Abstract
Unfractionated heparin (UFH) is a widely used anticoagulant that has long been known to potentiate platelet responses to subthreshold doses of platelet agonists. UFH has been reported to bind and induce modest conformational changes in the major platelet integrin, αIIbβ3, and induce minor changes in platelet morphology. The mechanism by which UFH elicits these platelet-activating effects, however, is not well understood. We found that both human and murine platelets exposed to UFH, either in solution or immobilized onto artificial surfaces, underwent biochemical and morphologic changes indicative of a potentiated state, including phosphorylation of key cytosolic signaling molecules and cytoskeletal changes leading to cell spreading. Low molecular weight heparin and the synthetic pentasaccharide, fondaparinux, had similar platelet-potentiating effects. Human or mouse platelets lacking functional integrin αIIbβ3 complexes and human platelets pretreated with the fibrinogen receptor antagonists eptifibatide or abciximab failed to become potentiated by heparin, demonstrating that heparin promotes platelet responsiveness via its ability to initiate αIIbβ3-mediated outside-in signaling. Taken together, these data provide novel insights into the mechanism by which platelets become activated after exposure to heparin and heparin-coated surfaces, and suggest that currently used glycoprotein IIb-IIIa inhibitors may be effective inhibitors of nonimmune forms of heparin-induced platelet activation.
Introduction
Unfractionated heparin (UFH) is a negatively charged, heterogeneous mixture of sulfated polysaccharide chains derived from the mucous membranes of porcine intestines, and is clinically the most widely used anticoagulant.1 Almost as soon as heparin was introduced into the clinic in 1935, it was reported to cause an immediate small, but consistent, reduction in platelet count.2 In the majority of cases, this phenomenon is independent of any immune reaction, mild and transient in nature, and not associated with clinical bleeding or thrombosis.
In addition to inducing mild, transient thrombocytopenia, studies performed over a nearly 40-year period have consistently observed that addition of heparin to platelets in suspension enhances their responsiveness to weak stimuli, such as adenosine 5′-diphosphate (ADP).3,4 These so-called nonimmune effects are much more moderate than the kind of platelet activation events that are precipitated by the binding to platelet FcγRIIa of patient antibodies that react with platelet factor 4 (PF4)/heparin complexes in the disorder heparin-induced thrombocytopenia (HIT),5,6 although heparin binding-mediated platelet activation has been suggested, via secretion of platelet α-granule localized PF4, to play a role in the pathogenesis of immune HIT.7 Although the precise mechanism by which heparin potentiates platelet activation in the absence of antibody/heparin/PF4 complexes is not well understood, there are numerous reports that heparin associates directly with the platelet surface,8,9 produces minor,10 though not always observed,11 changes in platelet morphology, promotes a low level of P-selectin expression,4 as well as the binding of fibrinogen12 or the fibrinogen-mimetic antibody PAC-1,4 to the major platelet integrin, αIIbβ3. Similar to other forms of agonist-induced platelet aggregation, heparin-potentiated platelet aggregation requires αIIbβ3, as it can be blocked with anti-αIIbβ3-specific monoclonal antibodies, and does not occur in platelets from patients missing this integrin receptor.13
Like other members of the integrin family, αIIbβ3 functions as a bidirectional signaling molecule that, in response to cellular activation, adopts a high-affinity conformation that allows binding of large molecular weight adhesive ligands, such as fibrinogen, fibronectin, and von Willebrand factor. After ligand binding, αIIbβ3 is also capable of transmitting into the platelet biochemical signals that initiate a wide variety of cellular events, ranging from activation of protein tyrosine and lipid kinases to gross morphologic changes in cell shape and migration, a process often referred to as “outside-in” integrin signaling.14 Because αIIbβ3-mediated outside-in signaling is able to amplify the effects of weak stimuli downstream from integrin engagement and because heparin has been shown to interact with and activate αIIbβ3, the purpose of the present investigation was to determine whether binding of UFH to the platelet surface acts directly on αIIbβ3 to initiate outside-in integrin signaling. By detecting the phosphorylation of Akt, a downstream substrate of phosphatidylinositol-3 kinase (PI3K), which plays an early role in platelet activation downstream of agonist15 and adhesion receptor16,17 engagement, and focal adhesion kinase (FAK), which reports early events after integrin αIIbβ3-mediated outside-in signaling,18 we show that soluble injectable heparin induces Akt phosphorylation in resting human and mouse platelets and potentiates agonist-induced platelet aggregation. When platelets are exposed to surfaces coated with immobilized-heparin, FAK and Akt become phosphorylated, and the platelets spread. Activation of platelets by either soluble or immobilized heparin requires the integrin αIIbβ3, and the β3 cytoplasmic domain appears to play an essential role in this “outside-in signaling” process.
Methods
Materials
Antibodies specific for Akt and FAK were purchased from Santa Cruz Biotechnology. Antibodies specific for glycogen synthase kinase 3-β (GSK3-β), serine9-phosphorylated GSK3-β, serine473-phosphorylated Akt, and tyrosine397-phosphorylated FAK were purchased from Cell Signaling Technology. The antiphosphotyrosine monoclonal antibody PY20 was purchased from Invitrogen. Protease inhibitor cocktails (2-aminoethyl-benzenesulfonylfluoride hydrochloride, aprotinin, E-64 protease inhibitor, ethylenediaminetetraacetic acid disodium, leupeptin and hemisulfate) and phosphatase inhibitor cocktails (imidazole, sodium fluoride, sodium molybdate, sodium orthovanadate, and sodium tartrate dehydrate) were purchased from EMD Biosciences. Soluble and injectable UFH, low molecule weight heparin, fondaparinux (Arixtra), eptifibatide, and abciximab were obtained by prescription from a local pharmacy. ADP was purchased from Sigma-Aldrich. Wortmannin was purchased from EMD Biosciences. Collagen-related peptide RGDW and thrombin receptor activating peptide (TRAP = SFLLRN) were synthesized by the Blood Research Institute's Protein Chemistry Core Laboratory.
Platelet isolation
For some studies, small blood samples were obtained from a child diagnosed with variant Glanzmann thrombasthenia whose platelets expressed 100% normal levels of a mutant αIIbβ3 integrin complex that lacked most of the β3-cytoplasmic domain.19,20 All other blood samples from healthy volunteers who had not taken any anticoagulant in the past 2 weeks were donated as approved by the Institutional Review Board of the BloodCenter of Wisconsin, and informed consent was obtained in accordance with the Declaration of Helsinki. Blood was drawn into sodium citrate, pH 7.4, supplemented with 50 ng/mL prostaglandin E1 (PGE1) and spun at 200g for 10 minutes. Platelet-rich plasma was collected, and after the addition of 50 ng/mL PGE1, platelets were pelleted at 700g for 10 minutes. Platelets were washed twice in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 12mM NaHCO3, 137mM NaCl, 2.7mM KCl, 5mM glucose, 0.25% bovine serum albumin [BSA]) buffer containing 50 ng/mL PGE1 and 1mM ethylenediaminetetraacetic acid, pH 7.4, and finally resuspended in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid to a final concentration of 2.5 × 108/mL.
Washed mouse platelets were prepared as previously described21 with minor modifications. Briefly, 1.0 to 1.5 mL of blood from each of 3 or 4 tribromoethanol-anesthetized mice was drawn from the inferior vena cava into 0.1 mL of 0.13M sodium citrate, pH 7.4, pooled, diluted with an equal volume of modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, and centrifuged at 200g for 10 minutes at room temperature. Platelets were collected by centrifuging the platelet-rich plasma at 700g for 10 minutes in the presence of 1mM ethylenediaminetetraacetic acid and 50 ng/mL PGE1. Platelets were resuspended in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer to a concentration of 2.5 × 108 platelets/mL, supplemented with 1mM CaCl2, and allowed to rest for 30 minutes at room temperature before experimentation.
Platelet activation assays
ADP-induced platelet activation was measured in a whole-blood lumi-aggregometer (Chronolog). Human platelet-rich plasma was prepared by centrifuging citrate-anticoagulated whole blood at 200g for 10 minutes. After removal of platelet-rich plasma, the remaining blood was spun at 700g for another 10 minutes to obtain platelet-poor plasma. Platelets in platelet-rich plasma were adjusted to 2.5 × 108/mL with platelet-poor plasma, supplemented with 1mM CaCl2, and placed in a siliconized glass cuvette at 37°C with constant stirring at 1000 rpm. After preincubation with 0.2 U/mL UFH or low molecule weight heparin for 1 minute, platelet activation was initiated by addition of 2 to 3μM ADP.
Activation of Akt by heparin was assessed by incubating washed human or mouse platelets (2.5 × 108/mL) with various doses of UFH, low molecule weight heparin, or fondaparinux at 37°C for 15, 30, 60, or 120 seconds under stirring conditions (1000 rpm). In some experiments, platelets were preincubated with eptifibatide or wortmannin for 2 minutes at 37°C under stirring conditions before addition of UFH. Platelet lysates were prepared as described in “Platelet spreading assays” and used to determine the phosphorylation state of Akt and GSK3-β.
Immunoprecipitation and Western blot analysis
Platelet detergent lysates were prepared by adding an equal volume of ice-cold 2× lysis buffer (30mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 300mM NaCl, 20mM ethyleneglycoltetraacetic acid, 0.2mM MgCl2, 2% Triton X-100) containing 2× protease and phosphatase inhibitor cocktail directly to the aggregometer cuvette. Akt, phosphoSer473-Akt, GSK3-β, phosphoSer9-GSK3-β, FAK, and phosphotyrosine397-FAK were examined by Western blot analysis of total platelet lysates, whereas the total phosphorylation state of FAK was measured after immunoprecipitation with anti-FAK antibody, followed by capture of immune complexes using protein G-Sepharose beads (GE Healthcare). Immunoprecipitated proteins were separated by SDS-PAGE, transferred to polyvinylidene fluoride membranes, and visualized using an ECL detection kit (Pierce Chemical).
Platelet spreading assays
Fibrinogen (100 μg/mL), 10 U/mL UFH, or 10 U/mL low molecular weight heparin (LMWH) was incubated in 8-chamber glass tissue-culture slides (BD Biosciences) overnight at 4°C. After washing twice with phosphate-buffered saline, the slides were blocked with 1% fatty acid-free purified BSA in phosphate-buffered saline for 1 hour at room temperature and washed twice with Tyrode buffer. A total of 20 μL of washed human or mouse platelets at 2.5 × 108/mL that had been preincubated with the indicated reagents for 20 minutes at room temperature was incubated at 37°C for 45 minutes. After removal of unbound platelets, adhered platelets were fixed, permeabilized, and stained with tetramethylrhodamine isothiocyanate-conjugated phalloidin, as described previously.19 Samples were mounted in Vectashield mounting medium (Vector Laboratories), and images were acquired with a Photometrics SenSys camera (Photometrics) from 3 consecutive fields using a Zeiss Axioscop microscope (Carl Zeiss) with a Zeiss 100× oil-immersion lens.
For biochemical analysis, platelets were incubated at 37°C for 45 minutes in 10-cm tissue-culture dishes and lysed directly with 2× lysis buffer (30mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 300mM NaCl, 20mM ethyleneglycoltetraacetic acid, 0.2mM MgCl2, 2% Triton X-100) containing 2× protease and phosphatase inhibitor cocktails, and subjected to SDS-PAGE immunoprecipitation and/or immunoblot analysis.
Results
Heparin potentiates platelet responsiveness by initiating platelet signal amplification pathways
Previous studies have shown that UFH is capable of potentiating ADP-induced platelet aggregation,4,11 although the mechanism by which this occurs is poorly understood. To determine whether exposure of platelets to heparin, either in suspension or immobilized on a surface, results in the generation of biochemically detectable activation events, human platelets in plasma were pretreated with either soluble heparin in suspension or incubated in microtiter wells to which heparin had been immobilized. After exposure, platelets were lysed and subjected to immunoblot analysis. As shown in Figure 1A, although addition of heparin by itself induced neither aggregation nor granule secretion, it potentiated both reactions in the presence of low-dose ADP, confirming previous observations.4,22 LMWH and the synthetic pentasaccharide fondaparinux (Arixtra) had similar potentiating effects (supplemental Figures 1A, 2A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Human platelets also bound to and spread on immobilized heparin (Figure 1B) and fondaparinux (supplemental Figure 2C), suggesting that adhesion to heparin and heparin-like substances, much like exposure to them in solution, results in the transmission of outside-in signals that potentiate cytoskeletal rearrangements associated with platelet activation.

Heparin potentiates platelet activation in suspension and on surfaces by activating signal amplification pathways. (A) Heparin synergizes with ADP to augment aggregation and granule release. Human platelets (2.5 × 108/mL) in citrate-anticoagulated plasma were stimulated with 2μM ADP in the presence (UFH, 0.2 unit/mL) or absence (NaCl) of UFH at 37°C under stirring conditions. Although heparin alone did not stimulate aggregation (top panel) or granule secretion (bottom panel), platelets exposed to both ADP and heparin underwent irreversible aggregation and low-level granule release. Washed platelets in the presence of 200 μg/mL of exogenously added fibrinogen showed a similar synergistic aggregation response (not shown). (B) Platelets spread on immobilized heparin. Human platelets were added to 8-chamber glass tissue culture slides that had been coated with BSA (1%), fibrinogen (100 μg/mL), or heparin (10 units/mL), and allowed to spread for 45 minutes at 37°C. Note that platelets bound to and spread on immobilized heparin, although with slightly different morphology than on fibrinogen. (C) Akt and GSK3-β become phosphorylated in platelets exposed to heparin. Washed human platelets (2.5 × 108/mL) were placed in an aggregometer cuvette at 37°C in the presence or absence of heparin (1 unit/mL) for 2 minutes, lysed in SDS sample buffer, and subjected to immunoblot analysis using the indicated antibodies. Note that exposure to heparin alone was sufficient to initiate the Akt → GSK3-β signal amplification pathway. (D) Akt and FAK become phosphorylated in platelets exposed to immobilized heparin. Platelets bound to immobilized fibrinogen or heparin for 45 minutes, or those nonadherent to BSA, were subjected to SDS-PAGE/immunoblot analysis using the indicated antibodies. Note that both Akt and FAK, reporters of integrin-mediated outside-in signaling, were phosphorylated in platelets bound to heparin.

Heparin potentiates platelet activation in suspension and on surfaces by activating signal amplification pathways. (A) Heparin synergizes with ADP to augment aggregation and granule release. Human platelets (2.5 × 108/mL) in citrate-anticoagulated plasma were stimulated with 2μM ADP in the presence (UFH, 0.2 unit/mL) or absence (NaCl) of UFH at 37°C under stirring conditions. Although heparin alone did not stimulate aggregation (top panel) or granule secretion (bottom panel), platelets exposed to both ADP and heparin underwent irreversible aggregation and low-level granule release. Washed platelets in the presence of 200 μg/mL of exogenously added fibrinogen showed a similar synergistic aggregation response (not shown). (B) Platelets spread on immobilized heparin. Human platelets were added to 8-chamber glass tissue culture slides that had been coated with BSA (1%), fibrinogen (100 μg/mL), or heparin (10 units/mL), and allowed to spread for 45 minutes at 37°C. Note that platelets bound to and spread on immobilized heparin, although with slightly different morphology than on fibrinogen. (C) Akt and GSK3-β become phosphorylated in platelets exposed to heparin. Washed human platelets (2.5 × 108/mL) were placed in an aggregometer cuvette at 37°C in the presence or absence of heparin (1 unit/mL) for 2 minutes, lysed in SDS sample buffer, and subjected to immunoblot analysis using the indicated antibodies. Note that exposure to heparin alone was sufficient to initiate the Akt → GSK3-β signal amplification pathway. (D) Akt and FAK become phosphorylated in platelets exposed to immobilized heparin. Platelets bound to immobilized fibrinogen or heparin for 45 minutes, or those nonadherent to BSA, were subjected to SDS-PAGE/immunoblot analysis using the indicated antibodies. Note that both Akt and FAK, reporters of integrin-mediated outside-in signaling, were phosphorylated in platelets bound to heparin.
To explore whether the potentiating effect of heparin might be the result of activation of one or more cytosolic signaling cascades that function to lower the threshold for platelet activation, platelets in suspension were lysed and probed for phosphorylation of 2 downstream reporters of phosphatidylinositol 3-kinase (PI3K) activation: Akt15 and GSK 3-β.23 These enzymes became phosphorylated in platelets that were exposed to UFH in solution at doses ranging from 0.1 U/mL (supplemental Figure 3) to 1 U/mL (Figure 1C). LMWH and fondaparinux were also able to induce Akt phosphorylation (supplemental Figures 1B, 2B). In platelets exposed to surface-immobilized heparin, both Akt and FAK, a key reporter of αIIbβ3 ligation,18,24 became phosphorylated (Figure 1D). In particular, FAK Y397, a residue that coordinates activation of Src-family kinases downstream of integrin engagement,25 became phosphorylated in platelets adherent to immobilized fibrinogen. Taken together, these data suggest that heparin, both in soluble and immobilized form, potentiates platelet responsiveness by initiating the activation of key platelet signal amplification pathways.
To further examine the importance of PI3K in heparin-mediated platelet activation, washed human platelets were treated with the PI3K inhibitor wortmannin before exposure to heparin. As shown in Figure 2A, platelet spreading on immobilized heparin was largely abolished by wortmannin, as was the phosphorylation of Akt and GSK3-β in platelets exposed to UFH in solution (Figure 2B). These data further support the notion that heparin potentiates platelet activation by activating early intermediates in platelet signal transduction.

Platelet activation by either immobilized or solution-phase heparin requires PI3K. (A) Inhibition of platelet spreading on heparin. Washed human platelets at a concentration of 2.5 × 108/mL were preincubated for 3 minutes with 3μM wortmannin (right panels), or its vehicle control dimethyl sulfoxide (DMSO; left panels) before being added to chamber slides that had been coated with either fibrinogen (Fg; 100 μg/mL) or UFH (10 units/mL) under the same conditions described in Figure 1. Platelets were allowed to spread for 45 minutes at 37°C before being photographed. Note that platelet spreading on immobilized heparin was markedly attenuated in the presence wortmannin. (B) Inhibition of heparin-induced platelet potentiation by wortmannin. SDS-PAGE/immunoblot analysis of washed human platelets (2.5 × 108/mL) that had been pretreated with 3μM wortmannin or its DMSO vehicle control for 3 minutes before addition of 1 U/mL of unfractionated, pharmaceutical-grade heparin (Hep). Note that the strong activation of Akt and GSK3-β induced by exposure to heparin in solution is eliminated by wortmannin.

Platelet activation by either immobilized or solution-phase heparin requires PI3K. (A) Inhibition of platelet spreading on heparin. Washed human platelets at a concentration of 2.5 × 108/mL were preincubated for 3 minutes with 3μM wortmannin (right panels), or its vehicle control dimethyl sulfoxide (DMSO; left panels) before being added to chamber slides that had been coated with either fibrinogen (Fg; 100 μg/mL) or UFH (10 units/mL) under the same conditions described in Figure 1. Platelets were allowed to spread for 45 minutes at 37°C before being photographed. Note that platelet spreading on immobilized heparin was markedly attenuated in the presence wortmannin. (B) Inhibition of heparin-induced platelet potentiation by wortmannin. SDS-PAGE/immunoblot analysis of washed human platelets (2.5 × 108/mL) that had been pretreated with 3μM wortmannin or its DMSO vehicle control for 3 minutes before addition of 1 U/mL of unfractionated, pharmaceutical-grade heparin (Hep). Note that the strong activation of Akt and GSK3-β induced by exposure to heparin in solution is eliminated by wortmannin.
αIIbβ3 transmits the signals that initiate heparin-induced platelet potentiation
Heparin is known to bind to the surface of both resting and activated platelets, either in buffer or in plasma,9 and one report used heparin-Sepharose affinity chromatography and chemical cross-linking analysis to show that heparin interacts directly with αIIbβ3.12 In addition, FAK, an early reporter of αIIbβ3 engagement,24,26 becomes strongly phosphorylated in platelets adherent to immobilized heparin (Figure 1D). To examine whether αIIbβ3 is required for heparin-initiated signal amplification leading to platelet potentiation, platelets from αIIbβ3-deficient versus wild-type mice were compared for their ability to bind to and spread on immobilized heparin, and for the ability of soluble heparin in solution to activate the PI3K → Akt signal amplification pathway in platelets in suspension. As shown in Figure 3, both of these reactions were severely compromised in platelets from β3-knockout mice. Adhesion and spreading of murine platelets on LMWH or on fondaparinux also required functional αIIbβ3 (supplemental Figures 1C and 2C). αIIbβ3 was also found to be required for heparin-induced potentiation of human platelets because, when they were preincubated with 2 different αIIbβ3 antagonists, eptifibatide and abciximab (before their addition to heparin-coated microtiter wells), spreading on immobilized heparin was substantially reduced (Figure 4A). Pretreatment with eptifibatide and/or abciximab also suppressed Akt phosphorylation in platelets exposed to either soluble heparin (Figure 4B) or immobilized heparin (supplemental Figure 4), as well as their ability to spread on immobilized fondaparinux (supplemental Figure 2C). Finally, platelets from a patient with Glanzmann thrombasthenia that expressed 100% levels of a mutant αIIbβ3 complex lacking much of the β3 cytoplasmic domain19,20 failed to increase the phosphorylation of Akt on exposure to soluble UFH (Figure 4C). Taken together, these data demonstrate that the mechanism by which heparin potentiates platelet activation is via outside-in signaling through the major platelet integrin, αIIbβ3 (Figure 5).

Heparin-induced potentiation of murine platelets is mediated by integrin αIIbβ3. (A) Platelets from β3 knockout mice cannot spread on immobilized heparin. Washed mouse platelets from wild-type (WT) and αIIbβ3-deficient (β3−/−) mice were added to glass slides that had been coated with 1% BSA, 100 μg/mL fibrinogen (Fg), or 10 U/mL heparin (Hep) in the presence of 2μM ADP to activate the integrin. Note that the failure of αIIbβ3-deficient platelets to spread on heparin is nearly identical to their well-characterized inability to spread on immobilized fibrinogen. (B) Heparin-induced phosphorylation of Akt in murine platelets requires αIIbβ3. Platelets from WT and integrin-β3-deficient mice were placed in an aggregometer cuvette in the presence of the indicated concentrations of UFH, allowed to stir at 1000 rpm for 2 minutes at 37°C, lysed in SDS sample buffer, and subjected to immunoblot analysis using antibodies specific for Akt antigen or phosphoserine473 Akt. Note that heparin failed to induce dose-dependent Akt phosphorylation in αIIbβ3-deficient platelets.

Heparin-induced potentiation of murine platelets is mediated by integrin αIIbβ3. (A) Platelets from β3 knockout mice cannot spread on immobilized heparin. Washed mouse platelets from wild-type (WT) and αIIbβ3-deficient (β3−/−) mice were added to glass slides that had been coated with 1% BSA, 100 μg/mL fibrinogen (Fg), or 10 U/mL heparin (Hep) in the presence of 2μM ADP to activate the integrin. Note that the failure of αIIbβ3-deficient platelets to spread on heparin is nearly identical to their well-characterized inability to spread on immobilized fibrinogen. (B) Heparin-induced phosphorylation of Akt in murine platelets requires αIIbβ3. Platelets from WT and integrin-β3-deficient mice were placed in an aggregometer cuvette in the presence of the indicated concentrations of UFH, allowed to stir at 1000 rpm for 2 minutes at 37°C, lysed in SDS sample buffer, and subjected to immunoblot analysis using antibodies specific for Akt antigen or phosphoserine473 Akt. Note that heparin failed to induce dose-dependent Akt phosphorylation in αIIbβ3-deficient platelets.

αIIbβ3 is required for heparin-induced potentiation of human platelets. (A) Fibrinogen receptor antagonists inhibit platelet spreading on immobilized heparin. Washed human platelets were added to heparin-coated glass slides in the presence of either a control IgG Fab fragment (left panel) or glycoprotein IIb-IIIa receptor antagonists, 10 μg/mL abciximab (middle panel) or 6.67 μg/mL eptifibatide (left panel). Note that platelet spreading 45 minutes later was abolished by either fiban. (B) Eptifibatide blocks heparin-induced platelet potentiation in solution. Washed platelets were stirred at 1000 rpm at 37°C alone or in the presence of heparin with or without 6.67 μg/mL eptifibatide, lysed, and subjected to immunoblot analysis as described in the legend to Figure 1. (C) Functional αIIbβ3 complexes are required for heparin-induced platelet potentiation. Platelets from a normal person or from a patient with variant Glanzmann thrombasthenia whose platelets express 100% levels of αIIbβ3 lacking most of the C-terminus of the β3 cytoplasmic domain were stirred in the presence or absence of UFH. Note that the increase in Akt phosphorylation normally induced by heparin exposure does not take place in platelets expressing signaling-defective αIIbβ3.

αIIbβ3 is required for heparin-induced potentiation of human platelets. (A) Fibrinogen receptor antagonists inhibit platelet spreading on immobilized heparin. Washed human platelets were added to heparin-coated glass slides in the presence of either a control IgG Fab fragment (left panel) or glycoprotein IIb-IIIa receptor antagonists, 10 μg/mL abciximab (middle panel) or 6.67 μg/mL eptifibatide (left panel). Note that platelet spreading 45 minutes later was abolished by either fiban. (B) Eptifibatide blocks heparin-induced platelet potentiation in solution. Washed platelets were stirred at 1000 rpm at 37°C alone or in the presence of heparin with or without 6.67 μg/mL eptifibatide, lysed, and subjected to immunoblot analysis as described in the legend to Figure 1. (C) Functional αIIbβ3 complexes are required for heparin-induced platelet potentiation. Platelets from a normal person or from a patient with variant Glanzmann thrombasthenia whose platelets express 100% levels of αIIbβ3 lacking most of the C-terminus of the β3 cytoplasmic domain were stirred in the presence or absence of UFH. Note that the increase in Akt phosphorylation normally induced by heparin exposure does not take place in platelets expressing signaling-defective αIIbβ3.
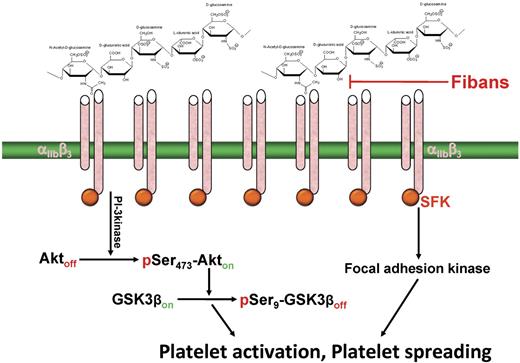
Schematic of the mechanism by which heparin induces outside-in, αIIbβ3-dependent platelet potentiation. Multivalent heparin is proposed to interact with the integrin at or near its ligand-binding site, resulting in microclustering of αIIbβ3 complexes on the platelet surface, transactivation of integrin-associated Src-family kinases (SFK), and subsequent activation of downstream signaling pathways that either potentiate (solution heparin) or induce (immobilized heparin) platelet activation. Fibans, such as abciximab and eptifibatide, might be effective in blocking this antibody-independent form of heparin-induced platelet activation.

Schematic of the mechanism by which heparin induces outside-in, αIIbβ3-dependent platelet potentiation. Multivalent heparin is proposed to interact with the integrin at or near its ligand-binding site, resulting in microclustering of αIIbβ3 complexes on the platelet surface, transactivation of integrin-associated Src-family kinases (SFK), and subsequent activation of downstream signaling pathways that either potentiate (solution heparin) or induce (immobilized heparin) platelet activation. Fibans, such as abciximab and eptifibatide, might be effective in blocking this antibody-independent form of heparin-induced platelet activation.
Discussion
UFH is widely used as an anticoagulant in the treatment and prevention of venous and arterial thrombosis, largely because of its low cost, rapid onset of action, efficacy, relatively short half-life, and ease of laboratory monitoring. However, heparin therapy has been associated with a number of side effects, including systemic and local allergic reactions, osteoporosis, bleeding, thrombocytopenia, and thrombosis.27,28 Of these, thrombocytopenia after heparin administration is by far the most common and has been classified into 2 types: nonimmune heparin-induced thrombocytopenia and antibody-mediated heparin-induced thrombocytopenia, the latter commonly referred to as HIT.29 Although the molecular mechanism of platelet activation in immune-mediated HIT has been elegantly elucidated over the past 20 years,5,30 the biologic basis of nonimmune heparin-mediated platelet activation remains poorly understood.
A high but not uncommonly used bolus dose of 5000 units of heparin in an adult would achieve, at least transiently, a circulating concentration in of 0.2 to 1 U/mL of heparin, the dose used in this study to elicit platelet potentiating effects. A series of papers published more than 25 years ago suggested that circulating heparin might be able to augment platelet aggregation, leading to platelet clearance by virtue of its ability to bind and tie up the naturally occurring, endothelial-derived platelet inhibitor prostacyclin.31,32 This mode of action, however, is not operable in the present studies because there is no PGI2 in our experimental system for heparin to neutralize that might explain its platelet-potentiating effects.
Mild nonimmune heparin-associated thrombocytopenia has been reported to occur in 10% to 30% of patients receiving heparin33 and was proposed more than 25 years ago to result from a direct action of heparin on some component of the platelet surface.34 Because of its net negative charge, heparin, like other glycosaminoglycans, is able to bind and concentrate growth factors and chemokines on the cell surface.35 It has also been exploited to bind and purify numerous protein components of platelet α-granules, including von Willebrand factor,36 fibronectin,37 P-selectin,38 thrombospondin,39 vitronectin,40 and PF4.41 Interestingly, and relevant to this discussion, heparin has been shown to bind a number of transmembrane glycoproteins, including the Ig-superfamily member G6b,42 as well as the cell-surface integrins αvβ343,44 and α5β1.44 Whether heparin similarly binds αIIbβ3 has been controversial, as Fitzgerald et al purified αIIbβ3 from heparin-binding contaminants by allowing it to flow unbound through heparin-Sepharose beads as part of an early chromatographic purification protocol for this integrin,45 whereas Sobel et al reported that not only could detergent solubilized αIIbβ3 specifically bind to and become eluted from this affinity matrix, tritiated heparin became photoaffinity cross-linked to cell surface αIIbβ3 in intact platelets.12 Our observation that either of 2 different anti-glycoprotein IIb-IIIa receptor antagonists, abciximab and eptifibatide, are able to prevent platelet spreading on immobilized heparin (Figure 4A) and downstream heparin-initiated signal transduction (Figure 4B; supplemental Figure 4), taken together with the observation that both murine (Figure 3) and human (Figure 4C) platelets lacking functional αIIbβ3 receptors fail to react with or become activated by immobilized UFH, LMWH (supplemental Figure 1), or fondaparinux (supplemental Figure 2), all under conditions in which the major αIIβ3 ligand, fibrinogen, is absent, provide compelling evidence that these clinically used compounds interact directly with αIIbβ3, and not another platelet receptor, to initiate outside-in signaling. This is not to imply that αIIbβ3 is the only binding site on the platelet surface for heparin, as platelets lacking this major platelet integrin still bind heparin.46 Our data do show, however, that functional αIIbβ3 complexes are required for heparin and heparin-like compounds to promote platelet responsiveness.
The mechanism by which platelets become activated by soluble versus immobilized heparin does not appear to be the same (Table 1). Thus, signals initiated by the interaction of platelet αIIbβ3 with heparin in solution are, in the absence of additional stimuli, themselves insufficient to reach the threshold necessary for platelet activation and require addition of costimulatory agonists, such as low-dose ADP or thrombin3,4 (Figure 1A). In contrast, heparin immobilized on a surface can support platelet adhesion and spreading in the absence of additional stimuli, perhaps because polyvalent heparin is able to bring fluid plasma membrane αIIbβ3 complexes into close enough proximity to catalyze the generation of outside-in signaling reactions of sufficient strength to initiate cytoskeletal rearrangements and cell spreading. That FAK Y397, a reliable reporter of αIIbβ3-mediated outside-in signaling,18,26 became strongly tyrosine phosphorylated in platelets adherent to immobilized heparin (Figure 1) would appear to support this notion.
Where on the αIIbβ3 complex heparin specifically interacts is not known. The observation that both abciximab and eptifibatide prevent platelet spreading on immobilized heparin (Figure 4A) strongly suggests that heparin interacts with αIIbβ3 at or near the ligand-binding site within the integrin head domain. In this regard, Vorup-Jensen et al have recently localized the heparin binding domain on the leukocyte integrin αXβ2 to the α-subunit I domain,47,48 which is homologous to the βA ligand-binding domain of the integrin β3-subunit. Future studies aimed at determining the exact site (β3-subunit βA domain, αIIb subunit β-propeller domain, or both) at which heparin binds αIIbβ3 might facilitate design of specific antagonists to prevent nonimmune heparin-initiated platelet activation.
Finally, in addition to its widespread use as a soluble anticoagulant, heparin is also widely used to modify the surface of medical devices, such as cardiopulmonary bypass circuits49 and, much less commonly, drug-eluting metallic stents used to restore patency and prevent subacute thrombosis and restenosis of coronary arteries.50 Although heparin-coated stents were previously reported,51 with some exception,52 to reduce the incidence of subacute stent thrombosis compared with bare metal stents, a small percentage (< 1%) of patients experienced low levels of platelet activation leading to clinical thrombosis. Studies aimed at determining whether the molecular mechanisms of nonimmune platelet potentiation described herein contribute to the residual platelet deposition and thrombus formation observed on heparin-coated surfaces in vivo may be an interesting area of future investigation.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part in abstract form at the XXII Congress of the International Society on Thrombosis and Hemostasis, July 11-16, 2009.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Richard Gumina (The Ohio State University) for his critical reading of the manuscript relative to the use of heparin-coated stents.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant HL-44612; P.J.N.).
National Institutes of Health
Authorship
Contribution: C.G. designed and performed research, analyzed results, and wrote the paper; B.B. performed research and analyzed results; J.F. performed research; D.A.W. analyzed data; D.K.N. analyzed results; and P.J.N. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: P.J.N. is a consultant for Novo Nordisk and a member of the Scientific Advisory Board for the New York Blood Center. The remaining authors declare no competing financial interests.
Correspondence: Cunji Gao, Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, 638 N 18th St, Milwaukee, WI 53201; e-mail:cunji.gao@bcw.edu.

