Abstract
We recently described paratarg-7 (P-7), a protein of unknown function, as the target of 15% of immunoglobulin A (IgA) and IgG paraproteins in monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. To determine the frequency of P-7 as a paraprotein target in IgM-MGUS and Waldenström macroglobulinemia (WM), sera from patients with IgM-MGUS/WM were tested for reactivity with recombinant P-7 by enzyme-linked immunoabsorbent assay. The specificity of the paraprotein-mediated reaction was shown by absorption studies and cloning of the respective B-cell receptor. The paraproteins of 18 (9 WM and 9 IgM-MGUS) of 161 patients (11%) reacted with P-7. Isoelectric focusing and phosphatase treatment showed that P-7 was hyperphosphorylated (pP-7) in all patients with an anti–P-7-specific IgM paraprotein tested. Because only 4 of 200 healthy controls (2%) were carriers of pP-7, pP-7 carrier state is associated with a significantly increased risk (odds ratio = 6.2; P = .001) for developing IgM-MGUS/MW. Family analyses showed that the pP-7 carrier state is inherited as a dominant trait. After IgA/IgG-MGUS and multiple myeloma, IgM-MGUS/WM is the second neoplasia associated with pP-7 carrier state. The dominant inheritance of pP-7 explains cases of familial IgM-MGUS/WM and enables the identification of family members at increased risk.
Introduction
Waldenström macroglobulinemia (WM) is classified as an immunoglobulin M (IgM)–secreting B-cell non-Hodgkin lymphoma characterized primarily by lymphoplasmacytic infiltrates in the bone marrow accompanied by hypersecretion of monoclonal IgM. It corresponds to the lymphoplasmacytic lymphoma (LPL) as defined by the World Health Organization classification.1 Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic precursor condition commonly preceding multiple myeloma (MM), and IgM-MGUS may precede development of WM.2 Although age, race, sex, and preexisting IgM-MGUS are recognized risk factors, the cause of WM is largely unknown. Several studies have shown a significantly increased risk of WM after infections with hepatitis B virus, immunodefinciency virus, and rickettsiosis and found an increased risk of WM among persons with a personal history of autoimmune disease.3
Genetic factors play an important role, with 20% of patients having a familial predisposition. Several studies of multiple affected families have been published, showing familial clustering of LPL and WM.4-6 A recent study showed that family members of patients with MGUS and LPL-WM have a significant higher risk of MGUS/LPL-WM.7 Environmental influences, chance occurrence, and inherited factors may all contribute to familial clusters. The evidence of somatic immunoglobulin gene mutations in WM indicates a role for antigenic stimulation in the development of WM.8-10 A causal relationship between MGUS/WM and chronic antigenic stimulation has been suggested by the results of several studies.8-12 Because of the rarity of LPL-WM, only a few studies have assessed the role of various types of chronic antigenic stimulatory conditions in relation to risk of developing MGUS/WM, and their results have been conflicting3,13-15 ; hence, the identification of the antigenic stimuli of B-cell neoplasms might be of considerable importance.
In a systematic study covering a broad spectrum of potential antigens with the use of a modified approach of serologic identification of antigens by expression cloning, which allows for the systematic screening of putative antibody-antigen interactions, even if neither the antigen nor the antibody is known.16 In a complementary approach that used a human fetal brain-derived macroarray and IgA or IgG paraprotein-containing sera, the paraproteins of 29 of 192 consecutive patients with MGUS and MM (15.1%) reacted with paratarg-7 (P-7).17 P-7 is identical to STOML2 (stomatin [EPB72]–like), also known as HSPC108 or stomatin-like-protein and SLP-2,18 a protein of unknown function, which is expressed in all human tissues. In an extension of our earlier study, the high frequency of P-7–specific paraproteins in the sera of patients with MGUS/MM (35 of 252; 14%) was confirmed in a subsequent study.19 Moreover, it was shown that all patients with P-7–specific paraproteins were carriers of a hyperphosphorylated version of the protein (pP-7) and that the carrier state of pP-7 is inherited in a dominant fashion.19 Because only 2% of healthy Germans are carriers of pP-7, pP-7 carrier state is associated with an increased risk (odds ratio, 7.9) to develop IgA/IgG-MGUS/MM. Thus, pP-7 is the first molecularly defined inherited risk factor known for any hematologic neoplasm to date. Because of the autosomal-dominant inheritance of pP-7, we set out to determine the prevalence of pP-7 carrier state and the frequency of pP-7–specific paraproteins in other malignancies.
Methods
Patients and controls
This study was approved by the local ethical review board (“Ethikkommission der Ärztekammer des Saarlandes”) and conducted according to the Declaration of Helsinki. Between January 2005 and February 2009 serum samples from 67 consecutive patients treated at Saarland University Medical School; 44 patients from the Bing Center for Waldenstrom Macroglobulinemia, Dana-Farber Cancer Institute, Harvard Medical School; and 50 patients from the University of Athens School of Medicine, Department of Clinical Therapeutics were included in this study in which serum protein electrophoresis had identified a monoclonal spike that was shown to contain a monoclonal IgM paraprotein by immunofixation. The control group consisted of 200 healthy employees of Saarland University Medical School. Healthy was defined as having no monoclonal immunoglobulin by serum electrophoresis and immunofixation and being healthy as diagnosed by the Medical Officer of Saarland University at the checkup before donation. Whenever possible, human materials were obtained during routine diagnostic or therapeutic procedures and stored at −80°C until use. Written informed consent was obtained from patients, their relatives, and controls for studying P-7 in lysates of their whole peripheral blood.
Family analyses
All relatives, identified by pedigree analysis, were contacted through the patients. Human materials were obtained from 25 relatives of 4 families from patients with IgM-MGUS/WM (2 families with MGUS and 2 families with WM).
Isoelectric focusing
Blood samples were centrifuged and washed with phosphate-buffered saline (PBS) followed by lysis in lysis buffer containing 8M urea, 0.1M NaH2PO4, 0.01M Tris [tris(hydroxymethyl)aminomethane]–HCL, and 0.1% NP40 (15 minutes, 20°C) and stored at −20°C until use. Equal volumes of sample and loading buffer were mixed. Samples were analyzed by isoelectric focusing (IEF) on a gel with a fixed pH gradient (pH 3-10) according to the manufacturer's instructions (Novex pH 3-10; Invitrogen), followed by an immunoblot screening.
Immunoblot screening
After lysates from whole peripheral blood were separated by IEF or sodium dodecyl sulfate–polyacrylamide gel electrophoresis, the proteins were transferred to a Immobilon-P polyvinylidene difluoride membrane (Millipore Immobilon) by semidry blotting. The membrane was blocked overnight at 4°C in TBST/milk buffer [10% (vol/vol) milk in 10mM Tris/HCl, pH 7.5, 150mM NaCl, and 0.1% (vol/vol) Tween 20], washed, and incubated for 1 hour at room temperature with serum in TBST (paraprotein-containing serum from patients at a dilution of 1:108 and from controls at a dilution of 1:103). After 3 washings in TBST, the membranes were incubated for 1 hour at room temperature with mouse anti–human IgG-POX antibody (Bio-Rad) diluted 1:3000 in TBST, subsequently washed in TBST, and followed by detection with the use of Pharmacia enhanced chemiluminescence system (General Electric).
Phosphatase treatment of P-7
Blood samples were centrifuged and washed with PBS followed by lysis in LS buffer (10mM Tris-HCL pH 8; 30 minutes, 4°C). After increasing the concentration of Tris-HCl to 100mM, alkaline phosphatase was added (1 U/μL per 500 μL lysate) and incubated at 37°C overnight. The phosphatase was inactivated by heating at 80°C for 10 minutes. Equal volumes of sample and loading buffer were mixed, followed by IEF and immunodetection as described above.
P-7 enzyme-linked immunoabsorbent assay
The P-7 enzyme-linked immunoabsorbent assay (ELISA) that used full-length recombinant P-7 was performed as described by us previously.20 For testing the P-7 specificity of the recombinant B-cell receptor (BCR), the P-7 ELISA was performed according to standard protocols with the use of the expressed and purified recombinant Fab antibodies.
Absorption studies
Recombinant His6-tagged P-7 protein or total blood lysate was immobilized on HiTRAP-chelating columns (Pharmacia) containing 1 mL of nickel-nitrilotriaceticacid-agarose (QIAGEN), following a published procedure (www.flemingtonlab.com). Patients' serum (100 μL) was diluted 1:2 (vol/vol) in PBS and depleted by passing 3 times over the P-7 column. The flow-through was checked by immunofixation and serum protein electrophoresis.
Cloning and expression of BCRs
DNA was isolated from formalin-fixed paraffin-embedded (FFPE) lymph node or bone marrow biopsies according to the manufacturer's instructions (QIAamp DNA FFPE Tissue Kit; QIAGEN). DNA was subjected to a seminested PCR for VH and VK/Vλ genes. The protocol and primers used for the amplifications were described previously.21 Products were analyzed by electrophoresis on agarose gels and sequenced. Sequencing results were analyzed online with the use of IMGT/V-QUEST22 on IMGT, the international ImMunoGeneTics information system (http://www.imgt.org).23
Results
Carrier state of pP-7 in hematologic neoplasias and solid tumors
Because pP-7 is inherited as a dominant trait and is expressed in all cells of pP-7 carriers, we looked for the prevalence of the pP-7 carrier state in various hematologic neoplasias and solid tumors (Table 1). No signal was observed in any of the tumor types investigated for an increased prevalence of pP-7 carriers except for IgM-MGUS and WM.
P-7 as the target of IgM paraproteins
Serum samples from 161 consecutive patients with MGUS and WM in Germany (n = 67), the United States (n = 44), and Greece (n = 50) with a monoclonal spike in the serum electrophoresis which was confirmed by immunofixation to be a monoclonal IgM paraprotein were included in this study. There was no difference with respect to age, sex (all patients), progression, M-protein level, and stage of the disease between patients with a P-7–specific paraprotein and patients having a non–P-7-specific paraprotein. The sera were tested at a dilution of 1:108 for anti–P-7 reactivity with the use of a P-7 ELISA. The paraproteins of 9 of 51 patients with IgM-MGUS (17.6%) and of 9 of 110 patients with WM (8.2%) reacted specifically with P-7, resulting in an overall rate of 18 of 161 or 11.2% (Table 2), proving P-7 as a paraprotein target in a significant proportion of patients with IgM-MGUS/WM. The anti–P-7 reactivity of these sera had titers ranging from 1:108 to 1:1010. None of the sera from healthy controls reacted at a dilution of ≤ 1:102. Lower serum dilutions were not tested because they cause too much background in the P-7 ELISA.
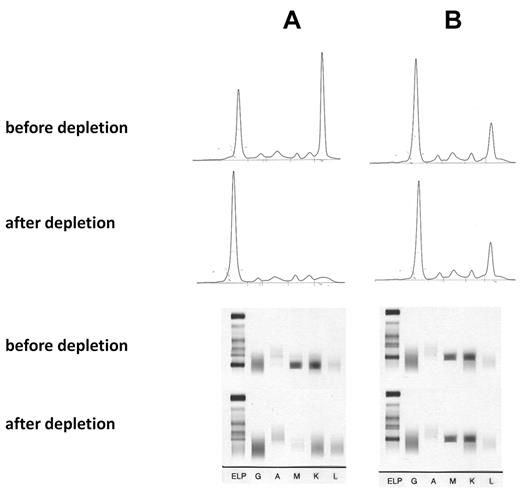
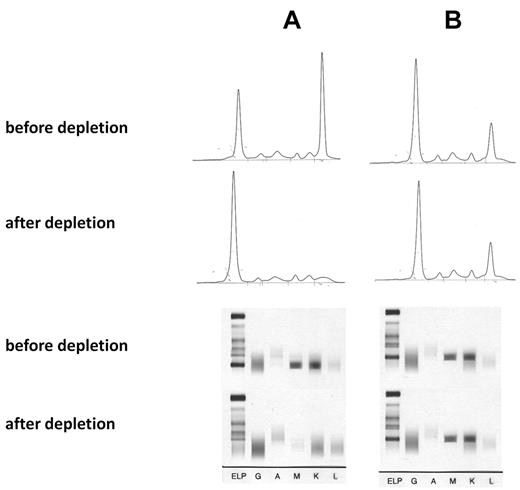
The specificity of the paraprotein-mediated reaction was shown by absorption studies with recombinant P-7 and by cloning the BCR. For the absorption study recombinant His6-tagged P-7 protein was immobilized on a Ni2+ agarose. After 3 passages of the anti–P-7-positive IgM paraprotein-containing sera over the P-7 column, the monoclonal spike in the electrophoresis of the flow-through and the clonal band in the immunofixation had disappeared, whereas this was not the case with a serum containing an IgM paraprotein with a non–P-7 reactivity (Figure 1). There was no relevant overall difference in the serum levels of the monoclonal IgM paraproteins when the 9 patients with pP-7 MGUS and the 9 patients with pP-7 WM were compared with the corresponding pP-7–negative patients. For cloning and expression of the BCR DNA was isolated from bone marrow cells and paraffin-embedded lymph nodes, respectively, from 3 patients with an anti–P-7-specific paraprotein and 5 BCRs with non–P-7 specificity as controls. The immunoglobulin genes (VH, VK, Vλ) were characterized by PCR and cloned into a phagemid vector to produce Fab fragments. The Fab fragments were characterized by protein gel electrophoresis and Western blot, and their specificity was shown by an ELISA with recombinant P-7 as a coat (Figure 2).
Absorption of IgM paraproteins with recombinant P-7. (A) IgM paraprotein with P-7 reactivity and absorption with a P-7 column. (B) IgM paraprotein with non–P-7 reactivity and absorption by a P-7 column (control). (Top) Serum electrophoresis before and after absorption, (bottom) immunofixation before and after absorption with P-7. The absorption studies of 3 sera containing a P-7 reactive IgM paraprotein and 2 IgM control paraproteins with P-7 nonreactivity yielded identical results.
Absorption of IgM paraproteins with recombinant P-7. (A) IgM paraprotein with P-7 reactivity and absorption with a P-7 column. (B) IgM paraprotein with non–P-7 reactivity and absorption by a P-7 column (control). (Top) Serum electrophoresis before and after absorption, (bottom) immunofixation before and after absorption with P-7. The absorption studies of 3 sera containing a P-7 reactive IgM paraprotein and 2 IgM control paraproteins with P-7 nonreactivity yielded identical results.
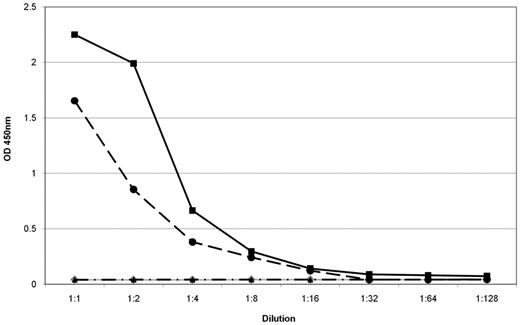
Reactivity and specificity of expressed P-7–specific BCRs. For this ELISA recombinant P-7 was used as a coat. The BCR of a patient with a P-7–specific IgM paraprotein that was cloned from the patient's lymph node involved by WM (black circles) had the same anti–P-7 specificity as the patient's serum containing the IgM paraprotein (black squares). There was no reactivity in the serum of a patient with an IgM-MGUS with a non–P-7-specific paraprotein (gray rhombus). The BCR of a patient with chronic lymphocytic leukemia with specificity for the FAM32A protein showed no reactivity (control; black triangle). The starting dilution of the sera was 108 and the starting concentration of the expressed BCRs was 10 μg/mL.
Reactivity and specificity of expressed P-7–specific BCRs. For this ELISA recombinant P-7 was used as a coat. The BCR of a patient with a P-7–specific IgM paraprotein that was cloned from the patient's lymph node involved by WM (black circles) had the same anti–P-7 specificity as the patient's serum containing the IgM paraprotein (black squares). There was no reactivity in the serum of a patient with an IgM-MGUS with a non–P-7-specific paraprotein (gray rhombus). The BCR of a patient with chronic lymphocytic leukemia with specificity for the FAM32A protein showed no reactivity (control; black triangle). The starting dilution of the sera was 108 and the starting concentration of the expressed BCRs was 10 μg/mL.
Analysis of P-7
Sequence analyses of P-7 from all patients analyzed (n = 6) were identical, excluding mutations or single nucleotide polymorphisms as the reason for the autoimmunogenicity of P-7 in the respective patients. However, 2-dimensional gel electrophoresis, IEF, and phosphatase treatment showed that P-7 was hyperphosphorylated in all patients with an anti–P-7-specific IgM paraprotein. To date all patients with a P-7–specific paraprotein (> 50 IgA/IgG paraproteins and 14 IgM paraproteins), from whom peripheral blood cells were available, were shown to be carriers of pP-7. Conversely, no pP-7 carrier was found among > 500 patients with a paraprotein of specificity other than pP-7. Of the patients included in this study, peripheral blood cells were not available from the 4 patients from Boston with P-7–specific paraproteins. All other patients with P-7–specific paraproteins were shown to be carriers of pP-7. In contrast, only 4 of 200 healthy blood donors (2.0%) were carriers of pP-7 (Table 2). Thus, carriers of pP-7 have a significantly increased risk (odds ratio = 6.2; 95% confidence interval, 2.0-18.6; P = .001) for developing IgM-MGUS/MW (Table 2).
Inheritance of pP-7
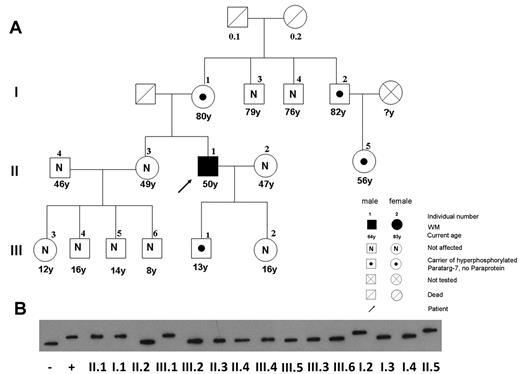
The analysis of relatives of patients with IgM-MGUS/WM with an anti–P-7-specific paraprotein showed that the hyperphosphorylated state of this protein is inherited as a dominant trait (Figures 3 and 4). The members of 4 families with patients who had a P-7–specific IgM paraprotein in their serum gave written consent to be studied for pP-7 carrier state. The pedigree in Figure 3A shows the family of a 50-year-old man with WM (II.1). He had a P-7–specific IgM paraprotein (titer, 1:109) and was a carrier of pP-7. His mother (I.1) and his son (III.1) did not have a paraprotein or an anti–P-7 reactivity in their serum at a dilution of 1:102, but IEF showed that they were also carriers of the pP-7 protein. Moreover, in the family shown here, a male-to-male transmission (from II-1 to III-1) was observed, allowing use to state the mode of inheritance more precisely as autosomal dominant. An uncle of the patient (I.2) and his daughter (II.5) were also carriers of pP-7, but they did not have a P-7–specific paraprotein. In all 4 families tested, the analysis of relatives of patients with MGUS/WM with an anti–P-7-specific paraprotein confirmed that the hyperphosphorylated state of P-7 is inherited in a dominant fashion.
Pedigree of a patient with WM with a P-7–specific IgM paraprotein carrying the pP-7. (A) The pedigree shows the family of a 50-year-old man with WM (II.1), having a P-7–reactive paraprotein and carrying the hyperphosphorylated state of this protein. The pattern of a pP-7 carrier state in this family is consistent with an autosomal dominant trait. (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (I.3; I.4; II.2; II.3; II.4; III.2; III.3; III.4; III.5; III.6) and pP-7 (I.1; I.2; II.1; II.5; III.1) after IEF. The numbers indicate family members in different generations.
Pedigree of a patient with WM with a P-7–specific IgM paraprotein carrying the pP-7. (A) The pedigree shows the family of a 50-year-old man with WM (II.1), having a P-7–reactive paraprotein and carrying the hyperphosphorylated state of this protein. The pattern of a pP-7 carrier state in this family is consistent with an autosomal dominant trait. (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (I.3; I.4; II.2; II.3; II.4; III.2; III.3; III.4; III.5; III.6) and pP-7 (I.1; I.2; II.1; II.5; III.1) after IEF. The numbers indicate family members in different generations.
Pedigree of a patient with MGUS with a P-7–specific IgM paraprotein carrying the pP-7. (A) The pedigree shows the family of a 77-year-old man with IgM-MGUS (I.1), having a P-7–reactive paraprotein and carrying the hyperphosphorylated state of this protein. (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (I.2; III.1) and pP-7 (I.1; II.1; III.2) after IEF. The numbers indicate family members in different generations.
Pedigree of a patient with MGUS with a P-7–specific IgM paraprotein carrying the pP-7. (A) The pedigree shows the family of a 77-year-old man with IgM-MGUS (I.1), having a P-7–reactive paraprotein and carrying the hyperphosphorylated state of this protein. (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (I.2; III.1) and pP-7 (I.1; II.1; III.2) after IEF. The numbers indicate family members in different generations.
Discussion
In this study 18 of 161 (11.2%) paraproteins from IgM-MGUS/WM (9 from patients with WM and 9 from patients with IgM-MGUS) reacted specifically with P-7 (Table 2), proving P-7 as the first antigen identified as a paraprotein target in a significant proportion of patients with IgM-MGUS/WM. The specificity of the paraprotein-mediated reaction was shown by absorption studies with recombinant P-7 and by cloning the BCR from involved tissues of patients with a P-7–specific paraprotein. Sequence analyses of P-7 from all patients analyzed (n = 6) were identical, excluding mutations or single nucleotide polymorphisms as the reason for the autoimmunogenicity of P-7 in the respective patients. Moreover, IEF and phosphatase treatment showed that all analyzed patients with P-7–specific IgM paraproteins were carriers of a hyperphosphorylated version of the protein (pP-7). Because only 2.0% of healthy controls are carriers of pP-7, pP-7 carrier state is associated with a significantly increased risk of developing IgM MGUS/WM.
Although it is well established that family members of patients with WM face an increased risk of developing WM and related B-cell malignancies,7 the genetic basis of enhanced susceptibility in these families remains undefined. Family analyses of relatives of patients with IgM MGUS/WM with an anti–P-7-specific paraprotein showed that the hyperphosphorylated state of this protein is inherited as a dominant trait. There have been several studies of familial aggregation of WM, implicating both environmental and inherited factors.4-7,24 Several reports suggest that gene mutations or genetic polymorphisms may be associated with the risk of WM.4,6,13 However, results have been inconsistent, and significant findings have not been replicated convincingly. pP-7 is the first molecularly characterized structure that provides a plausible explanation for the familial clustering of cases of IgM MGUS/WM, at least in cases with a P-7–specific paraprotein as shown for one of our families analyzed in this study (Figure 3). The index patient, a 50-year-old man, was a carrier of pP-7 and had a paraprotein with specificity for this antigenic target. His mother and one of his uncles were also carriers of pP7, but they did not have a paraprotein or an anti–P-7 reactivity in their serum at a dilution of 1:102. Moreover, in the family shown here, a male-to-male transmission (from II-1 to III-1) was observed, allowing us to describe the mode of inheritance more precisely as autosomal dominant. It is now possible to investigate whether previously reported cases of familial WM4-7,13,25 could also be explained by the carrier state of pP-7.
The frequency of the carrier state of pP-7 among patients with IgM-MGUS/WM and in healthy controls show a 6.2 times increased risk of developing MGUS/WM for carriers of the hyperphosphorylated protein. This is, to the best of our knowledge, the highest odds ratio of a single risk factor reported to date for IgM-MGUS/WM. Because the pP-7 carrier state is inherited as a dominant trait and expressed lifelong, age does not affect the prevalence of the pP-7 carrier state. According to the hypothesis of chronic antigenic stimulation, increasing age would be associated with prolonged antigenic stimulation and thus an increased incidence of pP-7–associated MGUS and WM, respectively. Indeed, the youngest patient with a pP-7–associated MGUS/WM in this study was 37 years old. Median age was 67.8 years (range, 37-90 years). There was no difference with respect to age, sex (all patients), M-protein level, stage of the disease between patients with an anti–P-7-specific paraprotein, and the rest. Because the control group (median age, 40 years) was 25 years younger than the patients with paraproteins (60 years), we cannot exclude that the 4 of 200 healthy pP-7 carriers will develop an anti–paratarg-specific paraprotein on longer follow-up. The same applies to family members with the pP-7 carrier state. If this were the case, however, it would further increase the odds ratio of pP-7 carriers to develop IgM-MGUS-WM.
The results obtained in IgM-MGUS/WM are similar to recent observations made in 252 patients with IgG- or IgA-MGUS/MM when pP-7 was also associated with a significantly increased risk of developing IgG- and IgA-MGUS/MM (odds ratio, 7.9; P < .001) and was also shown to be inherited in a dominant fashion.19 There was no indication that pP-7 carrier state is associated with any hematologic neoplasia or solid tumor other than MGUS, MM, and WM (Table 1), and it is intriguing that pP-7 is associated with 2 B-cell neoplasms that present with quite a different biology and very different clinical findings. This suggests that B-cells with anti–P-7 specificity are prone to malignant transformation during different stages of their maturation.
In contrast to the carrier state of pP-7, which is under exclusive genetic control, the nature of the immune response against pP-7 is complex and might involve both genetic and environmental factors. The frequency of pP-7 as an antigenic target or stimulus for paraprotein-producing clones, and the availability of many families with patients with MGUS/WM and MM with the pP-7 carrier state now allow for the analysis of tumor-host interactions in the presence and absence of the antigen in the respective patients and family members and to study more specifically the role of environmental factors and immunoregulatory deficiencies, such as the recently reported dysfunction of regulatory T cells26 in patients with MGUS and MM. Finally, the fact that pP-7 functions as the antigenic target of the paraproteins of all patients with MGUS/WM with pP-7 suggests that the hyperphosphorylated protein plays a role in the development of IgM-MGUS/WM. The hyperphosphorylation of P-7 appears to be the most obvious and probable reason for its autoimmunogenicity. Indeed, it has been shown that phosphoepitopes have a higher binding affinity to the major histocompatibility complex (MHC) and induce stronger CD8+27 and CD4+ T-cell responses.28 Whether pP-7 induces the development of MGUS/WM by chronic antigenic stimulation or whether it is only a marker or an epiphenomenon of another dominantly inherited susceptibility to develop MGUS/WM can now be investigated in the respective patients and their (not yet) affected relatives.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all Bostonian, Greek, and German patients and their families for participating in the study. We thank Prof Dr R. M. Bohle, Director of the Institute of Pathology of Saarland University Medical School, and Prof Dr H. Merz, Director of the Institute of Pathology of Luebeck University Medical School, for providing paraffin-embedded lymph nodes from patients with a Paratarg-7–specific IgM paraprotein.
This work was supported by Förderverein Krebsforschung Saar-Pfalz-Mosel, HOMFOR, and Wilhelm Sander-Stiftung.
Authorship
Contribution: S.G. designed the experiments and wrote the manuscript; K.-D.P. designed the experiments; A.W. performed the experiments and analyzed the results; E.T. and M.A.D. recruited and analyzed Greek patients for the study; S.P.T. and Z.R.H. recruited and analyzed Bostonian patients for the study; M.Z. did the statistical analysis; N.F. and E.R. performed and analyzed the experiments; D.N. and Y.Y. collected and tested the patients with neoplasias other than MGUS/MM/WM and solid tumors; M.P. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: Two of the authors (K.-D.P. and M.P.) have applied for a patent related to the work that is described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, José-Carreras-Center for Immuno- and Gene Therapy, Department of Internal Medicine I, Saarland University Medical School, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.