Abstract
The engagement of TCR induces T-cell activation, which initiates multiple characteristic changes such as increase in cell size, cell division, and the production of cytokines and other effector molecules. The mammalian target of rapamycin (mTOR) regulates protein synthesis, transcription, cell survival, and autophagy. Critical roles of mTOR in T-cell activation and effector/memory differentiation have been revealed using chemical inhibitors or by genetic ablation of mTOR in T cells. However, the connection between mTOR signaling and other signaling cascades downstream of TCR is unclear. We demonstrate that diacylglycerol (DAG) and TCR engagement activate signaling in both mTOR complexes 1 and 2 through the activation of the Ras–mitogen-activated protein kinase/extracellular signal–regulated kinase 1/2 (Mek1/2)–extracellular signal–regulated kinase 1/2 (Erk1/2)–activator protein 1 (AP-1), known collectively as the Ras-Mek1/2-Erk1/2-AP-1 pathway. Deficiency of RasGRP1 or inhibition of Mek1/2 activity drastically decreases TCR-induced mTOR activation, whereas constitutively active Ras or Mek1 promotes mTOR activation. Although constitutively active Akt promotes TCR-induced mTOR activation, such activation is attenuated by Mek1/2 inhibition. We demonstrated further that DAG kinases (DGKs) α and ζ, which terminate DAG-mediated signaling, synergistically inhibit TCR-induced mTOR activation by inhibiting the Ras-Mek1/2-Erk/12 pathway. These observations provide novel insights into the regulation of mTOR activation.
Introduction
The signal from the TCR plays crucial roles in T-cell development and peripheral T-cell function. In the thymus, maturation from CD4+CD8+ double-positive (DP) T cells to the CD4+CD8− and CD4−CD8+ single-positive (SP) T cells requires the engagement of functionally rearranged TCRs with self-peptide MHC complexes presented by thymic epithelial cells. In the periphery, engagement of the TCR with foreign peptides loaded on MHC molecules on APCs triggers serial events that result in T-cell activation and differentiation to effector/memory T cells. In both thymocytes and peripheral T cells, TCR signaling triggers a plethora of events, such as the increase of cell size and metabolism, proliferation, and the production of cytokines and other effector molecules that are important for maturation or effector functions.
TCR engagement triggers the activation of the Src and Syk families of tyrosine kinases, including Lck and Zap70, respectively, which phosphorylate many substrates, including adaptor proteins and enzymes, causing the formation of multimolecular signaling complexes that can lead to the activation of phospholipase C-γ1 (PLC-γ1).1,2 Activated PLC-γ1 hydrolyzes the membrane-bound phospholipid phosphatidylinositol 4,5-bisphosphate to generate two well-characterized second messengers, inositol 1,4,5-trisphosphate and diacylglycerol (DAG). These 2 second messengers activate multiple signaling cascades that are pivotal for T-cell development, activation, and effector functions. Soluble inositol 1,4,5-trisphosphate triggers calcium signaling to activate calcineurin, leading to nuclear translocation of nuclear factor of activated T-cells.3 The membrane-bound DAG can activate Ras guanyl nucleotide–releasing protein 1 (RasGRP1) and protein kinase Cθ (PKCθ) by binding to their cysteine-rich (C1) domains, resulting in activation of the Ras–mitogen-activated protein kinase/extracellular signal–regulated kinase 1/2 (Mek1/2)–extracellular signal–regulated kinase 1/2 (Erk1/2)–activator protein 1 (AP-1), known collectively as the Ras-Mek1/2-Erk1/2-AP-1 pathway, and the IκB kinase–NF-κB pathways.4,5 In addition to these signaling cascades, TCR stimulation activates the PI3K/Akt pathway, which is further strengthened in the presence of the CD28 costimulatory signal.6,7
The mammalian target of rapamycin (mTOR), a serine/threonine protein kinase, integrates numerous environmental stimuli, including growth factors, nutrients, and stress-activated signals, to regulate cell metabolism, survival, growth, and proliferation.8 A growing body of evidence suggests that mTOR signaling proceeds through two signaling complexes: mTOR complex 1(mTORC1), a rapamycin-sensitive complex associated with regulatory associated protein of mTOR (raptor),9 and mTOR complex 2 (mTORC2), a rapamycin-insensitive complex associated with rapamycin-insensitive companion of mTOR (rictor).10 In addition to raptor, mTORC1 consists of the Ras homolog enriched in brain (Rheb), the GβL adaptor subunit, mLST8, and PRAS40. GTP-bound RheB, the activity of which is further regulated by an upstream tuberous sclerosis complex (TSC), a bipartite protein complex of hamartin (TSC1), and tuberin (TSC2), positively activates mTOR kinase activity in mTORC1.11 In cell line models, the PI3K/Akt pathway has been shown to activate mTORC1 signaling through phosphorylation of TSC2.12,13 Phosphorylation of TSC2 triggers the dissociation of TSC2 from TSC1, leading to activation of mTORC1 signaling via Rheb.14 mTORC1 promotes cell growth and proliferation through phosphorylation and activation of the 70-kDa ribosomal S6 kinase (S6K1) and the translational repressor 4 elongation factor–binding protein 1 (4E-BP1).11,15,16 Activated S6K1 phosphorylates S6 and other translational regulators such as eIF2 kinase and eIF-4B1 to regulate the initiation of translation.17,18 Phosphorylation of 4E-BP1 releases eukaryotic initiation factor 4E (eIF4E) to promote the recruitment of ribosome machinery in protein translation.19 In addition to rictor, mTORC2 contains an upstream positive-signaling protein called mSin1, the GβL adaptor subunit, mLST8, and protein observed with rictor (protor).20 mTORC2 phosphorylates protein kinase B, or Akt, specifically at Serine 473 (S473) to increase Akt activity, further promoting nutrient uptake and cell survival.21
Both genetic and pharmacologic studies have demonstrated that mTOR plays crucial roles during T-cell activation, anergy, lineage commitment, and other immune responses.22 TCR stimulation and cytokine treatment have been shown to induce mTOR activity in T cells, whereas anergized T cells displayed decreased mTOR activity in vitro.23 Furthermore, treatment of T cells with rapamycin, an inhibitor of mTOR signaling during antigen stimulation, induced anergy in vitro.24 Ectopic expression of the rapamycin-resistant mTOR mutant protein in a CD4+ cell line failed to produce T-cell anergy.23 In addition, mTOR has been shown to inhibit the production of inducible regulatory T cells (iTregs), because rapamycin treatment has been found to increase the induction of iTregs in vitro25 and in vivo.26 Similarly, mTOR-deficient T cells are predisposed to iTregs, but exhibit impaired differentiation to effector T-cell lineages.27 Studies using rapamycin and RNA interference have demonstrated enhanced memory T-cell responses to microbial pathogens.28 In addition, mTOR has been shown to play an important role in T-cell trafficking in vivo by regulating the expression of CCR7.29 Given the importance of mTOR in T cells, it is critical to understand how mTOR is regulated in these cells. Whereas evidence shows that TCR stimulation induces mTOR activity,30 how TCR signaling leads to mTOR activation remains unclear.
DAG kinases (DGKs), a family of ten enzymes in mammals, catalyze the phosphorylation of DAG to produce phosphatidic acid (PA). Both DAG and PA are important second messengers and play important roles in signal transduction and cellular function.31,32 It has been proposed that DGK enzymes serve as an intracellular switch to initiate PA signaling by effectively converting DAG into PA while terminating DAG signaling. In cell line models and in mice, DGKα and DGKζ inhibit DAG-mediated signaling in T cells after TCR engagement.33-35 Deficiency of both DGKα and DGKζ causes elevated Ras-Erk1/2 activation in developing T cells and the developmental blockage of T cells at the DP stage in the thymus. DGKα and DGKζ negatively regulate peripheral T-cell activation and contribute to T-cell anergy.36,37 DGKα and DGKζ also have a tumor suppressor function, because a decrease in DGK activity promotes thymic lymphomagenesis.35 In non-T-cell line models and tumor cells, the PI3K/Akt pathway has been found to be the major pathway responsible for growth factor- and insulin-induced mTOR activation.38 In addition, DGKζ- and phospholipase D–derived PA, but not DGKα-derived PA, have been reported to enhance mTOR activity.39-41 However, the role of DAG-mediated signaling and the physiologic function of DGKs in mTOR activation are unclear.
In the present study, we demonstrate that the DAG-mediated RasGRP1-Ras-Mek1/2-Erk1/2 pathway is critical for TCR-induced mTORC1 and mTORC2 activation in both T-cell line models and in primary T cells. Deficiency of RasGRP1 or overexpression of a dominant-negative form, Mek1 (dnMek1), causes impairment in TCR-induced mTORC1 and mTORC2 activation, which is correlated with decreased Erk1/2 activation. Complementarily, constitutively active Ras or Mek1 promotes mTORC1 and mTORC2 activation. Moreover, DGKα and DGKζ synergistically inhibit TCR-induced mTOR activation in T cells. We demonstrate that overexpression of DGKζ inhibits TCR-induced mTORC1 and mTORC2 activation in a kinase activity–dependent manner. Alternately, the deficiency of both DGKα and DGKζ increases TCR-induced mTOR activation, an effect that is correlated with elevated activation of the Ras-Mek1/2-Erk1/2 pathway and can be abolished by inhibition of Mek1/2 activity. Our studies provide new insights into the mechanisms involved in mTOR activation in T cells.
Methods
Mice and cell lines
C56BL/6 and CaKRas mice were obtained from The Jackson Laboratory. RasGRP1−/−, DGKα−/−, and/or DGKζ−/− mice were described previously.5,34-36 All mice were generated and used in accordance with protocols approved by the institutional animal care and use committee at Duke University. Murine 2B4 T-cell hybridomas were grown in RPMI 1640 medium supplemented with 10% FBS (HyClone), penicillin, streptomycin, and glutamine (RPMI 10).
Constructs and establishing 2B4 cell lines
Retrovirus-mediated gene transfer was used to express the caMek1, dnMek1, Myr-Akt, Akt-DD, DGKζ1, DGKζ2, and DGKζ1-KD proteins in the 2B4 cell line. Packaging cells (Phoenix-Eco) were transfected using the calcium phosphate method, and the viral supernatant was collected 48 hours after transfection. For infection, 5 × 105 2B4 cells in 0.5 mL of RPMI 10 was mixed with 0.5 mL of viral supernatant in a 24-well plate, and 5 μg/mL of Polybrene was added. Cells were centrifuged at 2500g for 1.5 hours at room temperature. Medium was changed with fresh RPMI 10 at 7 and 24 hours after infection. After 48 hours of infection, green fluorescent protein-positive (GFP+) cells were sorted using the MoFlo sorter.
Cell preparation, stimulation, and Western blot analysis
Single-cell suspensions from thymi and spleens of mice were prepared in IMDM supplemented with 10% FCS, penicillin/streptomycin, and l-glutamine. T cells were purified from splenocytes using a T-cell enrichment kit (Miltenyi Biotec) according to the manufacturer's protocol, with purity usually > 90%. Five to 10 million thymocytes, splenic T cells, or 2B4 cells were washed 2 times with PBS and rested in 0.5 mL of PBS at 37°C for 30 minutes. During this period, cells were preincubated in the presence or absence of LY294002 (40μM), U0126 (20μM), or rapamycin (500nM; all from EMD Biosciences). Rested cells were stimulated with 5 μg/mL of anti-CD3 (500A2) for the indicated time points.
After stimulation, cells were lysed in 1% non-diet P-40 lysis buffer (1% NP-40, 150mM NaCl, 50mM Tris, pH 7.4) with freshly added protease and phosphatase inhibitors. Proteins in lysates were separated by SDS/PAGE and transferred onto nitrocellulose membranes. Membranes were probed for the following antibodies: anti–phospho-p44/42 mitogen-activated protein kinase T202/Y204 (197G2), anti–phospho-p90RSK T359/S363, anti–phospho-p70S6K T421/424, anti–phospho-S6 S235/236 (2F9), anti–phospho-S6 S240/244, anti–phospho-4E-BP1 T37/S45, anti–phospho-Akt T308, anti–phospho-Akt S473 (193H12), anti-Akt, and anti–phospho-Foxo1 S256 (all from Cell Signaling Technology); anti-RasGRP1 (from Santa Cruz Biotechnology); and anti–β-actin (from Sigma-Aldrich). The membranes were further probed with HRP-conjugated secondary antibodies (Bio-Rad) and developed with ECL Western blot substrate (PerkinElmer). Western blot films were scanned and phosphorylation intensities were quantified using ImageJ version 1.43u software. The values of phosphorylation intensities for different proteins from multiple experiments were analyzed statistically with ANOVA using Prism 5 software.
Results
Activation of mTOR by TCR and DAG in thymocytes
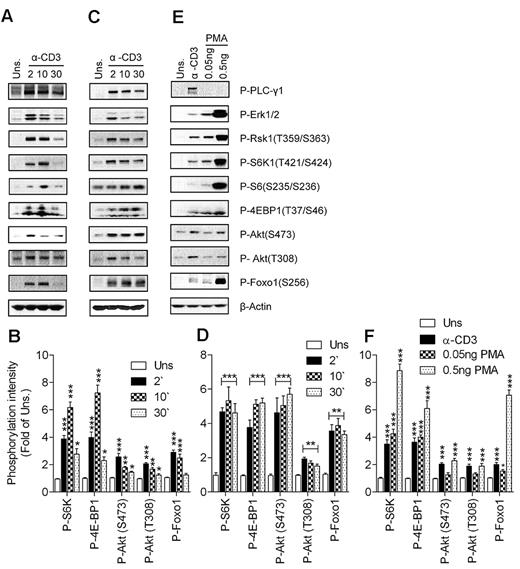
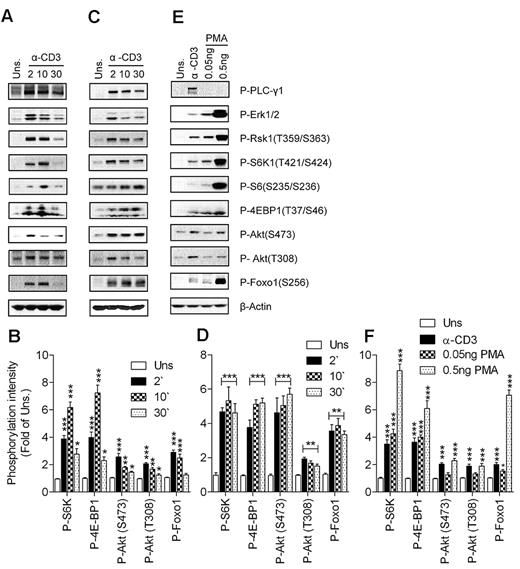
S6K1 and 4E-BP1 phosphorylation have been used as the canonical downstream substrates to study mTORC1 signaling in cell line models after treatment with nutrients and growth factors.42 We assessed anti-CD3–induced mTOR signaling in freshly isolated T cells from thymi and spleens of wild-type (WT) mice. As shown in Figure 1A and B, engagement of the TCR on thymocytes induced phosphorylation of S6K1 and 4E-BP1. Consistent with S6K1 activation, phosphorylation of S6, a substrate of S6K1, was also elevated after TCR stimulation in thymocytes compared with unstimulated conditions. TCR stimulation also induced Akt phosphorylation at T308 and S473, catalyzed by PDK1 and mTORC2, respectively, indicating that both PI3K and mTORC2 are activated after TCR engagement. Activation of Akt by TCR engagement was assessed by the increased phosphorylation status of Foxo1, a substrate of Akt. Similar results were obtained when peripheral T cells were used for assessing mTOR activity after T-cell stimulation (Figure 1C-D), although the phosphorylation of some of these proteins appeared to last longer in splenic T cells than in thymocytes. Correlated with mTOR and PI3K activation, phosphorylation of PLC-γ1, Erk1/2, and Rsk1 (a substrate of Erk1/2) was induced by TCR in thymocytes and splenic T cells after TCR engagement. Thus, TCR stimulation is sufficient to induce mTORC1, mTORC2, and PI3K/Akt activation.
TCR- and DAG-induced mTORC1 and mTORC2 activation. WT thymocytes (A-B) and splenic T cells (C-F) were left unstimulated or stimulated with an anti-CD3 antibody (500A2; A-D) at 37°C for the indicated times (in minutes) or with PMA (E-F) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of at least 3 experiments. Panels B, D, and F show quantification for phosphorylation levels relative to unstimulated controls (means ± SEM of 3 independent experiments; *P < .05; **P < .01; ***P < .001 relative to unstimulated control as indicated by ANOVA).
TCR- and DAG-induced mTORC1 and mTORC2 activation. WT thymocytes (A-B) and splenic T cells (C-F) were left unstimulated or stimulated with an anti-CD3 antibody (500A2; A-D) at 37°C for the indicated times (in minutes) or with PMA (E-F) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of at least 3 experiments. Panels B, D, and F show quantification for phosphorylation levels relative to unstimulated controls (means ± SEM of 3 independent experiments; *P < .05; **P < .01; ***P < .001 relative to unstimulated control as indicated by ANOVA).
As mentioned previously, DAG is one of the most important second messengers generated after TCR engagement. We sought to determine whether DAG-mediated signaling can activate mTORC1 and mTORC2 in T cells. As shown in Figure 1E and F, stimulation of thymocytes by low concentrations of phorbol 12-myristate 13-acetate (PMA), a functional analog of DAG, can induce S6K1 and 4E-BP1 phosphorylation. In addition, PMA induced Akt phosphorylation at S473 at a higher concentration compared with the concentration at which mTORC1 is activated. Furthermore, Foxo1 phosphorylation was also induced after PMA stimulation. Foxo1 phosphorylation could be detected after PMA (0.05 ng/mL) stimulation, whereas at this concentration of PMA, Akt phosphorylation was only slightly increased, possibly due to the high sensitivity of Foxo1 phosphorylation to a small increase in Akt activity. Similar data were also observed in splenic T cells (data not shown). These data suggest that DAG-mediated signaling is sufficient to induce both mTORC1 and mTORC2 activation in T cells.
Defective mTOR and Akt activation in RasGRP1-deficient thymocytes
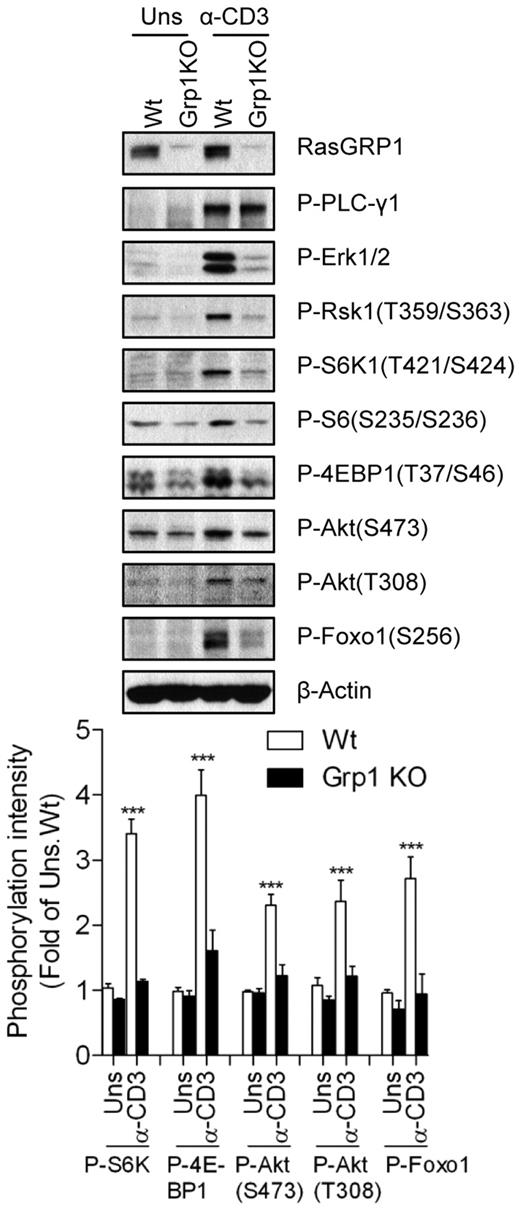
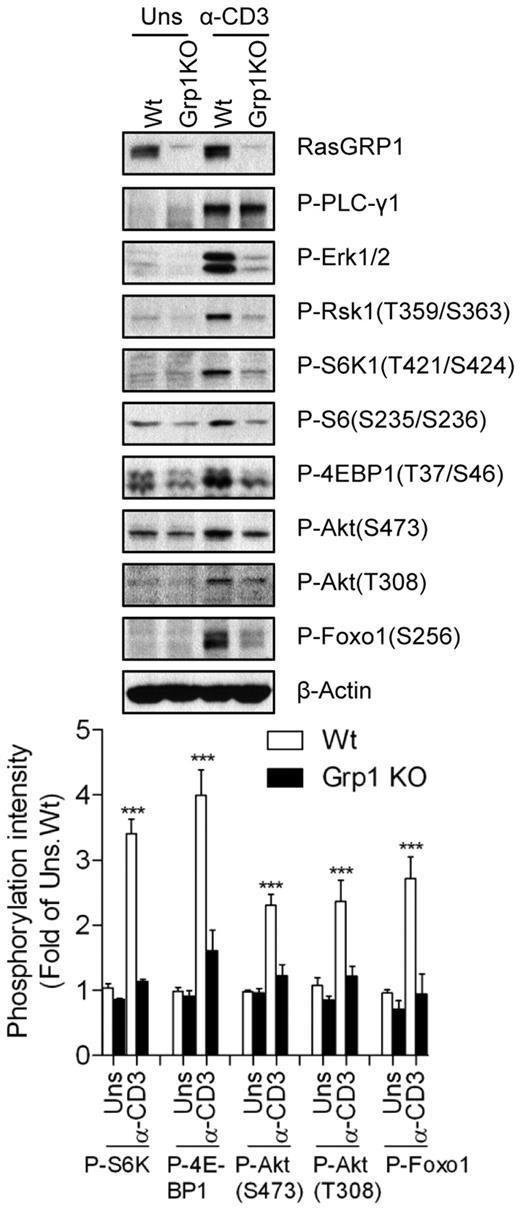
The RasGRP1-Ras-Mek1/2-Erk1/2 signaling cascade is a major downstream effector pathway for DAG signaling in T cells after TCR engagement. We examined the role of RasGRP1 in TCR-induced mTOR activation in RasGRP1-deficient thymocytes. RasGRP1 deficiency resulted in a blockage of T-cell development at the CD4+CD8+ DP stage in mice.5 TCR-induced Erk1/2 activation was defective, which is consistent with previous results,5 whereas PLC-γ1 phosphorylation, an event upstream of DAG production, was preserved in RasGRP1-deficient thymocytes (Figure 2). However, TCR-induced phosphorylation of S6K1, S6, and 4E-BP1 was significantly decreased in RasGRP1-deficient thymocytes. Phosphorylation of Akt at T308 and S473 and its downstream target Foxo1 was also decreased in these cells after TCR engagement. These results reveal for the first time that that RasGRP1 is critical for TCR-induced mTORC1 and mTORC2 activation in thymocytes. The data also suggest that RasGRP1 is involved in TCR-induced activation of the PI3K-PDK1-Akt pathway, because Akt phosphorylation at T308 was decreased in the absence of RasGRP1.
Critical role of RasGRP1 in TCR-induced mTORC1, mTOR2, and PI3K/Akt activation. Thymocytes from WT (Wt) and RasGRP1 KO (Grp1 KO) mice were left unstimulated or stimulated with anti-CD3 (500A2) for 5 minutes at 37°C. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown and bar graphs are representative of/quantified from 3 experiments. *P < .05; **P < .01; ***P < .001 indicate statistical significance between WT and RasGRP1KO after anti-CD3 stimulation.
Critical role of RasGRP1 in TCR-induced mTORC1, mTOR2, and PI3K/Akt activation. Thymocytes from WT (Wt) and RasGRP1 KO (Grp1 KO) mice were left unstimulated or stimulated with anti-CD3 (500A2) for 5 minutes at 37°C. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown and bar graphs are representative of/quantified from 3 experiments. *P < .05; **P < .01; ***P < .001 indicate statistical significance between WT and RasGRP1KO after anti-CD3 stimulation.
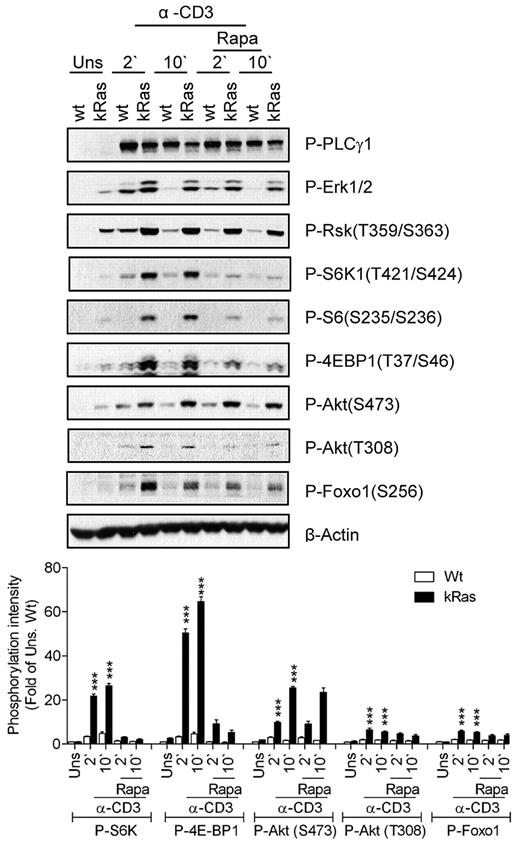
Effects of elevated Ras activity on mTOR activation
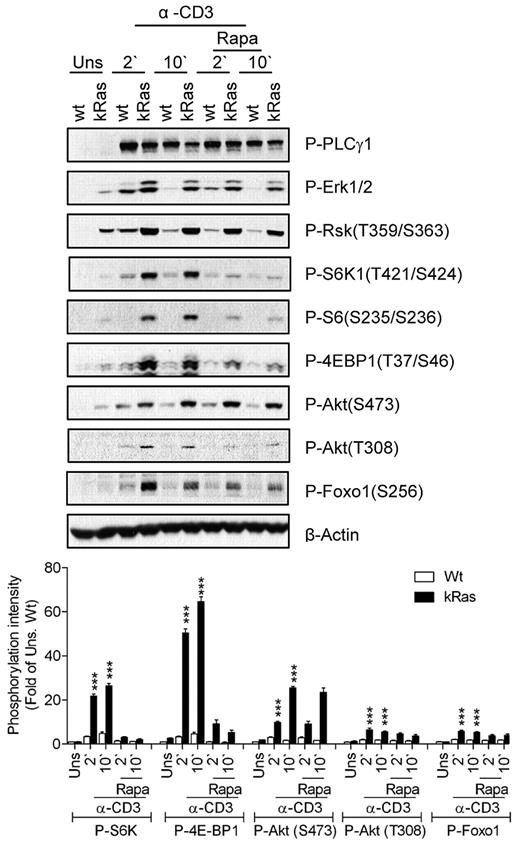
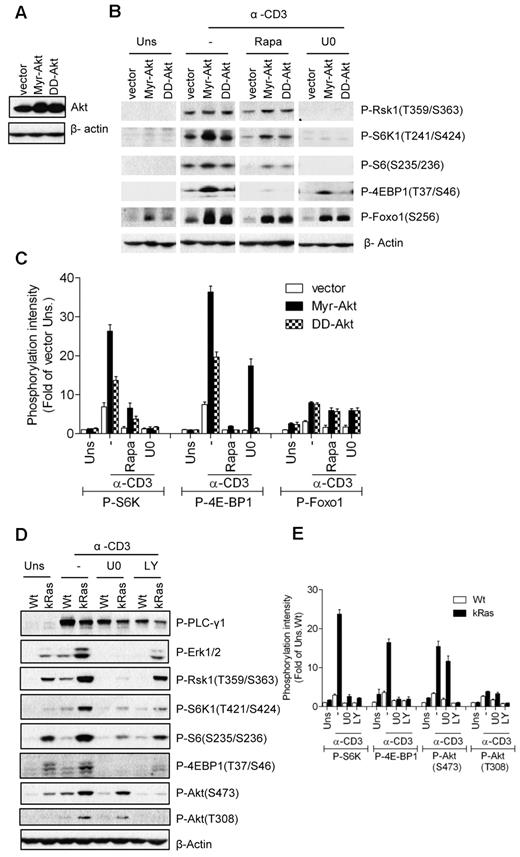
We further examined the role of Ras signaling in TCR-induced mTOR activation using T cells from mice carrying a constitutively active K-Ras (caKRas) allele. In these mice, caKRas can only be expressed after Cre-mediated deletion of a floxed transcription stop cassette in front of the gene. We bred caKRas mice with CD4-Cre transgenic mice so that caKRas was only expressed in T cells starting at the CD4+CD8+ DP stage of thymocyte development (thus named caKRas-T). The expression of caKRas does not obviously affect T-cell populations in the thymus based on CD4 and CD8 staining (data not shown). Elevated phosphorylation of Erk1/2 and Rsk1 was detected in caKRas-T thymocytes before and after TCR stimulation, confirming the constitutive activity of Ras (Figure 3). In contrast, PLC-γ1 phosphorylation was slightly decreased in caKRas-T thymocytes, possibly due to negative feedback inhibition caused by a constitutively activated Ras-Erk1/2 pathway. Constitutive phosphorylation of S6K1, S6, and 4E-BP1 was observed during resting conditions and was further enhanced after TCR stimulation in caKRas-T thymocytes compared with their WT counterparts. Treatment of rapamycin inhibited TCR-induced S6K1, S6, and 4E-BP1 phosphorylation in both WT and caKRas-T thymocytes, indicating that the enhanced phosphorylation of these molecules is dependent on mTORC1.
Constitutive Ras activation promotes mTORC1, mTORC2, and PI3K/Akt signaling. Thymocytes from WT and caKRas-T (KRas) mice were either not pretreated or pretreated with rapamycin (Rapa) for 30 minutes at 37°C and were left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 2 or 10 minutes, respectively. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown and bar graphs are representative of/quantified from 3 experiments. *P < .05; **P < .01; ***P < .001 indicate statistical significance between WT and KRas after anti-CD3 stimulation.
Constitutive Ras activation promotes mTORC1, mTORC2, and PI3K/Akt signaling. Thymocytes from WT and caKRas-T (KRas) mice were either not pretreated or pretreated with rapamycin (Rapa) for 30 minutes at 37°C and were left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 2 or 10 minutes, respectively. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown and bar graphs are representative of/quantified from 3 experiments. *P < .05; **P < .01; ***P < .001 indicate statistical significance between WT and KRas after anti-CD3 stimulation.
Akt phosphorylation at T308 and S473 was enhanced in caKRas-T thymocytes, suggesting that KRas also promotes PI3K and mTORC2 activity. Consistent with increased Akt phosphorylation, Foxo1 phosphorylation at S473 was significantly increased and prolonged. These observations indicate that enhanced Ras signaling in thymocytes leads to enhanced mTORC1, mTORC2, and PI3K/Akt activities.
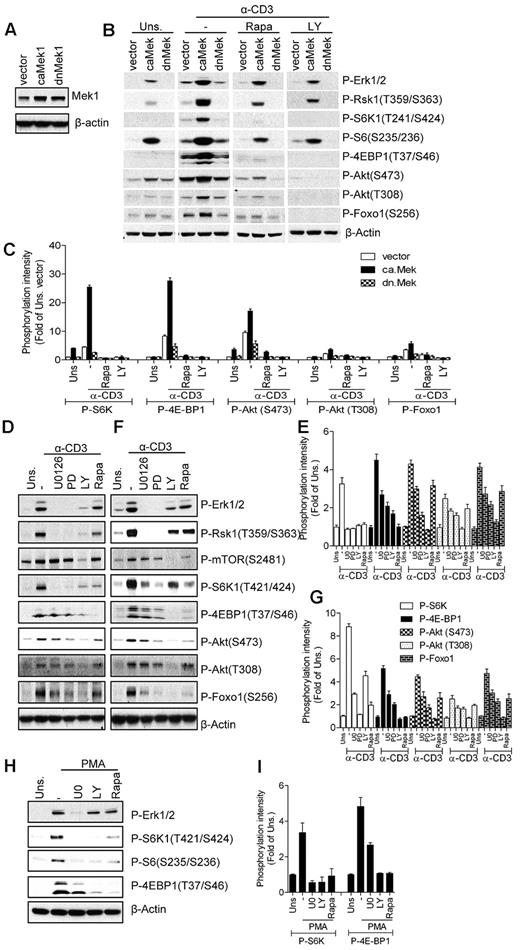
Mek1 activity modulates TCR-induced mTOR activation
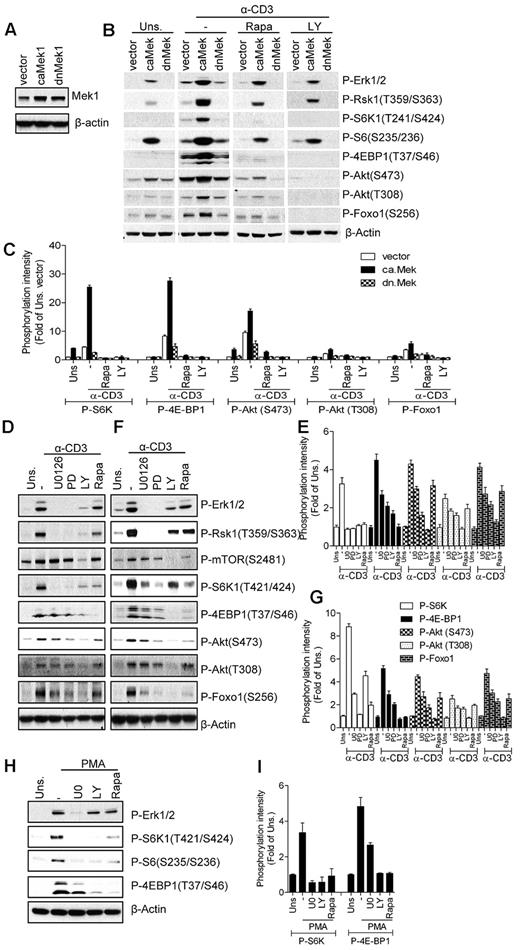
Ras signaling is processed through multiple effector molecules, such as Raf-1, PI3K, PKCϵ, Ras interaction/interference protein-1 (RIN-1), and Ral.43 The best-characterized Ras effector is Raf-1, which activates the Mek1/2-Erk1/2-Rsk pathway.44 Because both the PI3K/Akt and the Raf1-Mek1/2-Erk1/2 pathways are enhanced in caKRas-T thymocytes, we investigated the roles of these two pathways in TCR-induced mTOR activation. We overexpressed constitutively active or dominant-negative forms of Mek1 (caMek1 or dnMek1) in 2B4 T cells, a murine T-cell hybridoma, through retroviral-mediated infection. Stable cell lines were generated by sorting infected GFP+ cells using FACS, and their expression was confirmed by Western blot analysis (Figure 4A). We used the 2B4 T-cell line instead of primary T cells because primary T cells are difficult to transfect or infect without preactivation. As expected, Erk1/2 activation was elevated or slightly suppressed in caMek1- or dnMek1-expressing cells, respectively, after TCR stimulation (Figure 4B-C). In caMek1-expressing cells, S6K1, 4E-BP1, and S6 phosphorylation were enhanced after TCR stimulation. In addition, Akt phosphorylation at S473 and T308 and Foxo1 phosphorylation were slightly elevated in caMek1-expressing 2B4 cells before TCR stimulation, but were significantly enhanced after TCR stimulation. In contrast to caMek1, dnMek1 resulted in decreased S6K1 and 4E-BP1 phosphorylation, which was correlated with attenuated Erk1/2 activation and Rsk1 phosphorylation. Erk1/2 activation in unstimulated caMek1-2B4 cells was similar to TCR-stimulated vector control 2B4 cells. However, compared with TCR-stimulated vector control 2B4 cells, only very low levels of S6K1 phosphorylation could be detected in unstimulated caMek1-2B4 cells. Furthermore, inhibition of PI3K decreased mTORC1 signaling in caMek1-2B4 cells and in vector-2B4 cells. Therefore, although caMek1 enhances TCR-induced mTOR activation, caMek1 itself does not sufficiently activate mTOR signaling. TCR-induced S6 phosphorylation is decreased but not abolished by rapamycin, suggesting that both mTORC1-dependent and mTORC1-independent mechanisms (eg, Rsk1-mediated phosphorylation30 ) are involved in S6 phosphorylation at S235/236. Consistent with the effects of overexpressing caMek1 and dnMek1 on TCR-induced mTOR activation, treatment of thymocytes and peripheral T cells with Mek1/2 inhibitors U0126 (U0) or PD98059 (PD) inhibited TCR-induced mTORC1, mTORC2, and PI3K activation (Figure 4D-G). Furthermore, these Mek1/2 and PI3K inhibitors also decreased PMA-induced S6K1 and 4E-BP1 phosphorylation, suggesting that Mek1/2 and PI3K activities are important for DAG-mediated mTORC1 activation (Figure 4H-I). These observations suggest that Mek1/2 can function as an upstream activator of mTORC1 and mTORC2 and the PI3K/Akt pathway.
Mek activity modulates TCR-induced mTOR activation. (A) Establishment of caMek1- and dnMek1-expressing 2B4 T-cell lines. 2B4 T cells were infected with retrovirus expressing caMek1 and dnMek1, respectively. Mek1/2 expression in infected cells after sorting for positive GFP was determined by Western blot with an anti-Mek1/2 antibody. The blot was also probed with an anti–β-actin antibody for a loading control. (B-C) caMek1- or dnMek1-expressing 2B4 T cells were either not pretreated or pretreated with rapamycin (Rapa) or LY294002 (LY) for 30 minutes at 37°C, and then left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 2 experiments. (D-G) Effects of Mek1/2 and PI3K inhibition on TCR-induced mTOR activation. WT thymocytes (D-E) and splenic T cells (F-G) were not pretreated or pretreated with U0126 (U0) or LY294002 (LY) at 37°C for 30 minutes, and were then left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. (H-I). Effects of PI3K and Mek1/2 inhibitors on PMA-induced mTORC1 activation. WT thymocytes were pretreated with U0 or LY for 30 minutes, followed by stimulation with PMA for 5 minutes. Data shown are representative of/quantified from 3 experiments.
Mek activity modulates TCR-induced mTOR activation. (A) Establishment of caMek1- and dnMek1-expressing 2B4 T-cell lines. 2B4 T cells were infected with retrovirus expressing caMek1 and dnMek1, respectively. Mek1/2 expression in infected cells after sorting for positive GFP was determined by Western blot with an anti-Mek1/2 antibody. The blot was also probed with an anti–β-actin antibody for a loading control. (B-C) caMek1- or dnMek1-expressing 2B4 T cells were either not pretreated or pretreated with rapamycin (Rapa) or LY294002 (LY) for 30 minutes at 37°C, and then left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 2 experiments. (D-G) Effects of Mek1/2 and PI3K inhibition on TCR-induced mTOR activation. WT thymocytes (D-E) and splenic T cells (F-G) were not pretreated or pretreated with U0126 (U0) or LY294002 (LY) at 37°C for 30 minutes, and were then left unstimulated or stimulated with an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. (H-I). Effects of PI3K and Mek1/2 inhibitors on PMA-induced mTORC1 activation. WT thymocytes were pretreated with U0 or LY for 30 minutes, followed by stimulation with PMA for 5 minutes. Data shown are representative of/quantified from 3 experiments.
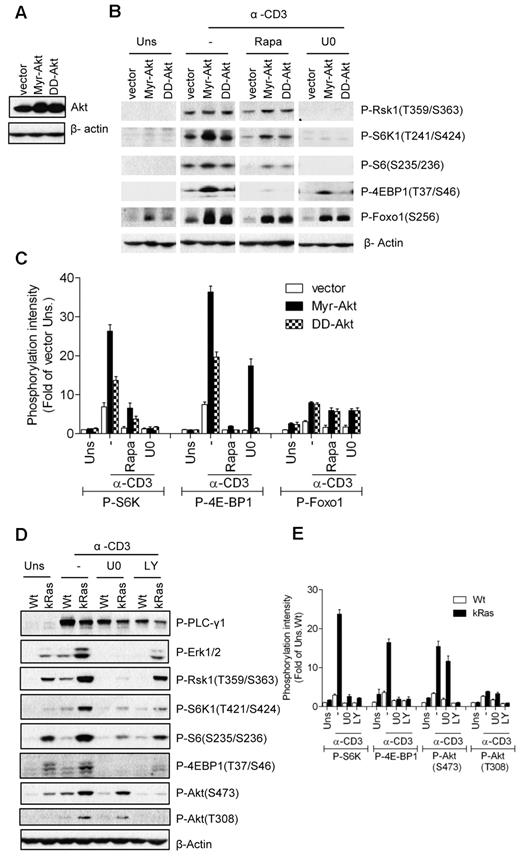
Effects of constitutively active Akt on TCR-induced mTOR activation
In non-T-cell line models, the PI3K/Akt pathway promotes mTORC1 activation through phosphorylation of TSC2.12 To investigate the role of Akt in TCR-induced mTOR activation, we generated a 2B4 T-cell line expressing constitutively active forms of Akt, myristoylated Akt (Myr-Akt), and S473D/T308D Akt (DD-Akt; Figure 5A). As expected, elevated Foxo1 phosphorylation was detected in 2B4 cells expressing either Myr-Akt or DD-Akt without TCR stimulation, with Myr-Akt showing stronger activity than DD-Akt (Figure 5B-C). In addition, Foxo1 phosphorylation in Myr-Akt- and DD-Akt–expressing cells was further enhanced after TCR stimulation. S6K1 and 4E-BP1 phosphorylation was significantly elevated in Myr-Akt–expressing cells, but was only weakly increased in DD-Akt cells after TCR stimulation. Whereas Myr-Akt and DD-Akt were able to phosphorylate Foxo1 in unstimulated cells, they were not able to induce obvious S6K1 and 4E-BP1 phosphorylation. Furthermore, TCR-induced S6K1 and 4E-BP1 phosphorylation in Myr-Akt- and DD-Akt–expressing 2B4 cells were significantly decreased when the cells were treated with a Mek1/2 inhibitor (U0). These data, together with the observations from caMek1-expressing cells, suggest that Akt activity promotes TCR-induced mTOR activity and that both Mek1/2 and PI3K/Akt activities are required for efficient mTORC1 and mTORC2 activation after TCR engagement.
Requirement of Mek1/2 activity for caAkt- or caKRas-mediated enhancement of TCR-induced mTOR signaling. (A) Establishment of Myr-Akt- and DD-Akt–expressing 2B4 cells was similar to the method described in Figure 4A except that retroviruses expressing Myr-Akt or DD-Akt were used. Akt expression in infected cells after sorting for GFP+ cells was determined by Western blot with an anti-Akt antibody. The blot was also probed with an anti–β-actin antibody for a loading control. (B-C) Akt-expressing 2B4 cells were not pretreated or pretreated with the indicated inhibitors, followed by anti-CD3 stimulation and immunoblot analysis as in Figure 4B. (D-E) Wt and caKRas-T (KRas) thymocytes were not pretreated or pretreated with the indicated inhibitors, followed by anti-CD3 stimulation and immunoblot analysis as in Figure 4B. Data shown are representative of/quantified from 3 experiments.
Requirement of Mek1/2 activity for caAkt- or caKRas-mediated enhancement of TCR-induced mTOR signaling. (A) Establishment of Myr-Akt- and DD-Akt–expressing 2B4 cells was similar to the method described in Figure 4A except that retroviruses expressing Myr-Akt or DD-Akt were used. Akt expression in infected cells after sorting for GFP+ cells was determined by Western blot with an anti-Akt antibody. The blot was also probed with an anti–β-actin antibody for a loading control. (B-C) Akt-expressing 2B4 cells were not pretreated or pretreated with the indicated inhibitors, followed by anti-CD3 stimulation and immunoblot analysis as in Figure 4B. (D-E) Wt and caKRas-T (KRas) thymocytes were not pretreated or pretreated with the indicated inhibitors, followed by anti-CD3 stimulation and immunoblot analysis as in Figure 4B. Data shown are representative of/quantified from 3 experiments.
Requirement of Mek1/2 and PI3K activities for Ras-mediated mTOR activation
Because elevated Ras activity promotes TCR-induced activation of the PI3K/Akt pathway, the Mek1/2-Erk1/2 pathway, and mTOR activation in thymocytes, we investigated whether Mek1/2 and PI3K activities are required for the enhanced mTOR activation in caKRas-T thymocytes. We treated the caKRas-T thymocytes with inhibitors of PI3K (LY) and Mek1/2 (U0) during TCR stimulation. As shown in Figure 5D and E, treatment with either LY or U0 inhibitors strongly suppressed the TCR-induced phosphorylation of S6K1, S6, and 4E-BP1, with a concomitant decrease of Akt (T308) or Erk1/2 phosphorylation, indicating that both PI3K and Mek1/2 activities contribute to the enhancement of mTORC1 activation in caKRas-expressing thymocytes. Phosphorylation of Akt at S473 was suppressed with LY treatment, but only weakly suppressed U0 treatment. These observations further support that both the Mek1/2- and PI3K-Akt–mediated signaling pathways are required for efficient mTOR activation after TCR engagement.
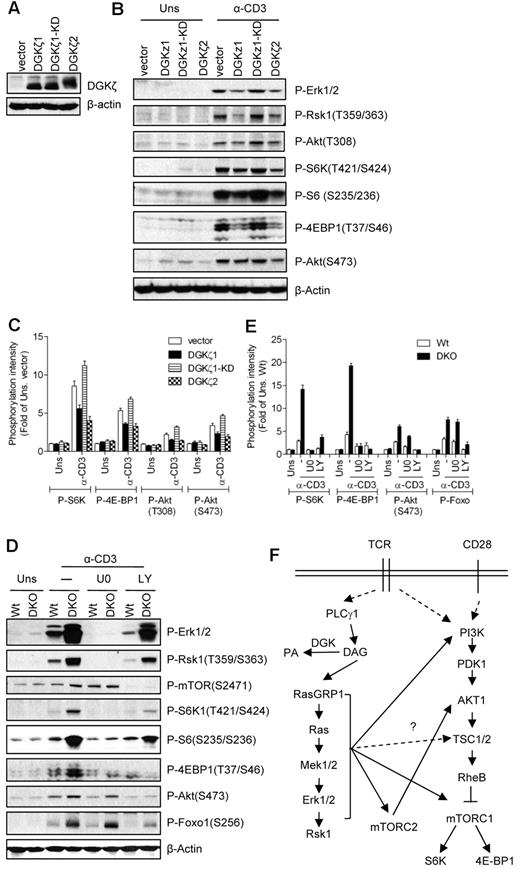
Overexpression of DGKζ inhibits TCR-induced mTOR activation
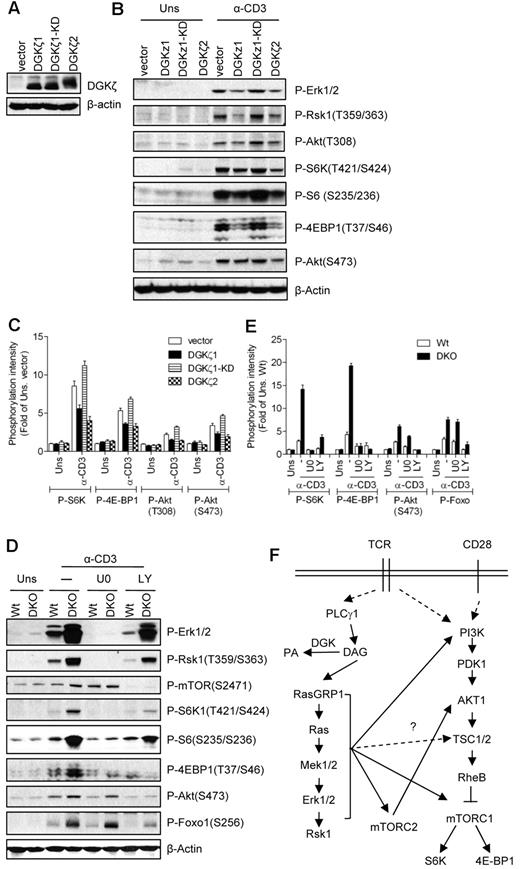
DGKs inhibit the Ras-Mek1/2-Erk1/2 pathway by converting subcellular DAG into PA. Our current data show that DAG-mediated activation of the RasGRP1-Ras-Mek1/2 pathway plays a critical role in TCR-induced mTOR activation. We hypothesize that DGK activity may negatively regulate the TCR-induced mTOR activation in T cells by terminating DAG signaling. However, both PLD- and DGK-derived PA have also been reported to promote mTOR signaling in non-T-cell line models.39,41 To investigate the role of DGK activity in TCR-induced mTOR activation, we generated 2B4 T-cell lines that overexpressed WT DGKζ1, kinase-dead DGKζ1 (DGKζ1-KD), or WT DGKζ2 (Figure 6A). DGKζ1 and DGKζ2 are alternatively spliced isoforms of DGKζ that differ only at their N-termini. Thymocytes express both isoforms, whereas peripheral T cells predominantly express DGKζ1. As shown in Figure 6B and C, TCR-induced Erk1/2 and Rsk1 phosphorylation was decreased in 2B4 cells expressing WT DGKζ1 or DGKζ2, but not in DGKζ1-KD–expressing cells. These data are consistent with our previous studies and also indicate that both DGKζ isoforms can inhibit the Ras-Erk1/2 pathway.33 S6K1, S6, and 4E-BP1 phosphorylation are also decreased in WT DGK ζ1- or DGKζ2-expressed cells, but not in DGKζ1-KD–expressed cells, compared with vector controls. Phosphorylation of Akt at T308 and S473 was slightly decreased in DGKζ1- or DGKζ2-overexpressed cells but not in DGKζ1-KD–expressed cells. These observations suggest that enhanced DGK kinase activity inhibits not only the Ras-Mek1/2 pathway, but also mTORC1, mTORC2, and PI3K/Akt signals.
Inhibition of TCR-induced mTOR activation by DGKα and DGKζ. (A) Establishment of DGKζ1-, KD-DGKζ1-, or DGKζ2-expressing 2B4 T-cell lines by retroviral infection as described in Figure 4A. (B-C) Enhanced DGK activity inhibits TCR-induced mTOR activation. 2B4 cells overexpressing DGKζ1, KD-DGKζ1, or DGKζ2 were left unstimulated or stimulated with anti-CD3 (500A2) 5 minutes at 37°C. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 4 experiments. (D-E) Enhanced TCR-induced mTOR activation in DGKα and DGKζ double-deficient thymocytes. WT and DGKαζDKO (DKO) thymocytes were not pretreated or pretreated with U0126 (U0) or LY294002 (LY) at 37°C for 30 minutes, and were then left unstimulated or stimulated an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 5 experiments. (F) Schematic illustration of mTOR activation in T cells (please see “Discussion” for details).
Inhibition of TCR-induced mTOR activation by DGKα and DGKζ. (A) Establishment of DGKζ1-, KD-DGKζ1-, or DGKζ2-expressing 2B4 T-cell lines by retroviral infection as described in Figure 4A. (B-C) Enhanced DGK activity inhibits TCR-induced mTOR activation. 2B4 cells overexpressing DGKζ1, KD-DGKζ1, or DGKζ2 were left unstimulated or stimulated with anti-CD3 (500A2) 5 minutes at 37°C. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 4 experiments. (D-E) Enhanced TCR-induced mTOR activation in DGKα and DGKζ double-deficient thymocytes. WT and DGKαζDKO (DKO) thymocytes were not pretreated or pretreated with U0126 (U0) or LY294002 (LY) at 37°C for 30 minutes, and were then left unstimulated or stimulated an anti-CD3 antibody (500A2) at 37°C for 5 minutes. Cell lysates were separated by SDS-PAGE followed by immunoblotting with the indicated antibodies. The blots were stripped and reprobed with an anti–β-actin antibody for a loading control. Data shown are representative of/quantified from 5 experiments. (F) Schematic illustration of mTOR activation in T cells (please see “Discussion” for details).
Synergistic inhibition of TCR-induced mTOR activation in thymocytes by DGKα and DGKζ
To further determine the role of DGK activity in mTOR regulation in primary cells, we analyzed thymocytes from mice deficient in either DGKα or DGKζ. TCR-induced S6K1 and 4E-BP1 phosphorylation was not obviously changed in DGKα- or DGKζ-deficient thymocytes and splenic T cells (data not shown). To investigate further whether DGKα and DGKζ play a redundant role in the regulation of mTOR, we compared TCR-induced mTOR activation in thymocytes from WT and DGKα and DGKζ double-knockout (DKO) mice. As shown in Figure 6B, DGKαζDKO thymocytes manifested elevated S6K1 and 4E-BP1 phosphorylation after TCR engagement, which was correlated with enhanced Erk1/2 and Rsk1 activation. DGKαζDKO cells displayed increased Akt phosphorylation at S473, indicating that mTORC2 activity was enhanced. Treatment of WT and DGKαζDKO thymocytes with a Mek1/2 inhibitor drastically decreased phosphorylation of S6K1 and 4E-BP1 and moderately decreased the phosphorylation of Akt (S473), suggesting that DGKs regulate mTOC1 signaling via Mek1/2. Treatment with LY suppressed phosphorylation of both mTORC1 and mTORC2 target proteins such as S6K1, 4E-BP1, and Akt, respectively. These data complement the aforementioned roles of RasGRP1 and Ras in TCR-induced mTOR activation, and indicate that DGKα and DGKζ synergistically inhibit TCR-induced mTORC1 and mTOC2 activation in thymocytes by controlling the DAG-RasGRP1-Ras-Mek1/2-Erk1/2 pathway.
Discussion
Increasing evidence is revealing the critical roles of mTOR in T cells45 ; however, how mTOR is activated in T cells has been poorly understood. In this study, we demonstrated that the DAG-RasGRP1-Ras-Mek1/2-Erk1/2 pathway plays a crucial role in mTOR activation in T cells in response to TCR stimulation. We showed that deficiency of RasGRP1 or constitutively active Ras in mice causes defective or enhanced mTORC1 and mTORC2 activation in thymocytes after TCR stimulation, respectively. We demonstrated further that Mek1/2 regulates TCR-induced activation of mTORC1 and mTORC2. Furthermore, we found that DGKα and DGKζ act as physiologic inhibitors for TCR-induced mTOR activation by down-regulating DAG-mediated signaling.
Previous studies have been focused on the role of PI3K/Akt in the activation of mTOR in T cells and many other cell types.29 Our data support that PI3K/Akt activity is required for TCR-induced mTORC1 and mTORC2 activation, because the inhibition of PI3K decreased TCR-induced activation of mTORC1 and mTORC2 T cells. In the HEK cell line, Akt phosphorylates TSC2 at S939 and S981 residues, which induces the formation of a binding pocket for a cytosolic anchoring protein, 14-3-3, to disrupt the TSC1/TSC2 complex and to promote RheB-mTORC1 activation.13 We also revealed that RasGRP1 is critical for TCR-induced activation of the PI3K/Akt pathway and that enhanced Ras activity promotes PI3K/Akt activation in thymocytes after TCR stimulation. Ras can directly associate with the p85-regulatory subunit of PI3K to promote PI3K activation.46 In addition, our data also suggest that RasGRP1 and Ras may promote PI3K/Akt signaling through Mek1/2, because Akt phosphorylation at T308 is increased in caMek1-expressing T cells. It remains to be determined how Mek1/2 may promote Akt activation and whether Akt promotes mTOR activation in T cells through TSC2 phosphorylation.
In addition to the PI3K-PDK1-Akt pathway, our data indicate that the RasGRP1-Ras-Mek1/2-Erk1/2 pathway is also critical for TCR-induced mTORC1 and mTORC2 activation. This conclusion is supported by multiple pieces of evidence. First, RasGRP1 deficiency caused defective Erk1/2 activation and mTOR activation in thymocytes, suggesting a correlation between these two signaling events. Second, caKRas enhanced TCR-induced mTOR activation and such an effect was dependent on Mek1/2 activity. Third, constitutive activity of Mek1 promoted TCR-induced mTORC1 and mTORC2 signaling and inhibition of Mek1/2 severely impaired TCR-induced mTORC1 and mTORC2 activation. Fourth, deficiency of both DGKα and DGKζ increased and prolonged DAG signaling, leading to enhanced Ras-Erk1/2 activation. In DGKαζDKO thymocytes, TCR-induced mTORC1 and mTORC2 activation was also enhanced, and such enhancement was dependent on Mek1/2 activity. Our findings are also consistent with the observation that Erk1/2 and Rsk1 can promote TCR-induced S6 phosphorylation in CD8 T cells.30 It has been reported previously that Erk1/2 can phosphorylate TSC2 at S540 and S664 to promote disruption of the TSC complex in HEK293 cells during serum or PMA stimulation conditions.14 In addition, Rsk1, one of the downstream effectors of Erk1/2, has been shown to phosphorylate TSC2 at S1791 and to inhibit TSC2 function by an unknown mechanism in HEK293 cells under growth factor stimulation.47 Rsk1 has also been shown to activate mTORC1 signaling by phosphorylating raptor at multiple sites.48 Based on these findings and on our current data, we propose that efficient activation of mTOR in T cells may require both PI3K/Akt-dependent and PI3K/Akt-independent mechanisms, and that the RasGRP1-Ras-Mek1/2-Erk1/2 pathway may activate mTOR activity by at least two mechanisms: by promoting PI3K/Akt activation and by directly modulating other mTOR regulators (Figure 6F). Further studies will determine whether the aforementioned mechanisms are involved in TCR-induced mTOR activation, and how altered RasGRP1, Ras, and Erk1/2 may affect these mechanisms in T cells.
The RasGRP1-Ras-Mek1/2-Erk1/2 pathway plays an essential role in T-cell development, especially during positive selection. Null mice of either RasGRP1 or Erk1/2 displayed T-cell developmental blockage at the CD4+CD8+ DP stage.5,49 The downstream mechanisms that result in the developmental defects in these mice are unclear. Our data showing that the requirement of this pathway for activation of both mTOR and PI3K/Akt suggest the possibility that diminished mTOR and PI3K/Akt activities may contribute to the developmental blockage in RasGRP1- or Erk1/2-deficient mice. In peripheral lymphoid organs, the RasGRP1-Ras-Mek1/2-Erk1/2 pathway is critical in the activation of T cells, and the impairment of this pathway can lead to T-cell anergy.50 Interestingly, anergic T cells showed decreased mTORC1 activity and rapamycin treatment promoted T-cell anergy in vitro and in vivo.23,24 Whereas the RasGRP1-Mek1/2-Erk1/2 pathway promotes T-cell activation and prevents T-cell anergy through AP1-mediated transcription activation, our data suggest the possibility that diminished mTOR signaling due to decreased Ras/Erk1/2 activity may also contribute to T-cell anergy.
DGKs regulate the subcellular concentration of both DAG and PA. Both PLD- and DGK-derived PA have been reported to promote mTOR signaling.39,41 Binding of PA to mTOR in vitro has been demonstrated to activate mTOR signaling.39 It has been reported that overexpression of DGKζ, but not DGKα, increased the serum-induced mTORC1 activity in HEK293 cells, whereas knockdown of DGKζ inhibited mTORC1 signaling. However, in DGKζ-overexpressing T cells, we have found that TCR-induced mTORC1 and mTORC2 activation is inhibited in a DGK kinase activity–dependent manner. Complementarily, deficiency of DGKα and DGKζ enhanced TCR-induced mTORC1 and mTORC2 signaling in thymocytes in a Mek1/2-dependent manner. The differences between these studies suggest that the mechanisms involved in mTOR activation are context-dependent, which may vary in different cell types and/or in response to stimulation of different receptors. Whereas we cannot firmly rule out that DGK-derived PA may participate in the regulation of TCR-induced mTOR signaling, our data indicate a predominantly inhibitory role of DGK activity on mTOR signaling in T cells.
In summary, the results of our study demonstrate that RasGRP1 and Ras are critical for TCR-induced mTORC1, mTORC2, and PI3K/Akt activation. In addition, DGK negatively regulates these signaling events by terminating DAG-mediated signaling. These data reveal that Mek1/2-Erk1/2 play critical roles in TCR-induced mTORC1 and mTORC2 activation and may function as upstream regulators to promote PI3K/Akt signaling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Harvey Lodish and Jeff Rathmell for providing caMek1- and caAkt-expressing vectors, Dr James Stone for providing RasGRP1-deficient mice, laboratory staff for generating reagents, and Wei Yang for editing the manuscript.
This study was supported by the National Institutes of Health (grants R01AI076357, R01AI079088, and R21AI079873), by the American Cancer Society, by the American Heart Association, and by the Food Allergy and Anaphylaxis Network (to X.-P.Z.).
National Institutes of Health
Authorship
Contribution: B.K.G. designed and performed research, analyzed data, and wrote the paper; C.-K.W. performed research; and X.-P.Z. supervised the project, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Ping Zhong, MD, PhD, Department of Pediatrics-Allergy and Immunology, Rm 133 MSRB, Research Dr, Box 2644, Duke University Medical Center, Durham, NC 27710; e-mail: zhong001@mc.duke.edu.