Abstract
The development and antigen-dependent differentiation of B lymphocytes are orchestrated by an array of growth factors, cytokines, and chemokines that require tight spatiotemporal regulation. Heparan sulfate proteoglycans specifically bind and regulate the bioavailability of soluble protein ligands, but their role in the immune system has remained largely unexplored. Modification of heparan sulfate by glucuronyl C5-epimerase (Glce) controls heparan sulfate-chain flexibility and thereby affects ligand binding. Here we show that Glce deficiency impairs B-cell maturation, resulting in decreased plasma cell numbers and immunoglobulin levels. We demonstrate that C5-epimerase modification of heparan sulfate is critical for binding of a proliferation inducing ligand (APRIL) and that Glce-deficient plasma cells fail to respond to APRIL-mediated survival signals. Our results identify heparan sulfate proteoglycans as novel players in B-cell maturation and differentiation and suggest that heparan sulfate conformation is crucial for recruitment of factors that control plasma cell survival.
Introduction
Heparan sulfate proteoglycans (HSPGs) are proteins with covalently attached polysaccharide heparan sulfate (HS) chains, which in the native form consist of alternating N-acetylated glucosamine and D-glucuronic acid units.1,2 These macromolecules are expressed in all mammalian tissues as extracellular matrix components (eg, perlecan) or as cell-membrane-bound glycoconjugates (eg, syndecans, glypicans). To exert their function, the HS chains undergo a series of processing reactions involving N-deacetylation/N-sulfation, epimerization, and O-sulfation.1,2 This endows HS chains with highly modified domains, which provide specific docking sites for a large number of bioactive molecules. Binding of these ligands, such as growth factors and cytokines, serves a variety of functions, ranging from immobilization and concentration to distinct modulation of signaling.2-4 In this way, HSPGs act as multifunctional scaffolds, regulating important biologic processes, including cell adhesion and migration, angiogenesis, and tissue remodeling.5
Genetic defects in humans and studies with genetically modified animals have unequivocally demonstrated the critical role of HSPGs and their correct modification by HS-modifying enzymes in development and tissue homeostasis.1,5-9 Altered HSPG core protein expression or defective HS modification can cause mistargeting of morphogens and growth factors, which may lead to developmental defects and can initiate or modulate tumor growth.3,6,10-12 The fact that many of the growth factors, cytokines, and chemokines critical for lymphocyte growth, differentiation, and positioning contain HS-binding domains suggests that HSPGs could also play a key role in the control of lymphocyte function.12-14 Consistent with this notion, previous studies from our own and other laboratories have demonstrated that HSPG expression by B lymphocytes is developmentally regulated and stimulation-dependent.14-16 Specifically, activation by B-cell antigen receptor (BCR) and CD40 ligation was shown to induce strong expression of cell surface CD44-HS, which act as functional coreceptors promoting growth factor signaling.14 In addition, B cells acquire strong expression of the HSPG syndecan-1 on their terminal differentiation into plasma cells.15,17 These observations indicate that dynamic regulation of HSPG expression in B cells could play a role in tuning their sensitivity to soluble signals from the microenvironment and might thus be crucial for B-cell development and antigen-dependent differentiation. To explore this hypothesis, we studied the effect of targeted deletion of the Glce gene, encoding glucuronyl C5-epimerase, which converts D-glucuronic acid to its stereoisomer L-iduronic acid (IdoA). This conversion releases the conformational constraints of the polysaccharide, creating chain flexibility and allowing the access of protein ligands to specific regions of the HS chains.18 Because Glce knockout (Glce−/−) mice die neonatally,6 we studied B-cell development and differentiation in alymphoid Rag-2−/−γc−/− mice,12 reconstituted with fetal liver hematopoietic stem cells (FLHSCs) derived from Glce−/− mice or wild-type littermates. We report here that Glce deficiency leads to attenuated B-cell maturation, diminished baseline- and antigen-specific immunoglobulin (Ig) levels, and reduced plasma cell numbers. Furthermore, we show that Glce deficiency impairs plasma cells to respond to a proliferation inducing ligand (APRIL)-mediated survival signals. Our data establish a role for HSPGs in the control of B-cell maturation and antigen-dependent differentiation in vivo.
Methods
Mice
C57BL/6 mice carrying a mutant allele for Glce, described previously,6 were bred under specified pathogen-free conditions. Offspring of Glce+/− couples was genotyped by PCR, using primers as described.6 Rag-2−/−γc−/− mice, which lack T, B, and NK cells,12 were bred under specified pathogen-free conditions and housed in individual ventilated cages. All mice were housed in the animal facility of the Academic Medical Center. All animal experiments were conducted according to Institutional Guidelines of the University of Amsterdam, after acquiring permission from the local Ethical Committee for Animal Experimentation and in accordance with current Dutch laws on animal experiments.
Antibodies and reagents
For immunohistochemistry and/or FACS analysis, primary anti–mouse antibodies used were unconjugated anti-CD45R/B220, clone RA3-6B2 (BD Biosciences PharMingen); anti-IgA, anti-IgM, anti-IgD, clone 11-26c.2, and anti–mouse κ (all from Southern Biotechnology); anti-FLAG (Sigma-Aldrich); anti-stromal-derived factor-α (SDF-1α or CXCL12α) and K15C (kindly provided by Dr F. Arenzana-Seisdedos, Grenoble, France); anti-hepatocyte growth factor (HGF) and anti-CXC chemokine ligand 13 (CXCL13) (both from R&D Systems); anti-CC chemokine ligand 19 (CCL19) and anti-CCL21 (kindly provided by Dr R. E. Mebius, Amsterdam, The Netherlands); a phage display-derived single-chain antibody vesicular stomatitis virus-tagged AO4B08 (anti-HS),19 biotin-conjugated peanut agglutinin (selective binding to germinal center [GC] B cells; Sigma-Aldrich); biotin- and FITC-conjugated antisyndecan-1, 281-2; FITC-conjugated anti-CD3 (Armenian hamster, 145-2c11, eBioscience); FITC-conjugated anti–c-kit; FITC-conjugated anti-IgM; phycoerythrin-conjugated anti-IgD; and allophycocyanin-conjugated anti-B220, RA3-6B2 (all from BD Biosciences PharMingen). Secondary antibodies used were Cy3-conjugated anti-vesicular stomatitis virus, P5D4 (Sigma-Aldrich); biotin-conjugated rabbit anti–rat Igs (Dako North America).
For ELISA, unconjugated and horseradish peroxidase-conjugated goat anti–mouse Igs used were anti–mouse IgM, IgG1, IgG2b, and IgA (all from Southern Biotechnology). For ELISA standards and quantification, purified mouse IgM, IgA, IgG1, and IgG2b (100 μg/mL) were used (all from Southern Biotechnology). Recombinant proteins used were APRIL, B cell activating factor (BAFF), HGF, SDF-1α, and CXCL13 (all from R&D Systems) and CCL19 and CCL21 (both from PeproTech). Transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI)–Ig was from Sigma-Aldrich. Trinitrophenylated keyhole limpet hemocyanin (TNP-KLH) and TNP-bovine serum albumin were obtained from Biosearch Technologies.
Immunohistochemistry
Immunohistochemistry on frozen and on formalin-fixed paraffin embedded tissues was performed as described previously.20 Bone (marrow) was fixed in formalin, embedded in plastic, and processed for immunohistochemistry as described.12 Immunochemistry micrographs were obtained with an Olympus BX51 light microscope, using a 20×/0.50 Plan FI or 40×/0.85 PlanApo objective connected to an Olympus DP70 camera. Images were captured with Olympus DPController software (Version 1.2.1.108) and further processed with Adobe Photoshop (Version 7.0).
Flow cytometry
Expression of membrane proteins was analyzed by single, double, or triple stainings, as described previously.14 Stainings were measured on a FACSCantoII flow cytometer system (BD Biosciences), interfaced to FACSDiva software (Version 6.0), and analyzed with FlowJo (TreeStar).
Growth factor and chemokine binding assay
Mouse embryonic fibroblasts (MEFs) were generated and cultured as described.21 To examine Glce-dependent HS binding, 2 × 105 MEFs were incubated with 100 μL of ΔR-APRIL-FLAG (2 μg/mL), HGF (2 μg/mL), SDF-1 (5 μg/mL), CXCL13 (10 μg/mL), CCL19 (5 μg/mL), or CCL21 (5 μg/mL) at 4°C for 90 minutes in RPMI 1640 medium containing 20nM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 1% BSA. Ligand concentrations used represent the minimal concentrations required to saturate binding to Glce+/+ MEFs. Subsequently, cells were washed and incubated with specific antibodies to determine binding to the cells by FACS analysis.
Adoptive transfer of FLHSCs
To obtain mice with a Glce-deficient lymphoid system, alymphoid Rag-2−/−γc−/− mice were transplanted with FLHSCs from Glce−/− or wild-type (Glce+/+) embryos. At embryonic day 14.5 (E14.5), fetal livers were harvested and gently smashed, and total cell suspensions were collected of each donor embryo. Cell suspensions were genotyped by PCR and stored at −80°C until used. The FLHSCs were injected into the liver of newborn Rag-2−/−γc−/− mice. After 4 to 5 weeks, the mice were analyzed for lymphoid reconstitution in the blood by FACS, using markers for B and T cells.
Immunizations
Mice were immunized intraperitoneally with 100 μg TNP-KLH (alum precipitated in PBS; Biosearch Technologies) in a 200-μL volume, for analysis of T cell–dependent B-cell differentiation. For induction of a secondary immune response, mice were boosted at day 28 and reboosted at day 42 after primary immunization. Blood samples were taken from every mouse at indicated time points.
Measurement of total and antigen-specific Ig levels
Mice were bled from the femoral vein and serum was isolated. Serum levels of distinct Ig subclasses were measured by a sandwich ELISA, using plates coated with unlabeled antimouse Ig isotype-specific antibodies (Southern Biotechnology) at 4°C overnight. For measurement of TNP-specific antibodies, ELISA plates were coated with TNP-bovine serum albumin at 4°C overnight. Serially diluted plasma samples, as indicated, were incubated at room temperature for 1 hour, followed by 1-hour incubation with antimouse Ig isotype-specific horseradish peroxidase-labeled antibodies at room temperature. As a liquid substrate, 3,3′,5,5′-tetramethylbenzidine was used. Absorbance was measured at 450 nm. For antibody concentration determination, purified Igs (Southern Biotechnology) were used as standards.
In vitro culture of BM plasma cells
Femurs of immunized mice were isolated, and total bone marrow (BM) was collected by flushing the bones. Total BM cells (1-2 × 106) were cultured in complete RPMI 1640 supplemented with 10% FCS containing APRIL (200 ng/mL), APRIL* (mutated such that it is unable to bind to HS, 200 ng/mL),22 BAFF (200 ng/mL), and/or TACI-Ig (2 μg/mL) for 8 to 10 days. At the end of the culture period, all cells were harvested and stained for plasma cells (CD138hiLy6C+B220−),23 and analyzed by FACS.
Results
Lymphoid reconstitution of alymphoid Rag-2−/−γc−/− mice with Glce−/− or Glce+/+ donor fetal liver cells
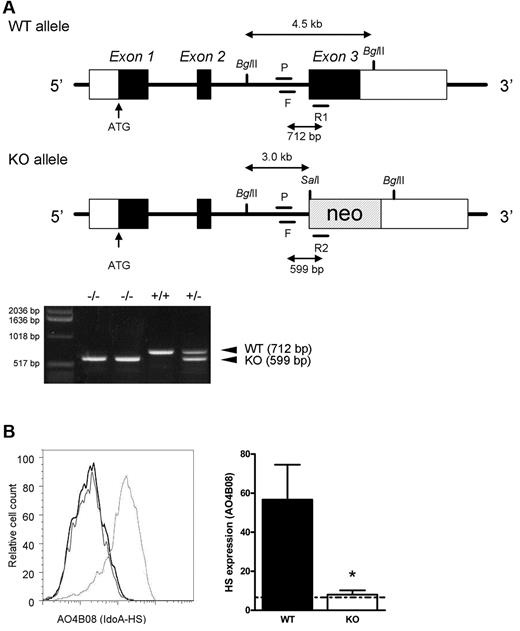
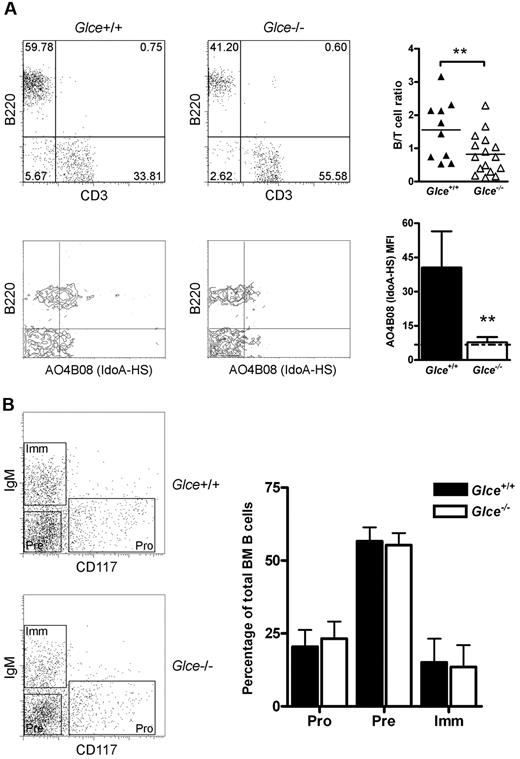
The generation and phenotype of the glucuronyl C5-epimerase-deficient (Glce−/−) mice used in this study has been described previously (Figure 1A).6,8 Importantly, these mice die at birth because of renal agenesis and pulmonary dysplasia. To study the role of HS-chain modification by Glce in lymphocyte development and antigen-dependent differentiation, we transferred FLHSCs from Glce−/− embryos or Glce+/+ littermates (E14.5) to alymphoid Rag-2−/−γc−/− mice. Rag-2−/−γc−/− mice completely lack B, T, and NK cells and, consequently, permit highly reproducible engraftment of lymphocytes.12 Preceding grafting, the FLHSC donor populations were genotyped by PCR (Figure 1A). In addition, the “Glce phenotype” of FLHSC was determined by FACS using antibody AO4B08, directed against an HS epitope that is dependent for its binding on the presence of IdoA, the product of Glce activity (Figure 1B).19 At 4 to 5 weeks after transfer of either Glce+/+ or Glce−/− FLHSCs, T and B lymphocytes were present in the peripheral blood of the recipients (Figure 2A top panels). Notably, however, the proportion of B cells in the blood of Glce−/− mice was significantly reduced (Figure 2A top panels). The peripheral blood B cells of mice reconstituted with Glce+/+ FLHSCs expressed IdoA-HS, whereas IdoA-HS was not expressed on B cells of Glce−/− reconstituted animals (Figure 2A bottom panels). These data show that B cells (but not T cells, data not shown) express Glce-modified HS moieties and suggest that Glce deficiency attenuates B-lineage development. From here on, alymphoid Rag-2−/−γc−/− mice reconstituted with Glce−/− or Glce+/+ FLHSCs will be referred to as Glce−/− and Glce+/+ mice, respectively.
Targeted disruption of Glce and loss of IdoA containing HS. (A) A neo-cassette was inserted into the endogenous Glce gene by homologous recombination. Glce wild-type (top, WT or Glce+/+) and mutant allele (bottom, KO or Glce−/−). Mice were genotyped by PCR. F indicates forward primer; R1/R2, reverse primers; and P, probe (for primer sequences, see “Mice”). Representative PCR reactions are shown. (B) Left panel: Expression of IdoA containing HS on the cell surface of fetal liver cells of Glce+/+ and Glce−/− mice (E14.5) shown, respectively, as the light gray and black line (overlying the background stain), detected with antibody AO4B08. Right panel: Bar diagram represents the mean fluorescence intensity (MFI) ± SD of AO4B08 staining of Glce+/+ and Glce−/− fetal liver cells (n = 4). The dotted line indicates background staining. *P < .05.
Targeted disruption of Glce and loss of IdoA containing HS. (A) A neo-cassette was inserted into the endogenous Glce gene by homologous recombination. Glce wild-type (top, WT or Glce+/+) and mutant allele (bottom, KO or Glce−/−). Mice were genotyped by PCR. F indicates forward primer; R1/R2, reverse primers; and P, probe (for primer sequences, see “Mice”). Representative PCR reactions are shown. (B) Left panel: Expression of IdoA containing HS on the cell surface of fetal liver cells of Glce+/+ and Glce−/− mice (E14.5) shown, respectively, as the light gray and black line (overlying the background stain), detected with antibody AO4B08. Right panel: Bar diagram represents the mean fluorescence intensity (MFI) ± SD of AO4B08 staining of Glce+/+ and Glce−/− fetal liver cells (n = 4). The dotted line indicates background staining. *P < .05.
Detection of blood lymphocytes and B-cell development in the BM. (A) Top panel: B (B220+) and T (CD3+) cells detected in the peripheral blood of Rag-2−/−γc−/− mice reconstituted with Glce+/+ and Glce−/− FLHSCs at 4 weeks after transplantation; representative mice are shown. Scatter plot represents individual mice measured and the mean B-/T-cell ratios (n ≥ 10). **P < .01. Bottom panel: Expression of IdoA containing HS on the cell surface of B220+ B cells was measured. Bars represent mean ± SEM (n = 4). The dotted black line indicates background staining. **P < .01. (B) BM of Glce+/+ and Glce−/− mice was isolated and stained for B220, CD117 (c-Kit), and IgM. The dot plots show 3 distinct B220+ B-cell populations; pro-B cells (Pro, CD117+/IgM−), pre-B cells (Pre, CD117−/IgM−), and immature B cells (Imm, CD117−/IgM+). Bar diagram represents mean ± SD of each subsets as percentage of the total B-cell population (n = 4).
Detection of blood lymphocytes and B-cell development in the BM. (A) Top panel: B (B220+) and T (CD3+) cells detected in the peripheral blood of Rag-2−/−γc−/− mice reconstituted with Glce+/+ and Glce−/− FLHSCs at 4 weeks after transplantation; representative mice are shown. Scatter plot represents individual mice measured and the mean B-/T-cell ratios (n ≥ 10). **P < .01. Bottom panel: Expression of IdoA containing HS on the cell surface of B220+ B cells was measured. Bars represent mean ± SEM (n = 4). The dotted black line indicates background staining. **P < .01. (B) BM of Glce+/+ and Glce−/− mice was isolated and stained for B220, CD117 (c-Kit), and IgM. The dot plots show 3 distinct B220+ B-cell populations; pro-B cells (Pro, CD117+/IgM−), pre-B cells (Pre, CD117−/IgM−), and immature B cells (Imm, CD117−/IgM+). Bar diagram represents mean ± SD of each subsets as percentage of the total B-cell population (n = 4).
Glce−/− mice have a reduced splenic follicular mature B-cell pool
B-cell development from committed precursors to mature immunocompetent B cells takes place in the BM and the spleen and proceeds through a number of developmental checkpoints. During early development in the BM, mouse B-cell precursors consecutively pass through the pro-B cell (B220+,c-kit+, IgM−), pre-B cell (B220+, c-kit−, IgM−), and immature B cell (B220+, c-kit−, IgM+) stages. Our flow cytometric analysis of the BM of Glce−/− mice and Glce+/+ littermates showed an identical representation of these subsets within the BM (Figure 2B) as well as cell numbers (data not shown), indicating that the early maturation of B cells in the BM is independent of Glce activity.
Once B cells have reached the immature B-cell stage, they will leave the BM and migrate to the spleen. Here, they will pass through the transitional type 1 (T1) and transitional type 2 (T2) stages to become follicular mature (FM) B cells. Interestingly, compared with their Glce+/+ littermates, Glce−/− mice showed a significant reduction of the total number of splenic B cells (B220+). This reduction in Glce−/− mice was accompanied by a nearly 2-fold increase in the percentage of immature (IgMlow, IgD−) and T1 (IgM+, IgDlow/−) B cells as well as a significant decrease in the percentage of FM B cells (IgMlow, IgDbright; Figure 3A-B). Consistent with these flow cytometric data, immunohistochemical studies showed a reduction in the size of the splenic B-cell areas in Glce−/− mice (Figure 3C). Hence, whereas maturation to the FM cell stage takes place in the absence of Glce activity, the reduced size of the FM B-cell pool suggests that naive mature B cells require Glce-modified HS for optimal development and/or survival.
Glce−/− mice have a reduced follicular mature B-cell pool. (A) B-cell maturation in the spleen of Glce+/+ and Glce−/− mice was analyzed by triple staining with B220, IgM, and IgD. Gates represent: immature B cells (IgMlo/IgD−); T1, transitional 1 B cells (IgM+/IgDlow/−); T2, transitional 2 B cells (IgM+/IgDhigh); FM, follicular mature B cells (IgMlow/IgDbright). (B) Left panel: Bar diagram showing the total number of splenic B cells in Glce+/+ and Glce−/− mice. Right panel: B-cell subsets quantified by FACS analysis, measuring the percentage of all B220+ B cells of the total spleen. The 4 different B220+ B-cell populations (as in panel A) are shown in a bar graph (right panel). Bars represent mean ± SD of 6 mice and are representative for 3 independent groups. *P < .05. **P < .01. (C) Serial cryosections of the spleens of Glce+/+ and Glce−/− stained for B220 and IgD to identify the B cells. T indicates T-cell area; f, B-cell follicle; and rp, red pulp.
Glce−/− mice have a reduced follicular mature B-cell pool. (A) B-cell maturation in the spleen of Glce+/+ and Glce−/− mice was analyzed by triple staining with B220, IgM, and IgD. Gates represent: immature B cells (IgMlo/IgD−); T1, transitional 1 B cells (IgM+/IgDlow/−); T2, transitional 2 B cells (IgM+/IgDhigh); FM, follicular mature B cells (IgMlow/IgDbright). (B) Left panel: Bar diagram showing the total number of splenic B cells in Glce+/+ and Glce−/− mice. Right panel: B-cell subsets quantified by FACS analysis, measuring the percentage of all B220+ B cells of the total spleen. The 4 different B220+ B-cell populations (as in panel A) are shown in a bar graph (right panel). Bars represent mean ± SD of 6 mice and are representative for 3 independent groups. *P < .05. **P < .01. (C) Serial cryosections of the spleens of Glce+/+ and Glce−/− stained for B220 and IgD to identify the B cells. T indicates T-cell area; f, B-cell follicle; and rp, red pulp.
Glce−/− mice have reduced basal serum Ig levels and show an attenuated antigen-dependent immune response
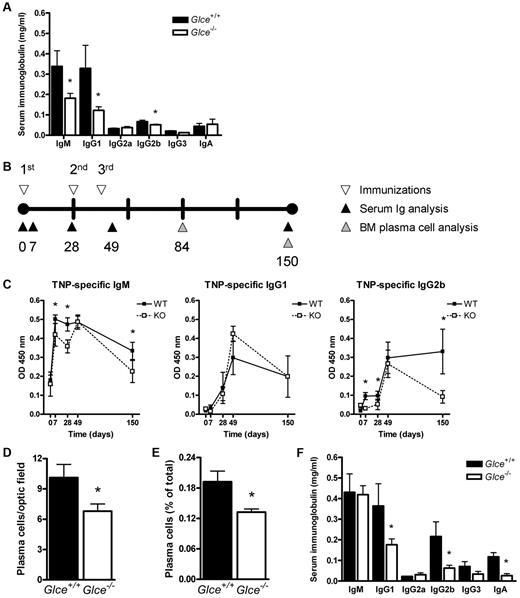
Under physiologic conditions, the immune system is continuously stimulated by antigens from the microenvironment, leading to differentiation of naive B cells into memory cells and antibody-forming plasma cells. The latter cells are responsible for the basal protective serum levels of IgG and IgM antibodies and are characterized by expression of high levels of the HSPG syndecan-1 on their cell surface, which may functions as a (co)receptor for survival signals from the microenvironment.12,24 To explore the possible effect of Glce deficiency on plasma cell function, we measured the concentration of Igs in the serum of 8- to 10-week-old nonimmunized Glce−/− mice and Glce+/+ littermates. Interestingly, the serum levels of IgM as well as the levels of IgG1 and IgG2b were significantly reduced in Glce-deficient mice (Figure 4A). This suggests that modification of HS-chains by Glce is involved in the regulation of plasma cell differentiation and/or plasma cell survival.
Glce−/− mice have reduced basal serum Ig levels, an attenuated antigen-dependent immune response, and reduced plasma cell numbers. (A) Serum Ig concentrations in nonimmunized Glce+/+ and Glce−/− mice. The concentration of Igs of different isotypes was measured by ELISA, preceding immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. (B) The timeline of the immunization protocol. Glce+/+ and Glce−/− mice were immunized at 3 (primary, boost, reboost) subsequent time points (▿). Blood samples were taken and serum was isolated (▲). Mice were killed at 84 and 150 days ( ) after primary immunization for analysis of the BM plasma cells. (C) TNP-specific antibody levels after immunization with TNP-KLH. ELISA was performed to measure the TNP-specific antibody titer of the IgM, IgG1, and IgG2b isotypes. ■ represents Glce+/+ mice; and □, Glce−/− mice. Outliers and nonresponders were statistically excluded. These graphs represent 3 independent experiments of at least 4 to 8 mice (mean ± SEM). (D) Number of BM plasma cells (day 84). Tissue sections of femurs obtained at autopsy were stained for κ-light chain to quantify the number of plasma cells per optic field (original magnification, × 40) (mean ± SEM; n = 4/group). (E) Number of BM plasma cells (day 150). Whole BM was isolated and BM plasma cells (B220−, syndecan-1+, Ly6c+) were quantified by FACS as percentage of total BM cells (mean ± SD, n = 4/group). (F) Total serum Ig concentrations in Glce+/+ and Glce−/− mice (day 49). The concentration of Igs of different isotypes was measured by ELISA after repeated immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. *P < .05.
) after primary immunization for analysis of the BM plasma cells. (C) TNP-specific antibody levels after immunization with TNP-KLH. ELISA was performed to measure the TNP-specific antibody titer of the IgM, IgG1, and IgG2b isotypes. ■ represents Glce+/+ mice; and □, Glce−/− mice. Outliers and nonresponders were statistically excluded. These graphs represent 3 independent experiments of at least 4 to 8 mice (mean ± SEM). (D) Number of BM plasma cells (day 84). Tissue sections of femurs obtained at autopsy were stained for κ-light chain to quantify the number of plasma cells per optic field (original magnification, × 40) (mean ± SEM; n = 4/group). (E) Number of BM plasma cells (day 150). Whole BM was isolated and BM plasma cells (B220−, syndecan-1+, Ly6c+) were quantified by FACS as percentage of total BM cells (mean ± SD, n = 4/group). (F) Total serum Ig concentrations in Glce+/+ and Glce−/− mice (day 49). The concentration of Igs of different isotypes was measured by ELISA after repeated immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. *P < .05.
Glce−/− mice have reduced basal serum Ig levels, an attenuated antigen-dependent immune response, and reduced plasma cell numbers. (A) Serum Ig concentrations in nonimmunized Glce+/+ and Glce−/− mice. The concentration of Igs of different isotypes was measured by ELISA, preceding immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. (B) The timeline of the immunization protocol. Glce+/+ and Glce−/− mice were immunized at 3 (primary, boost, reboost) subsequent time points (▿). Blood samples were taken and serum was isolated (▲). Mice were killed at 84 and 150 days ( ) after primary immunization for analysis of the BM plasma cells. (C) TNP-specific antibody levels after immunization with TNP-KLH. ELISA was performed to measure the TNP-specific antibody titer of the IgM, IgG1, and IgG2b isotypes. ■ represents Glce+/+ mice; and □, Glce−/− mice. Outliers and nonresponders were statistically excluded. These graphs represent 3 independent experiments of at least 4 to 8 mice (mean ± SEM). (D) Number of BM plasma cells (day 84). Tissue sections of femurs obtained at autopsy were stained for κ-light chain to quantify the number of plasma cells per optic field (original magnification, × 40) (mean ± SEM; n = 4/group). (E) Number of BM plasma cells (day 150). Whole BM was isolated and BM plasma cells (B220−, syndecan-1+, Ly6c+) were quantified by FACS as percentage of total BM cells (mean ± SD, n = 4/group). (F) Total serum Ig concentrations in Glce+/+ and Glce−/− mice (day 49). The concentration of Igs of different isotypes was measured by ELISA after repeated immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. *P < .05.
) after primary immunization for analysis of the BM plasma cells. (C) TNP-specific antibody levels after immunization with TNP-KLH. ELISA was performed to measure the TNP-specific antibody titer of the IgM, IgG1, and IgG2b isotypes. ■ represents Glce+/+ mice; and □, Glce−/− mice. Outliers and nonresponders were statistically excluded. These graphs represent 3 independent experiments of at least 4 to 8 mice (mean ± SEM). (D) Number of BM plasma cells (day 84). Tissue sections of femurs obtained at autopsy were stained for κ-light chain to quantify the number of plasma cells per optic field (original magnification, × 40) (mean ± SEM; n = 4/group). (E) Number of BM plasma cells (day 150). Whole BM was isolated and BM plasma cells (B220−, syndecan-1+, Ly6c+) were quantified by FACS as percentage of total BM cells (mean ± SD, n = 4/group). (F) Total serum Ig concentrations in Glce+/+ and Glce−/− mice (day 49). The concentration of Igs of different isotypes was measured by ELISA after repeated immunization with TNP-KLH. Bars represent the mean ± SD; n = 12. *P < .05.
To further explore the impact of Glce deficiency on antibody formation and plasma cell homeostasis, Glce−/− mice and Glce+/+ littermates were immunized with the T cell–dependent antigen TNP-KLH (Figure 4B), and the TNP-specific serum Ig levels were measured. On primary immunization, the Glce−/− mice showed an attenuated antibody response with significantly lower serum levels of TNP-specific IgM and IgG2b compared the Glce+/+ littermates (Figure 4C; days 7 and 28). However, after repeated antigenic challenge, the TNP-specific IgM and IgG response of the Glce−/− mice equaled that of their Glce+/+ littermates (Figure 4C; day 49). This delayed generation of TNP-specific plasma cells in Glce−/− mice could be related to the reduced size of their mature naive B-cell pool (Figure 3). Interestingly, however, reduced levels of TNP-specific IgM and IgG2b were also found at a late phase of the immune response (Figure 4C; day 150), suggesting a decreased life span of Glce−/− TNP-specific plasma cells.
The above data indicate that Glce deficiency affects plasma cell differentiation and/or survival. Consistent with this notion, immunohistochemical studies and FACS analysis revealed a significant reduction in the total number of BM plasma cells in Glce−/− mice at both day 84 (Figure 4D) and day 150 (Figure 4E). Furthermore, after repeated immunizations, the total concentration of IgG1 and IgG2b in the serum of the Glce−/− mice remained significantly lower compared with their Glce+/+ littermates, whereas IgA levels were also strongly reduced (Figure 4 F). Importantly, immunohistochemical studies of the spleens of the Glce−/− mice after TNP-KLH challenge (day 56) showed a small but significant decrease in plasma cell numbers (Figure 5), without any abnormalities in size or architecture of GCs (Figure 5A-B). Thus, modification of HS by Glce on B cells is required for optimal antibody formation and affects the fate of plasma cells, both short- and long-lived, but is not required for GC formation.
GC formation and splenic plasma cells in Glce+/+ and Glce−/− mice. (A) GC formation is undisturbed in Glce−/− mice. Glce+/+ and Glce−/− mice were killed after repeated immunization with KLH-TNP (day 56). Serial cryosections of the spleen were stained with anti-B220 and peanut agglutinin, respectively. T indicates T-cell area; f, B-cell follicle; wp, white pulp; and rp, red pulp. (B) Quantification of GCs in the spleen of Glce+/+ and Glce−/− mice. GCs were quantified per millimeter squared tissue section (mean ± SEM; n = 4/group). (B) Glce−/− mice show decreased splenic plasma cell numbers. Number of syndecan-1+ plasma cells in the spleen of Glce+/+ and Glce−/− mice quantified per millimeter squared (mean ± SEM; n = 4/group). *P < .05.
GC formation and splenic plasma cells in Glce+/+ and Glce−/− mice. (A) GC formation is undisturbed in Glce−/− mice. Glce+/+ and Glce−/− mice were killed after repeated immunization with KLH-TNP (day 56). Serial cryosections of the spleen were stained with anti-B220 and peanut agglutinin, respectively. T indicates T-cell area; f, B-cell follicle; wp, white pulp; and rp, red pulp. (B) Quantification of GCs in the spleen of Glce+/+ and Glce−/− mice. GCs were quantified per millimeter squared tissue section (mean ± SEM; n = 4/group). (B) Glce−/− mice show decreased splenic plasma cell numbers. Number of syndecan-1+ plasma cells in the spleen of Glce+/+ and Glce−/− mice quantified per millimeter squared (mean ± SEM; n = 4/group). *P < .05.
Glce deficiency impairs HS interaction with a subset of ligands
Unlike interactions of protein ligands with heparin, their interaction with natural HSPGs is thought to be highly selective and dependent on specific modifications of the HS-chains.1,2 Altered HS modification could thus cause loss of interaction with specific, rather than all, immune cytokines and chemokines. To explore whether Glce deficiency indeed selectively impairs ligand binding, we tested a panel of cytokines and chemokines with established HS-binding for interaction with MEFs from either Glce−/− or Glce+/+ mice.21 The panel consisted of APRIL, HGF, CXCL12α (SDF-1α), CXCL13 (BLC), and CCL21 (SLC), factors, which all have important functions in B-cell and plasma cell biology. Interestingly, although Glce−/− and Glce+/+ MEFs synthesize equal amounts of HS,21 the Glce−/− cells almost completely failed to bind the cytokines APRIL and HGF, as well as the chemokine SDF-1α (Figure 6). In marked contrast, the binding of the chemokines CXCL13 and CCL21 was hardly affected by Glce deficiency. FACS analysis ruled out that these chemokines were bound to the MEFs via their cognate receptors because these cells were devoid of CXCR5 (for CXCL13) and CCR7 (for CCL19/CCL21; data not shown). These findings demonstrate that modification of HS by Glce selectively affects ligand binding and that IdoA moieties are required for interaction of HS with a distinct subset of cytokines involved in B-cell and plasma cell functioning.
Glce deficiency impairs HS interaction with a subset of growth factors/chemokines. Binding of ligands to Glce+/+ and Glce−/− MEFs. MEFs from Glce+/+ and Glce−/− mice were incubated with (A) growth factors (HGF and APRIL) or (B) chemokines (CXCL12α, CXCL13, and CCL21), washed, and the binding was analyzed by FACS. Graphs represent the relative binding, compared with the Glce+/+ MEFs (relative mean fluorescence intensity [MFI] ± SEM; n = 3-5). **P < .01. ***P < .001. ΔR-APRIL indicates point mutant (R231A) that is unable bind to the high-affinity receptor BCMA and TACI.
Glce deficiency impairs HS interaction with a subset of growth factors/chemokines. Binding of ligands to Glce+/+ and Glce−/− MEFs. MEFs from Glce+/+ and Glce−/− mice were incubated with (A) growth factors (HGF and APRIL) or (B) chemokines (CXCL12α, CXCL13, and CCL21), washed, and the binding was analyzed by FACS. Graphs represent the relative binding, compared with the Glce+/+ MEFs (relative mean fluorescence intensity [MFI] ± SEM; n = 3-5). **P < .01. ***P < .001. ΔR-APRIL indicates point mutant (R231A) that is unable bind to the high-affinity receptor BCMA and TACI.
APRIL-mediated plasma cell survival is dependent on HS modification by Glce
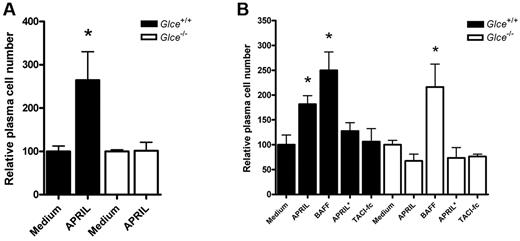
Recent studies have identified APRIL as an important survival factor for plasmablasts and BM plasma cells.23,25,26 Our current finding that Glce−/− mice have decreased serum antibody levels (Figure 4A) and diminished plasma cell numbers in BM and spleen (Figure 4D-E), together with our observation that Glce-deficient cells are unable to bind APRIL (Figure 6A), prompted us to explore whether Glce-deficient plasma cells are able to respond to APRIL-mediated survival signals. To this end, BM plasma cells from Glce−/− and Glce+/+ littermates were cultured in vitro in the presence or absence of APRIL and plasma cell survival was determined by measuring cell numbers.23 Whereas the survival of Glce+/+ plasma cells was strongly promoted by APRIL, stimulation with this protein did not have any effect on the survival of Glce−/− plasma cells (Figure 7A). Importantly, this lack of stimulation was not the result of a general defect in survival signaling because BAFF, a close relative of APRIL that acts in an HS-independent fashion,26 strongly promoted the survival of Glce+/+ as well as Glce−/− plasma cells (Figure 7B). Consistent with the HS-binding requirement for the activity of APRIL, a mutant form of APRIL containing 6 point mutations in regions responsible for HS binding and denoted as APRIL*,22 was unable to stimulate plasma cell survival. Furthermore, coincubation of APRIL with TACI-fc, which blocks APRIL binding to the high affinity receptors BCMA and TACI, abrogated the stimulatory effect of APRIL on the survival of the Glce+/+ plasma cells (Figure 7B). Taken together, we show that HS modification by Glce is required for APRIL binding to HS and is essential for APRIL-mediated survival of BM plasma cells.
Glce−/− BM plasma show impaired APRIL-mediated survival. (A) APRIL-mediated survival of Glce+/+ and Glce−/− BM plasma cells. BM was isolated from Glce+/+ and Glce−/− mice and cultured in vitro with or without APRIL (200 ng/mL). Bars represent the relative number of surviving plasma cells (B220−, Syndecan-1+, and Ly6c+) after 10 days of culture of 3 independent experiments (mean ± SEM). *P < .05. (B) Unlike APRIL-mediated survival, BAFF-mediated BM plasma cell survival is independent of Glce activity. Glce+/+ and Glce−/− BM plasma cells were isolated and cultured as described earlier in the Figure 7 legend. Bar diagram represents the relative number of surviving BM plasma cells at day 10 (mean ± SEM). *P < .05. BAFF (200 ng/mL); TACI-Ig (2 μg/mL); APRIL* (200 ng/mL) = APRIL mutant that cannot bind to HS.
Glce−/− BM plasma show impaired APRIL-mediated survival. (A) APRIL-mediated survival of Glce+/+ and Glce−/− BM plasma cells. BM was isolated from Glce+/+ and Glce−/− mice and cultured in vitro with or without APRIL (200 ng/mL). Bars represent the relative number of surviving plasma cells (B220−, Syndecan-1+, and Ly6c+) after 10 days of culture of 3 independent experiments (mean ± SEM). *P < .05. (B) Unlike APRIL-mediated survival, BAFF-mediated BM plasma cell survival is independent of Glce activity. Glce+/+ and Glce−/− BM plasma cells were isolated and cultured as described earlier in the Figure 7 legend. Bar diagram represents the relative number of surviving BM plasma cells at day 10 (mean ± SEM). *P < .05. BAFF (200 ng/mL); TACI-Ig (2 μg/mL); APRIL* (200 ng/mL) = APRIL mutant that cannot bind to HS.
Discussion
HSPGs are designed to specifically bind and regulate the bioactivity of soluble protein ligands1,2 and play an essential role in the spatial control of extracellular signals regulating cell growth, survival, and differentiation.1,5,27 Previous studies have demonstrated that B cells show developmentally regulated and stimulation-dependent expression of cell surface HSPGs,14-16 suggesting a role for HSPGs in the control of B-cell development and antigen-dependent differentiation. To explore this role, we studied the effect of targeted deletion of Glce, an enzyme that converts D-glucuronic acid to its stereoisomer IdoA, creating chain flexibility, thereby allowing access of protein ligands to specific regions of the HS chains.18 In addition to imposing conformational constraints, Glce deficiency results in a distorted sulfation pattern, which may also contribute to altered ligand binding.1,6,21 Glce−/− mice die neonatally.6 Therefore, the effect of Glce deficiency on B-cell development and differentiation was assessed in alymphoid Rag-2−/−γc−/− mice,12 immune reconstituted with FLHSCs derived from Glce−/− mice or Glce+/+ littermates. Our results show that mice with Glce-deficient lymphocytes display an attenuated B-cell maturation, as well as diminished baseline- and antigen-specific Ig levels and decreased plasma cell numbers. Moreover, we demonstrate that the response of Glce-deficient plasma cells to APRIL-mediated survival signals is impaired.
During B-cell development in the BM, precursor cells consecutively pass through the pro B, pre B, and immature B-cell stages. This early maturation was not affected by Glce deficiency. Pre-BCR and IL-7R–mediated signals are indispensable for B-cell development in BM.28,29 Notably, several studies have shown that HS can interact with both the pre-BCR30,31 and with IL-7,32 suggesting that they may play a role in controlling early B lymphopoiesis. However, the critical HSs in these in vitro studies were either exogenously added or expressed by supporting stromal cells, rather than by the B cells, the Glce-deficient cells in our current model. Whether B-cell development in vivo requires HSPG expression (and Glce modification of HS) by BM stromal cells remains to be established.
Immature B cells leave the BM and migrate to the spleen where they pass through the T1 and T2 stages to become FM B cells. Glce deficiency resulted in a significant size reduction of the splenic B-cell pool. This was accompanied by a markedly altered B-cell subset distribution with a nearly 2-fold increase in the proportion of immature and T1 B cells and a significant decrease in FM B cells. Hence, in the absence of Glce activity, splenic B-cell maturation is clearly attenuated, although not completely blocked. The maturation of T1 B cells to mature follicular B cells is critically dependent on signals emanating from the BCR and from interaction of BAFF-R (BR3) with its ligand BAFF (BLyS).33-37 These signals activate the nonclassic and classic NF-κB pathways, respectively, and crosstalk between these pathways is thought to orchestrate the selection and survival of mature B cells.36 Glce deficiency could potentially interfere with B-cell maturation by affecting either or both of these signaling routes. Because BAFF has the capacity to auto-multimerize and therefore does not require HSPGs for signaling,38,39 a contribution of defective BAFF-BAFF-R signaling in the B-cell maturation defect of Glce−/− mice seems unlikely. On maturation to the T2 and FM stage, however, T1 B cells acquire expression of the receptor TACI, in addition to BAFF-R. Besides serving as a receptor for BAFF, TACI can also engage APRIL and signaling requires APRIL multimerization by HSPGs.22,38,39 Because we found that Glce deficiency leads to an almost complete loss of HS interaction with APRIL, it is tempting to attribute the B-cell maturation defect of Glce−/− mice to defective APRIL-TACI signaling. However, APRIL mutant mice did not display clear defects in T1 to FM transition,40,41 indicating that this explanation may overestimate the role of APRIL in B-cell maturation, at least as a single factor. Conceivably, defective interaction with other ligands (eg, with CXCL12 or HGF) may also contribute to the maturation defect. In support of this notion, it was recently demonstrated that HGF-Met signaling specifically supports the survival of follicular mature B cells, in vitro and in vivo.42 In addition, we have previously shown that presentation of HGF by an HSPG variant of CD44 enhances HGF-Met signaling in B cells.14 Alternatively, Glce deficiency might also affect B-cell maturation by interfering with the organization of the BCR signalosome responsible for generating tonic survival signals. A potential key player in this scenario is CD19, which possesses an HS binding domain43 and, during B-cell activation, acts as an adaptor that transiently recruits signaling molecules, such as Vav, PI3K, and Lyn into BCR microclusters.44 Altered interaction between CD19 and the abnormally modified HSs on Glce−/− B cells could affect CD19 dynamics, leading to defective recruitment of CD19 to the BCR complex, attenuating survival signaling.
On stimulation by antigen, naive B cells can differentiate directly into plasmablasts or engage in a GC reaction to undergo affinity maturation, isotype switching, and differentiation into memory B cells or plasma cells. Antibody-secreting plasma cells subsequently migrate to the BM, where they reside in specific niches to maintain protective serum antibody levels.45 Plasma cells are characterized by expression of high levels of the HSPG syndecan-1.15,17 In multiple myeloma, a malignancy of long-lived plasma cells, syndecan-1 acts as a crucial mediator of tumor cell proliferation and survival,12,24 a function that involves recruitment of growth factors from the BM microenvironment. By engaging soluble factors produced in the plasma cell niche, syndecan-1 may similarly control the survival of normal plasma cells in the BM. The reduced basal serum IgM and IgG levels in Glce−/− mice indeed signify the presence of a disturbed plasma cell homeostasis, either caused by defective plasma cell formation in response to antigenic-stimulation and/or from impeded plasma cell survival or retention. The attenuated primary antibody response against TNP in Glce−/− mice suggests that Glce facilitates plasma cell differentiation. This might be explained by the reduced size of the mature naive B-cell pool, also because the peak responses on repeated antigenic stimulation were normal, but a contribution of altered activation of T cells or altered T-cell help to the observed reduced antigen-specific immune responses in vivo cannot be ruled out.46,47 On the other hand, the apparent inability of Glce−/− mice to maintain TNP-specific IgM and IgG antibody levels, suggests that Glce may be especially important for the longevity of plasma cells, a notion that is supported by the reduced numbers of BM plasma cells in Glce-deficient animals, as well as by the fact that the serum Ig concentrations remained reduced despite repeated immunizations. Whereas retention of plasma cells in the BM depends on the expression of CXCR4 by plasma cells and its ligand CXCL12 by the stromal cells,48 survival of plasma cells and plasmablasts in spleen and BM was recently shown to be critically dependent on BAFF/APRIL.23,25,26 In particular, APRIL appears to be a key player in promoting plasmablast/cell survival. On differentiation of plasmablasts into plasma cells, BAFF-R and TACI are lost, whereas BCMA, which has a much higher binding affinity for APRIL compared with BAFF, is up-regulated.49 APRIL requires HSPG interaction for effective signaling.38 The strongly reduced capacity of APRIL to interact with HS moieties lacking IdoA could thus render APRIL unable to convey survival signals to Glce−/− plasma cells, explaining the reduced antibody levels and plasma cell numbers in Glce−/− mice. Consistent with this hypothesis, we observed that Glce-deficient plasma cells were unable to respond to APRIL-mediated survival signals in vitro, whereas their survival response to BAFF was undisturbed. Besides perturbed APRIL signaling, impaired interaction with other soluble factors, including CXCL12 and HGF, might affect homing and survival of plasmablasts and plasma cells in vivo and thereby also contribute to the reduced antibody levels and plasma cell numbers in Glce−/− mice.
Apart from promoting survival of BM plasma cells, APRIL also plays an important role in IgA antibody responses to T cell-independent-1 antigens encountered in the mucosa of the intestine.40 The strongly reduced serum IgA levels in older Glce-deficient mice presumably reflect this role and may result from impaired binding of APRIL to HS lacking IdoA leading to defective APRIL-mediated IgA class switching40,50 and/or to reduced survival of IgA plasma cells in the intestinal lamina propria.51 Further studies are needed to discriminate between these possible mechanisms.
Our data show that Glce-deficient B cells can undergo maturation and can differentiate to plasma cells in response to stimulation with a T cell-dependent antigen, but they also reveal that Glce deficiency leads to distinct defects in B-cell and plasma cell maturation and homeostasis. In marked contrast to our findings, Garner et al recently reported that conditional inactivation of the HS polymerase exostin-1 at the pro B-cell stage does not significantly affect B-cell maturation and antigen-dependent differentiation.46 However, unlike in our model where all B lymphocytes contain the mutant Glce, the Cre-mediated deletion of exostin-1 in the study of Garner was incomplete, ranging from 50% to 85%. As also debated by these authors, positive selection of cells that escaped HS deletion could thus explain the absence of a B-cell phenotype in these mice. Unfortunately, no data on serum Ig levels and plasma cell numbers or survival were reported in this study. In conclusion, our results identify HSPGs as novel players in B-cell maturation and differentiation and imply that regulation of HS conformation is crucial for the recruitment of factors that control survival of BM plasma cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Dutch Cancer Society (M.S., S.T.P.), The Netherlands Organization for Health Research and Development (TOP grant, ZonMw/NOW; M.S., S.T.P.), and the Swedish Research Council (K2009-67X-21128-01-3; J.-P. L).
Authorship
Contribution: R.M.R. performed research, analyzed data, and wrote the paper; R.W.J.G., A.K., and F.C.K. performed research; K.W. provided the Rag-2−/−γc−/− mice and technical assistance; J.P.M. provided different forms of recombinant APRIL and discussed the data; J.-P.L. provided the Glce+/− mice; T.H.v.K. provided the phage display antibody AO4B08; and M.S and S.T.P. designed and supervised the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven T. Pals, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.t.pals@amc.uva.nl.
References
Author notes
M.S. and S.T.P. contributed equally to this study, sharing last authorship.



![Figure 6. Glce deficiency impairs HS interaction with a subset of growth factors/chemokines. Binding of ligands to Glce+/+ and Glce−/− MEFs. MEFs from Glce+/+ and Glce−/− mice were incubated with (A) growth factors (HGF and APRIL) or (B) chemokines (CXCL12α, CXCL13, and CCL21), washed, and the binding was analyzed by FACS. Graphs represent the relative binding, compared with the Glce+/+ MEFs (relative mean fluorescence intensity [MFI] ± SEM; n = 3-5). **P < .01. ***P < .001. ΔR-APRIL indicates point mutant (R231A) that is unable bind to the high-affinity receptor BCMA and TACI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/23/10.1182_blood-2010-12-325522/4/m_zh89991172260006.jpeg?Expires=1766466404&Signature=UyN8GR1AKAhmfkQCFiVbZVAJGtTCmaFNkTFvffQA-ou3B5w9WdCwOfBfWku-Aej3WwKPXoX9GGXjCXerxiCWx14bqYar1vpBGgBoNsNLzZuAh-HM8vn~bnSVI6Fg~c6agdFsHPAs~c7rM6TaTLnFB3-Qy79~1yaIJgyBwb~7FA2RQtLJU8La9HeImlOomw-E60SzoqiSwCVtN2KIOfPUdUuaTqKLe4kTevFJ6ZKjPlVfN~x7EvpzkzzvyI8lz6zsRgBeirYNygMtivI-u9VmZFnbh21NLV8BRPj0WagQIEUGk0JAto25EKrEktsNNL3Ubr3dYvLuw83vmDPwRxNspA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)