-cell depletion showing increased propensity to relapse,15 GVHD prophylaxis with ATG-F was not associated with increased risk of relapse. However, the study was not powered to detect differences in relapse risk among the 2 groups.
In addition to this graft-versus-leukemia effect, there was a trend that extensive cGVHD also raised NRM in our cohort, as reported by others.1,3,10,36-39 However, while decreasing 3-year NRM incidence by > 10% (on an absolute scale), ATG-F did not lead to a large improvement in overall survival on a relative scale (ATG-F 55.2% vs control 43.3%). Improved supportive care and antimicrobial therapies of patients with cGVHD40 most probably explain why decreased incidence and severity of cGVHD in the ATG-F arm do not yet translate to improved survival. Similarly, although the risk of severe bacterial infections was higher in the control group, as expected from cGVHD rates, neither the late bacterial infection rate nor bacterial-related deaths significantly differs between the 2 treatment groups.
Finally, as discussed elsewhere, the most stringent proof of any prophylactic or treatment efficacy in cGVHD is to compare its ability to provide quality-of-life-improved survival without immunosuppressive drug therapy between 2 treatment groups.37,41-44 Indeed, the cGVHD course is typified by flare and remission episodes, and the withdrawal of any drugs to attain immune tolerance is the ultimate goal in treating this disease. Our data demonstrate that patients in the ATG-F group had a 100% better chance than the controls of being able to stop IST. At 3 years, the ATG-F group's probability of being alive and free of IST was 3-fold higher than that of the control group.
Prognostic factors for developing cGVHD after adjusting for previous aGVHD and ATG-F treatment showed that patients with acute myelogenous leukemia had a slightly lower risk of developing cGVHD and that older donor's age and the use of a non–irradiation-based conditioning regimen raised the risk. We have no formal explanations for those results. These results must be confirmed because multiple comparisons were performed.
However, it needs to be made very clear that (1) these data do not necessarily apply to other brands of ATG, and (2) that they do apply only to ablative HSCT. Furthermore, in a recent retrospective Center for International Blood and Marrow Transplant Research analysis of RIC HSCT using, in a nonrandomized setting, other ATG brands showed worse outcomes with ATG.45 Thus, our results may not apply to RIC HSCT. Finally, most of the stem cell products used in our study were grafts from peripheral blood, although there were minorities of marrow infusions: thus, our data may not apply to marrow grafts in which there is a lower incidence of cGVHD.
In conclusion, the addition of ATG-F to standard GVHD prophylaxis significantly reduces the incidence, severity, and morbidity of cGVHD and the risk of receiving IST. It does not increase the risk of relapse and may ultimately provide a long-term survival advantage.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jan Beyersmann and Anika Buchholz, Institute of Medical Biometry and Medical Informatics, University Medical Center, Freiburg, Germany, for support of the statistical analysis, and Carole Cuerten, Department of Hematology and Oncology, University Medical Center, Freiburg, Germany, for editorial assistance.
The study was supported by Fresenius Biotech GmbH, Germany.
Authorship
Contribution: G.S., W.A.B., H.D.O., M.S., A.R.Z., L.V., T.R., D.A.H., R.S., K.K., J.M., J.A.M., W.L., E.H., V.K., M.B., H.E., H.-J.K., H.B., M.E., and J.F. contributed to the recruitment and treatment of patients in the trial; J.F., C.S., O.G., and G.S. contributed to the design and analysis of the trial; and all authors contributed to the interpretation of the data and saw and approved the final version of the manuscript.
A complete list of the ATG-Fresenius Trial Group members appears in the “Appendix.”
Conflict-of-interest disclosure: J.F. has received travel and lecture fees from Fresenius Biotech GmbH. W.A.B. has received consultancy and lecture fees from Fresenius Biotech GmbH. K.K. has received travel and lecture fees from Fresenius Biotech GmbH. H.B. has received travel and lecture fees from Fresenius Biotech GmbH. M.E. has received travel fees from Fresenius Biotech GmbH. The other authors declare no competing financial interests.
Correspondence: Gérard Socié, Hematology/Transplantation, Hospital Saint Louis & University paris VII, 1 Avenue Claude vellefaux 75475 Paris, Cedex 10, France; e-mail: gerard.socie@sls.aphp.fr.

![Figure 4. Effect of treatment on late bacterial infection. Treatment effect estimated from Cox model adjusted for disease status and stem-cell source (HR [ATG-F vs control]= 0.68, 95% CI, 0.39-1.17, P = .16).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/23/10.1182_blood-2011-01-329821/4/m_zh89991171840004.jpeg?Expires=1767803929&Signature=Ep~jdJ2TPBACJAClJ7tdFAJVP8grmFP13A4YT7Slh16t4Gg-enQHiMvq49LuumW6syJ7SDsdahQihqWMUcqkBO4OWki45ppQnz03JYEIj546CW2m0mvuCb1DTjjJC-WPZS8tuU-2XQSkKuiXr9eCNe4-OvOKRt4mD2XH4GdQuY4fvrPprQqSGmGG3etQ5vMtiqwBE7x601okLoQ09aDeVkT6B5r7sk2VblfKTdTNvOv3hMZbSqk7vmR6ClnZDIyBaU4qUZnwhdS9HQ2-ojsiY4Ve0oAKgPbB-uSCM6HkgGzKpAEBKHOsQ56GQNqlpQXDEHRPTgwY6Dx9Trwgcx8dCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Effect of treatment on late bacterial infection. Treatment effect estimated from Cox model adjusted for disease status and stem-cell source (HR [ATG-F vs control]= 0.68, 95% CI, 0.39-1.17, P = .16).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/23/10.1182_blood-2011-01-329821/4/m_zh89991171840004.jpeg?Expires=1768318509&Signature=OjJvbigUPIDtpcuTiD25ByPSU8qSOOY826AAV46KfSipbD7HSViPvLJawtfn9Eh2Qh9FoWtAna7--fxlTtn~4xTWEkYyMWOfPHeD2OPOi5aMCAY2sK0rPNqMfdJJLwElCQyD8~M0V2A47VoCKSFjihl2l-HpBE29nuchQm8XQ6NPmsS4~JPMVKi7QggHs36UW3mv9GVMOBMdx~l~XyITXm-pzbHlc8IpNPv8vG7uProdPZGpA-BFqXCfkA1OgRaiW0QXWlN9SqOTHgk0ep4uUjN3WiTRBLndW33DGGB~fl-JthBKliyrm-KzgOXc6jAahBhIy-KA0un-hWzeDu~~xQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)