Abstract
Currently dendritic cell (DC)–based vaccines are explored in clinical trials, predominantly in cancer patients. Murine studies showed that only maturation with Toll-like receptor (TLR) ligands generates mature DCs that produce interleukin-12 and promote optimal T-cell help. Unfortunately, the limited availability of clinical-grade TLR ligands significantly hampers the translation of these findings into DC-based vaccines. Therefore, we explored 15 commonly used preventive vaccines as a possible source of TLR ligands. We have identified a cocktail of the vaccines BCG-SSI, Influvac, and Typhim that contains TLR ligands and is capable of optimally maturing DCs. These DCs (vaccine DCs) showed high expression of CD80, CD86, and CD83 and secreted interleukin-12. Although vaccine DCs exhibited an impaired migratory capacity, this could be restored by addition of prostaglandin E2 (PGE2; vaccine PGE2 DCs). Vaccine PGE2 DCs are potent inducers of T-cell proliferation and induce Th1 polarization. In addition, vaccine PGE2 DCs are potent inducers of tumor antigen-specific CD8+ effector T cells. Finally, vaccine PGE2–induced DC maturation is compatible with different antigen-loading strategies, including RNA electroporation. These data thus identify a new clinical application for a mixture of commonly used preventive vaccines in the generation of Th1-inducing clinical-grade mature DCs.
Introduction
Dendritic cells (DCs) are the most potent professional antigen-presenting cells of the immune system. On infection or inflammation, immature DCs (imDCs) are activated and differentiate into mature DCs that instruct and activate B and T lymphocytes, the mediators of adaptive immunity.1 Currently, DC-based immunotherapy is explored in clinical trials, predominantly in cancer patients,2,3 including hematologic malignancies.4 Antigen-loaded autologous DCs are administered to patients with the intention of inducing antigen-specific T- and B-cell responses. Although DC-based immunotherapy induces immunologic responses, thus far, only a limited number of clinical responses have been observed.3 It remains unclear why some patients respond to DC-based immunotherapy and others do not, but it has been suggested that the current protocols used to generate mature DCs may not result in optimal Th1 responses. To date, most clinical studies use tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and prostaglandin E2 (PGE2) for DC maturation. However, murine studies have shown that activation of DCs by solely proinflammatory cytokines yields DCs that support CD4+ T-cell clonal expansion but fail to efficiently direct helper T-cell differentiation. DCs polarize immune responses via secretion of soluble factors, such as cytokines.5 IL-12p70 favors the differentiation of interferon-γ (IFN-γ)–producing T helper 1 cells.6,7 In addition, IL-12p70 is involved in the activation of CD8+ effector T cells8,9 and is thus relevant in enhancing in vivo antitumor responses.10,11 DCs matured with proinflammatory cytokines produce very little or no IL-12p70. In contrast, exposure of DCs to pathogen-associated molecular patterns (PAMPs), such as Toll-like receptor (TLR) ligands, induces DCs that produce high levels of IL-12p70 and promote efficient T-cell help.12-15
PAMPs are recognized by pattern recognition receptors. TLRs are part of this family of proteins and sense microbial and viral products. TLR engagement on DCs induces maturation and cytokine secretion, including IL-12p70.16 In humans, 11 TLRs have been described for which many specific ligands have been identified.17-19 The signaling pathways associated with ligation to each of these TLRs are not identical; therefore, distinct biologic responses are initiated on ligation.20 Human monocyte-derived DCs (moDCs) express TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, and TLR8.18 We recently demonstrated that a maturation cocktail combining the TLR3 ligand polyinosinic-polycytidylic acid (poly(I:C)) and the TLR7/8 ligand R848 supplemented with PGE2 yields DCs with both high migratory capacity and IL-12p70 production on T-cell encounter.12 TLR-mediated maturation of ex vivo–generated human moDCs may thus be used to improve immunologic and clinical responses in DC vaccination of cancer patients.
One possible drawback of the use of purified TLR ligands was recently reported. We showed that the presence of the TLR3 ligand poly(I:C) in the maturation cocktail activates innate immune mechanisms that induce an antiviral state in DCs.21,22 This may lead to RNA degradation or inhibition of protein synthesis, thus hampering protein expression of tumor antigen–mRNA after electroporation. Therefore, we searched for an alternative maturation cocktail containing clinically applicable TLR ligands that give rise to IL-12–producing mature DCs and allow electroporation with mRNA encoding tumor antigens.
Whereas several TLR ligands have been shown to yield mature Th1-directing DCs, limited availability in Good Manufacturing Practice conditions impedes the use of these TLR ligands for the generation of DCs for immunotherapy. Vaccines against infectious diseases frequently contain PAMPs derived from bacteria or viruses. These PAMPs may be recognized by pattern recognition receptors and may therefore be good candidates for stimulation of DCs via TLRs. Generally, vaccines consist of either live attenuated or killed microorganisms, subunits of microorganisms, or inactivated toxic compounds, often supplemented with adjuvants that further stimulate an immune response against the pathogens. The importance of TLR ligation for efficacy of vaccines is now underscored by investigators working on vaccine development,23,24 but for the majority of currently used vaccines the presence of TLR ligands has not been studied.25 In this study, we searched for new sources of clinical-grade TLR ligands that can be used for maturation of clinically applicable DCs. To activate DCs that express TLR receptors, we explored the use of commercially available prophylactic vaccines. We have identified vaccines that contain TLR ligands and are capable of inducing DC maturation. This knowledge provides a new application for these clinically applicable agents: clinical-grade DC stimulators.
Methods
Vaccines
Vaccines include the following: Act-HIB (Aventis Pasteur), BCG vaccin SSI (Nederlands Vaccin Instituut [NVI]), BMR vaccin (Bof- Mazelen-, Rubellavaccin, NVI), FSME-IMMUN (Baxter AG), Havrix (GlaxoSmithKline BV), HBVAXPRO (Sanofi Pasteur MSD), Infanrix-IPV + HIB (GlaxoSmithKline BV), Influvac 2007/2008 (Solvay Pharmaceuticals), Neisvac-C (Baxter AG), Pneumo-23 (Aventis Pasteur), Prevenar (Wyeth), Geïnactiveerd Rabiesvaccin Mérieux HDCV (Sanofi Pasteur MSD), Stamaril (Sanofi Pasteur MSD), Tetanus (NVI), and Typhim Vi (Sanofi Pasteur MSD). Table 1 gives detailed information about the contents of the vaccines.
TLR ligand screening
The presence of TLR ligands was tested on recombinant human embryonic kidney 293 (HEK293) cell lines functionally expressing a given TLR protein and a reporter gene driven by a nuclear factor-κB–inducible promoter. A recombinant HEK293 cell line for the reporter gene only was used as negative control. Positive control ligands are PAM2 (100 ng/mL) for HEK293-hTLR2, lipopolysaccharide (LPS); K12 (100 ng/mL) for HEK293-hTLR4, and Flagellin (1 μg/mL) for HEK293-hTLR5. Vaccines (10× diluted) were added to the reaction volume. TLR ligand screenings were performed by InvivoGen Europe.
Generation of DCs from peripheral blood precursors
DCs were generated from peripheral blood mononuclear cells (PBMCs) prepared from leukapheresis products or from buffy coats as described.26,27 Buffy coats were obtained from healthy volunteers according to institutional guidelines. Plastic-adherent monocytes from leukapheresis or buffy coats were cultured in X-VIVO 15 medium (Lonza Walkersville) supplemented with 2% pooled human serum (HS; Bloodbank Rivierenland), IL-4 (500 U/mL), and granulocyte-macrophage colony-stimulating factor (800 U/mL; both CellGenix). On day 6 or 7, DCs were either kept immature or one of the following maturation cocktails was added for 48 hours: 10 ng/mL recombinant TNF-α (CellGenix), 5 ng/mL IL-1β (ImmunoTools), 15 ng/mL IL-6 (CellGenix), and 10 μg/mL PGE2 (Pharmacia; conventional DCs [cDCs]); 20 μg/mL poly(I:C) (Sigma-Aldrich), and 5 μg/mL R848 (Axxora) without PGE2 (TLR DCs) or with 10 μg/mL PGE2 (TLR PGE2 DCs); BCG (4%), Typhim (4%), and Influvac (4%) without PGE2 (vaccine DCs) or with 10 μg/mL PGE2 (vaccine PGE2 DCs). Single vaccines were added at a concentration of 5%.
Flow cytometric analysis
The phenotype of DCs was determined by flow cytometry. The following primary monoclonal antibodies (mAbs) or appropriate isotype controls were used: anti–human leukocyte antigen (HLA)–ABC (W6/32), anti–HLA-DR/DP (Q5/13), and anti-CD80 (all BD Biosciences), anti-CD83 (Beckman Coulter), anti-CD86 (BD Biosciences PharMingen), anti-CCR7 (R&D Systems), followed by Alexa 488–conjugated goat anti–mouse IgG (Invitrogen). Intracellular staining of gp100 and tyrosinase was performed as described22 with NKI/beteb (IgG2b) and T311 (IgG2a; Cell Marque). Flow cytometry was performed with a FACSCalibur flow cytometer equipped with CellQuest software (BD Biosciences).
In vitro migration assays
For random migration on fibronectin, flat-bottomed 96-well plates (Corning Life Sciences) were coated with 20 μg/mL fibronectin (Roche Diagnostics) for 60 minutes at 37°C and blocked with 0.01% gelatin (Sigma-Aldrich) for 30 minutes at 37°C. A total of 4000 DCs per well were seeded on fibronectin-coated plates and recorded for 60 minutes at 37°C, after which migration tracks of individual DCs were analyzed using an automated cell-tracking system.28
For CCR7-mediated migration, transwell inserts with 5 μm pore size polycarbonate membranes (Corning Life Sciences) were preincubated with 100 μL of X-VIVO 15/2% HS in 24-well plates, each well containing 600 μL of medium. A total of 1 × 105 DCs were seeded in the upper compartment. CCL21 (10 or 100 ng/mL; R&D Systems) was added to the lower wells. Spontaneous migration and kinesis were measured by incubation of the cells in a transwell without or with 100 ng/mL CCL21 in both the upper and the lower well, respectively. DCs were allowed to migrate for 60 minutes in a 5% CO2, humidified incubator at 37°C. After 60 minutes, DCs were harvested from the lower chamber and counted by flow cytometry. All conditions were tested in duplicate.
Determination of chemokine and cytokine production
IL-12p70 production was measured in the supernatants 48 hours after induction of maturation using a standard sandwich enzyme-linked immunosorbent assay (ELISA; Pierce Biotechnology).
MIP1α, RANTES, and IP-10 production was measured using FlowCytomix Simplex kits (Bender MedSystems).
MLR
The ability of DCs to induce Th1 cells was studied in a mixed lymphocyte reaction (MLR). DCs were added to 1 × 105 freshly isolated allogeneic nonadherent PBLs from a healthy donor in the presence or absence of neutralizing anti–human IL-12 antibody (R&D Systems). Cytokine production was measured in MLR supernatants after 48 hours by cytometric bead array (Th1/Th2 Cytokine CBA 1; BD Biosciences PharMingen).
KLH-specific proliferation assay
Cellular responses against the protein keyhole limpet hemocyanin (KLH) were measured in a proliferation assay. In our vaccination studies, KLH is added to immature DC cultures as an immunomonitoring tool. Peripheral blood lymphocytes (PBLs) were isolated from blood samples from 3 patients taken after 4 biweekly vaccinations with mature DCs. The PBMCs were plated in a 96-well tissue-culture microplate with autologous cDCs, vaccine DCs or vaccinePGE2 DCs that were cultured with or without KLH. After 4 days of culture, 1 μCi/well of tritiated thymidine was added for 8 hours, and incorporation of tritiated thymidine was measured in a β-counter.
Generation of CD45RA+CD8+ gp100-specific T cells
The vectors pGEM4Z-TCRα296 and pGEM4Z-TCRβ296 are encoding TCR-α and -β chains originating from a gp100:280-288/HLA-A2-specific CTL clone. Gp100-specific T cells were generated by transferring the TCR α and β chain to T cells by electroporation of RNA, resulting in transient expression of the TCR chains as described previously.29 In vitro transcription of gp100 TCR RNA was performed as described.29
CD8+CD45RA+ T cells were isolated from PBMCs of an HLA-A2.1–positive donor. Monocytes were removed via adherence, and CD8+ T cells were isolated by positive isolation using fluorescein isothiocyanate (FITC)–conjugated anti–human CD8 (BD Biosciences) and anti-FITC microbeads (Anti-FITC Multisort kit; Miltenyi Biotec) according to the manufacturer's instructions. Subsequently, CD45RA+ T cells were isolated from the CD8+ T-cell fraction by negative selection using CD45RO microbeads (Miltenyi Biotec). Purity of CD8+CD45RA+ T cells was 90% to 95%, as assessed by double staining using FITC-conjugated anti–human CD8 and phycoerythrin (PE)–conjugated anti–human CD45RA mAbs (BD Biosciences).
For RNA electroporation, CD45RA+CD8+ T cells or CD8+ T cells were washed once with phosphate-buffered saline and once with OptiMEM without phenol red (Invitrogen). A total of 10 to 12 × 106 cells were incubated for 3 minutes with 15 to 20 μg of RNA in 200 μL OptiMEM in a 4-mm cuvette (Bio-Rad). Subsequently, cells were pulsed in a Genepulser Xcell (Bio-Rad). Pulse conditions were square-wave pulse, 500 V, 5 ms. Immediately after electroporation, the cells were transferred to X-VIVO 15 medium without phenol red (Cambrex) supplemented with 6% HS. After 4 hours of incubation at 37°C, cells were frozen in liquid nitrogen. Expression of the gp100 TCR was verified by flow cytometry using PE-conjugated anti-TCRVβ14 mAb from Coulter Immunotech (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Functionality of the gp100-specific T cells was shown by up-regulation of the early activation marker CD69 after overnight stimulation with gp100:280-288 peptide-loaded HLA-A2+ imDCs (supplemental Figure 1B).
Electroporation of DCs
Mature DCs were washed twice in phosphate-buffered saline and once in OptiMEM without phenol red (Invitrogen). A total of 20 μg of RNA (gp100 or tyrosine RNA, Curevac) was transferred to a 4-mm cuvette (Bio-Rad), and 10 × 106 DCs were added in 200 μL of OptiMEM and incubated for 3 minutes before being pulsed with an exponential decay pulse at 300 V, 150 μF in a Genepulser Xcell (Bio-Rad) as described previously.22 Immediately after electroporation, the cells were transferred to warm (37°C) X-VIVO 15 without phenol red (Cambrex) supplemented with 6% HS, and left for at least 2 hours at 37°C, before further manipulations were performed.
gp100-specific activation of CD45RA+CD8+ T cells and CD8+ T cells
moDCs from an HLA-A2.1+ donor were matured with different maturation cocktails for 48 hours and loaded with either specific peptide (gp100:280-288) or control peptide (gp100:154-167 or tyrosinase:369-376) for 1 hour (1 μg/7 × 103 DCs). DCs (7 × 103 per well) were washed and coincubated with CD45RA+CD8+ gp100:280-288-specific T cells derived from the same donor (5 × 104 per well) in round-bottom 96-well plates. T-cell stimulatory capacity of electroporated DCs was tested by coculturing 7 × 103 DCs 4 hours after electroporation with gp100-encoding or control (tyrosinase) mRNA with 5 × 104 CD8+ gp100:280-288-specific T cells. After overnight incubation, CD69 expression was measured by flow cytometry using FITC-conjugated mouse anti–human CD69 (BD Biosciences PharMingen), and IFN-γ production was measured using a standard sandwich ELISA (Pierce Chemical). After 4 days of culture, 1 μCi/well of tritiated thymidine was added for 8 hours, and incorporation of tritiated thymidine was measured in a β-counter. After 5 days, granzyme B expression was measured by flow cytometry using PE-conjugated mouse antihuman granzyme B antibodies (Sanquin). Antigen-specific degranulation was measured in gp100;280 TCR-expressing CD8+ T cells cocultured with differently matured DCs for 5 hours in the presence of Golgi-stop (monensin; BD Biosciences) and PE-Cy5–labeled anti-CD107a (BD Biosciences PharMingen) as described previously.30 CD107a expression was measured by flow cytometry.
RNA isolation
Total RNA was isolated from DC cultures using TRIZOL reagent (Invitrogen) according to the manufacturer's instructions, with minor modifications. RNA integrity was determined by analyzing the ribosomal 28S and 18S bands on 1% agarose gel. The reverse transcription reaction was performed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's instructions. For each sample, an “-RT” control was included in which the reverse transcriptase was replaced by diethyl pyrocarbonate–treated Milli-Q. cDNA was stored at −80°C until further use.
Quantitative PCR
Quantitative analysis of gene expression in DCs was performed using SYBR Green-based quantitative polymerase chain reaction (PCR). The quantitative PCR reactions were performed in a 25-μL volume containing 12.5 μL of SYBR Green mix (Applied Biosystems), 1.5 μL of forward/reverse primer (300nM end concentration), 4.5 μL of Milli-Q, and 5 μL of cDNA dilution. Reactions were performed on an ABI 7900HT Sequence Detection System (Applied Biosystems). Analysis was performed using Sequence Detection software (Version 2.0, Applied Biosystems). Primer sequences are available on request and were designed using the freely accessible Primer Bank program.31
Statistics
Data were analyzed using the Student t test and 1-way analysis of variance. P values less than .05 were considered to be statistically significant.
Results
Vaccines contain TLR ligands
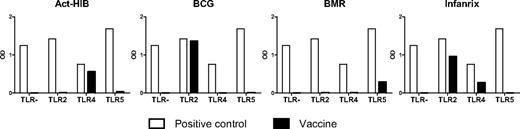
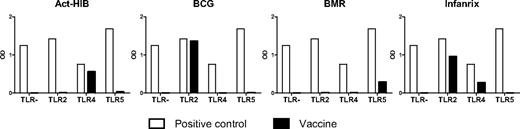
TLR ligands, such as LPS or poly(I:C), are frequently used for in vitro DC maturation. However, the availability of synthetic TLR ligands for clinical usage is limited. To search for alternative sources of clinical-grade TLR ligands for maturation of clinically applicable DCs, we tested 15 readily available vaccines (Table 1) for their capacity to interact with extracellular TLRs known to be expressed by moDCs (TLR2, TLR4, and TLR5). HEK293 cells stably transfected with plasmids encoding human TLR genes were used to investigate the 15 vaccines. The HEK293 cell line was selected for its null or low basal expression of endogenous TLR genes. Four of 15 vaccines were able to activate TLR-expressing HEK293 transfectants (Figure 1). TLR2-mediated activation was observed with BCG (tuberculosis vaccine containing live attenuated Mycobacterium bovis) and Infanrix (containing antigens of diphtheria, Clostridium tetani, pertussis, poliovirus, and Haemophilus influenzae type b), whereas Act-Hib (vaccine against H influenzae type b) and Infanrix were able to activate TLR4. BCG activated the TLR2-expressing HEK293 cell line as efficient as the TLR2 ligand PAM2. BMR, a vaccine composed for vaccination against measles, mumps, and rubella, was able to activate TLR5. The remaining 11 vaccines did not activate one of the TLRs tested (data not shown).
Vaccines contain TLR ligands. Vaccines were added (10× diluted) to recombinant HEK293 cell lines, functionally expressing a given TLR protein and a reporter gene driven by an nuclear factor-κB–inducible promoter. TLR stimulation was measured as activation of the reporter gene. The negative control value for each clone is the background signal. Positive control ligands used are: PAM2 for the HEK293-hTLR2 cell line, LPS K12 for the HEK293-hTLR4 cell line, and Flagellin for the HEK293-hTLR5 cell line. Data are expressed as optical density (OD) values.
Vaccines contain TLR ligands. Vaccines were added (10× diluted) to recombinant HEK293 cell lines, functionally expressing a given TLR protein and a reporter gene driven by an nuclear factor-κB–inducible promoter. TLR stimulation was measured as activation of the reporter gene. The negative control value for each clone is the background signal. Positive control ligands used are: PAM2 for the HEK293-hTLR2 cell line, LPS K12 for the HEK293-hTLR4 cell line, and Flagellin for the HEK293-hTLR5 cell line. Data are expressed as optical density (OD) values.
To exclude that vaccine adjuvants play a major role in the observed DC activation, vaccine preparations were compared that all share the same adjuvant (Table 1). Because we observed that neither all vaccines containing aluminum hydroxide nor all vaccines with aluminum phosphate induced TLR activation, we can exclude a prominent role for the adjuvants present in the vaccine formulations in activating TLRs.
Vaccines induce DC maturation and IL-12 production
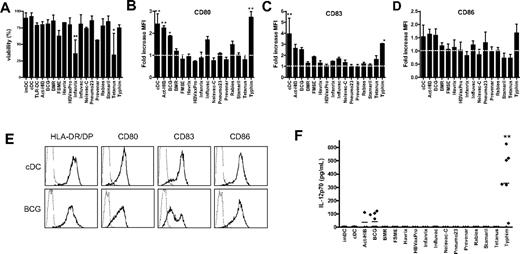
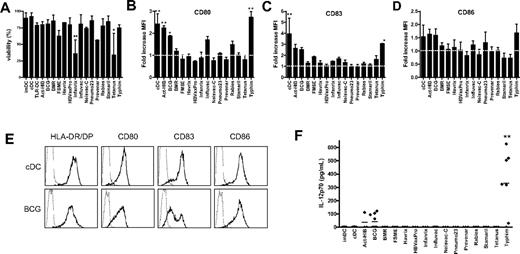
Because the TLR ligand screening revealed that several vaccines contain TLR ligands, we studied whether these vaccines can be used for DC maturation in vitro to obtain clinically applicable TLR-matured DCs. Given that, in addition to TLR, other pattern recognition receptors such as dectin-1 are involved in antigen-presenting cell maturation,32 we also included vaccines that did not activate TLRs tested in TLR ligand screening. The vaccines were added at 5% (vol/vol) concentration to the culture medium of imDCs at day 6 for 48 hours. The majority of the vaccines were nontoxic, and the viability was comparable with that of cDCs in the concentrations used (Figure 2A). Only Infanrix and tetanus strongly affected cell viability (25% viable cells), even when added at 1% (vol/vol; data not shown).
Vaccines induce DC maturation. imDCs were incubated with the conventional cytokine cocktail (TNF-α, IL-6, IL-1β, and PGE2) or with different preventive vaccines for 48 hours. (A) Viability was analyzed by Trypan blue exclusion. Data are mean ± SD of 3 independent experiments performed with DCs from different donors. **P < .01 compared with cDCs. (B-D) The expression of maturation markers HLA-DR/DP, CD80 (B), CD83 (C), and CD86 (D) was measured by flow cytometry. Results are shown as fold increase of mean fluorescence intensity relative to imDCs. Data are mean ± SEM of 3 experiments with different donors. *P < .05. **P < .01. The dotted line indicates fold increase 1.0 (unchanged fluorescence intensity compared with imDCs). (E) Example of expression of HLA-DR/DP, CD80, CD83, and CD86 (bold line) on cDCs and DCs treated with BCG. The thin line represents the isotype control. (F) At 48 hours after addition of the vaccines, IL-12p70 secretion was measured in the supernatant by ELISA. Per condition, each symbol represents 1 donor. Mean values are shown for each vaccine. **P < .01 compared with cDCs.
Vaccines induce DC maturation. imDCs were incubated with the conventional cytokine cocktail (TNF-α, IL-6, IL-1β, and PGE2) or with different preventive vaccines for 48 hours. (A) Viability was analyzed by Trypan blue exclusion. Data are mean ± SD of 3 independent experiments performed with DCs from different donors. **P < .01 compared with cDCs. (B-D) The expression of maturation markers HLA-DR/DP, CD80 (B), CD83 (C), and CD86 (D) was measured by flow cytometry. Results are shown as fold increase of mean fluorescence intensity relative to imDCs. Data are mean ± SEM of 3 experiments with different donors. *P < .05. **P < .01. The dotted line indicates fold increase 1.0 (unchanged fluorescence intensity compared with imDCs). (E) Example of expression of HLA-DR/DP, CD80, CD83, and CD86 (bold line) on cDCs and DCs treated with BCG. The thin line represents the isotype control. (F) At 48 hours after addition of the vaccines, IL-12p70 secretion was measured in the supernatant by ELISA. Per condition, each symbol represents 1 donor. Mean values are shown for each vaccine. **P < .01 compared with cDCs.
To study the effect of all 15 vaccines on DC maturation, the expression of major histocompatibility molecule HLA-DR/DP, costimulatory molecules CD80 and CD86, and the DC-maturation marker CD83 at the surface of DC was measured by flow cytometry. Act-HIB, BCG, and Typhim significantly increased the expression of CD80 on moDCs compared with imDCs (Figure 2B). Influvac and rabies caused a moderate up-regulation of CD80. In addition, Typhim significantly increased CD83 expression on moDCs (Figure 2C). Act-HIB, BCG, and Typhim slightly increased the expression of CD86 (Figure 2D). Figure 2E shows the expression of maturation markers on DCs matured with the conventional cytokine cocktail or with BCG. Although BMR contains a TLR5 ligand, it did not enhance the expression of maturation markers on moDCs.
On TLR triggering, DCs rapidly produce high levels of IL-12p70.12,33 We analyzed the IL-12p70 concentration in the culture medium of DCs 48 hours after addition of the 15 vaccines. Cytokine-matured DCs (IL-1β, TNF-α, IL-6, PGE2; cDCs) hardly produced any IL-12p70, whereas BCG and Typhim induced secretion of IL-12p70 (Figure 2F), suggesting that these vaccines activate TLRs on DCs.
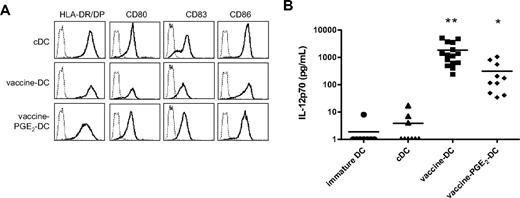
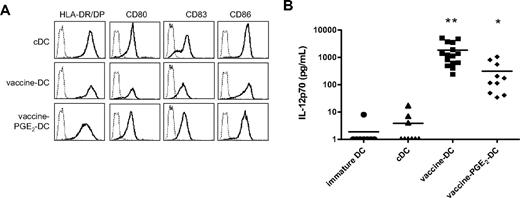
Combined vaccines have a synergistic effect on DC maturation
Previous studies described that different TLRs act in synergism and induce a stronger immune response when activated simultaneously or successively.33,34 Therefore, we combined vaccines that contain different TLR ligands and/or induced DC maturation. We compared different combinations of vaccines (data not shown) and the cocktail of BCG, Influvac, and Typhim (all 4% vol/vol) induced optimal expression of maturation markers and IL-12p70 production. This vaccine cocktail was chosen for further analysis. The morphology of DCs matured with BCG, Influvac, and Typhim (vaccine DCs) was comparable with that of DCs matured with the TLR ligands R848 or poly(I:C)12 (and data not shown). Vaccine DCs had an elongated phenotype and were more adherent to plastic than cDCs, which have a semiround appearance with multiple dendrites (data not shown). Vaccine DCs have a fully mature phenotype, and expression of maturation markers is comparable with the expression on cDCs (Figure 3A). The phenotype of vaccine DCs was stable for at least 24 hours after removal of vaccines and cytokines from the culture medium (data not shown). Compared with DCs treated with single vaccines, such as BCG or Typhim, IL-12p70 production of vaccine DCs was strongly enhanced, indicating a synergistic effect of the separate vaccines (Figure 3B).
Combined vaccines have a synergistic effect on DC maturation. DCs were matured for 48 hours with the conventional cytokine cocktail (TNF-α, IL-6, IL-1β, and PGE2), preventive vaccines (BCG, Typhim, and Influvac), or vaccines with PGE2, and the expression of maturation markers and IL-12p70 production was evaluated. (A) The expression of maturation markers HLA-DR/DP, CD80, CD83, and CD86 (bold line) was measured by flow cytometry. The thin line represents the isotype control. (B) IL-12p70 production was measured by ELISA in the supernatant of DC cultures 48 hours after maturation. Per condition, each symbol represents 1 donor. Mean values are shown for each maturation cocktail. *P < .05, **P < .01 compared with cDCs.
Combined vaccines have a synergistic effect on DC maturation. DCs were matured for 48 hours with the conventional cytokine cocktail (TNF-α, IL-6, IL-1β, and PGE2), preventive vaccines (BCG, Typhim, and Influvac), or vaccines with PGE2, and the expression of maturation markers and IL-12p70 production was evaluated. (A) The expression of maturation markers HLA-DR/DP, CD80, CD83, and CD86 (bold line) was measured by flow cytometry. The thin line represents the isotype control. (B) IL-12p70 production was measured by ELISA in the supernatant of DC cultures 48 hours after maturation. Per condition, each symbol represents 1 donor. Mean values are shown for each maturation cocktail. *P < .05, **P < .01 compared with cDCs.
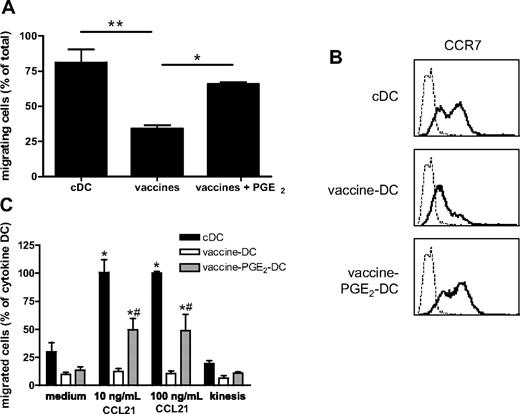
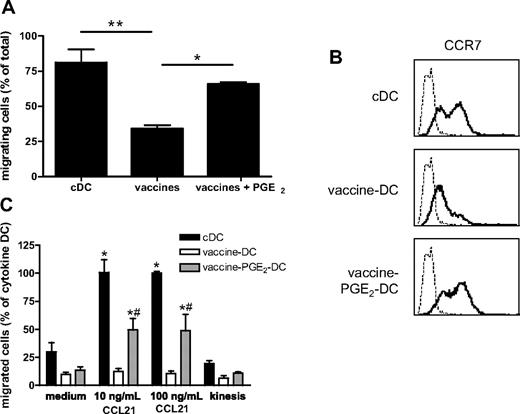
PGE2 is essential for migration of vaccine DC
For clinical studies, it is important that DCs are able to migrate from the injection site to the T-cell areas of lymph nodes to present tumor antigens to the targeted T cells, even when injected intranodally. Migration to the lymph nodes is mediated by the chemokines CCL19 and CCL21 and their receptor CCR7.35 The migratory capacity was tested 48 hours after vaccine-induced maturation in a random migration assay on the extracellular matrix protein fibronectin and in a transwell migration assay toward the lymph node chemokine CCL21. In the random migration assay, single cells were tracked with an automated cell-tracking system and their traversed paths were analyzed.28 Compared with cDCs, random migration of vaccine DCs was strongly decreased (Figure 4A). In addition, vaccine DCs showed reduced CCR7-mediated migration toward CCL21 compared with cDCs (Figure 4C). However, the addition of PGE2 to the vaccine cocktail, which increased expression of the chemokine receptor CCR7 (Figure 4B), restored the migratory capacity of vaccine DCs, both on fibronectin (Figure 4A) and toward CCL21 (Figure 4C). Addition of PGE2 did not affect the expression of maturation markers (Figure 3A). Although addition of PGE2 to the vaccines reduced IL-12p70 production, vaccine PGE2 DCs still produce 100-fold more IL-12p70 than cDCs (Figure 3B).
PGE2 is essential for migration of vaccine DCs. (A) Random migration on fibronectin. cDCs, vaccine DCs, and vaccine PGE2 DCs were added to a fibronectin-coated plate, and migration of individual cells was monitored for 60 minutes. Data represent the percentage of migrating cells (mean ± SEM) of 3 experiments with cells from different donors. For each experiment, migration of 50 cells per condition was monitored. *P < .05. **P < .01. (B) The expression of CCR7 (bold line) on cDCs, vaccine DCs, and vaccine PGE2 DCs was measured by flow cytometry. The thin line represents the isotype control. (C) CCR7-mediated chemotaxis of cDCs, vaccine DCs, and vaccine PGE2 DCs was determined by the number of cells that had migrated into the lower compartment of a transwell system containing 10 or 100 ng/mL CCL21, counted by flow cytometry. To measure spontaneous migration, cells were incubated in a transwell without CCL21 in the upper and lower compartment (medium) or with 100 ng/mL CCL21 in both compartments (kinesis). Migration of cDCs in the presence of 100 ng/mL CCL21 was regarded as 100% (100% corresponds to 26 190 ± 10 636 migrated cells). The graph represents means ± SEM from 3 experiments (with cells from different donors) performed in duplicate. *Significant difference (P < .05) compared with medium. #Significant difference (P < .05) compared with vaccine DCs.
PGE2 is essential for migration of vaccine DCs. (A) Random migration on fibronectin. cDCs, vaccine DCs, and vaccine PGE2 DCs were added to a fibronectin-coated plate, and migration of individual cells was monitored for 60 minutes. Data represent the percentage of migrating cells (mean ± SEM) of 3 experiments with cells from different donors. For each experiment, migration of 50 cells per condition was monitored. *P < .05. **P < .01. (B) The expression of CCR7 (bold line) on cDCs, vaccine DCs, and vaccine PGE2 DCs was measured by flow cytometry. The thin line represents the isotype control. (C) CCR7-mediated chemotaxis of cDCs, vaccine DCs, and vaccine PGE2 DCs was determined by the number of cells that had migrated into the lower compartment of a transwell system containing 10 or 100 ng/mL CCL21, counted by flow cytometry. To measure spontaneous migration, cells were incubated in a transwell without CCL21 in the upper and lower compartment (medium) or with 100 ng/mL CCL21 in both compartments (kinesis). Migration of cDCs in the presence of 100 ng/mL CCL21 was regarded as 100% (100% corresponds to 26 190 ± 10 636 migrated cells). The graph represents means ± SEM from 3 experiments (with cells from different donors) performed in duplicate. *Significant difference (P < .05) compared with medium. #Significant difference (P < .05) compared with vaccine DCs.
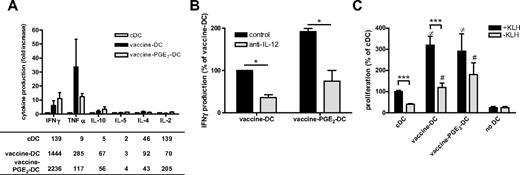
Vaccine PGE2 DCs have high stimulatory capacity
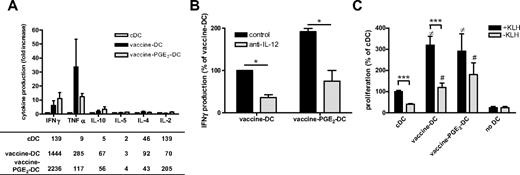
For effective immunotherapy, DCs need to have a high T-cell stimulatory capacity and induce Th1 cells. Vaccine DCs produce high levels of the inflammatory and Th1-attracting chemokines MIP1α, RANTES, and IP-10 (supplemental Figure 2). Although addition of PGE2 reduced the production of these chemokines, vaccine PGE2 DCs produce more MIP1α, RANTES, and IP-10 than imDCs and cDCs. To analyze whether vaccine DCs induce a Th1 type of immune response, we measured the secretion of cytokines by PBLs in supernatants of primary MLRs (Figure 5A). cDCs induced very low levels of the Th1 cytokines IFN-γ and TNF-α, whereas cocultures of vaccine DCs and vaccine PGE2 DCs showed considerably higher production of these cytokines. Neutralization of IL-12 reduced the production of IFN-γ in primary MLRs with vaccine DCs or vaccine PGE2 DCs, suggesting that IL-12 is involved in the induction of Th1 responses by vaccine DCs and vaccine PGE2 DCs (Figure 5B). The secretion of IL-10 was slightly increased in cocultures with both vaccine DCs and vaccine PGE2 DCs, but the secreted levels were less than 70 pg/mL. There were no differences in secretion of Th2 cytokines IL-4 and IL-5 between cDCs, vaccine DCs, and vaccine PGE2 DCs.
Vaccine PGE2 DCs have a high stimulatory capacity. (A) The profile of cytokines secreted by PBLs on contact with allogeneic cDCs, vaccine DCs, and vaccine PGE2 DCs was measured by cytokine bead array. The graph represents the fold change in cytokine production of vaccine DCs and vaccine PGE2 DCs relative to cDCs of 3 different donors. The table presents the mean concentration (picograms per milliliter) of each cytokine in absolute numbers for all conditions. (B) IFN-γ production by PBLs cocultured with vaccine DCs or vaccine PGE2 DCs in the absence ( ) or presence (
) or presence ( ) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.
) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.  represents DCs loaded with KLH; and
represents DCs loaded with KLH; and  represents DCs without KLH. *P < .05. ***P < .001. ≠Significant difference (P < .05) with cDCs with KLH. #Significant difference (P < .05) with cDCs without KLH.
represents DCs without KLH. *P < .05. ***P < .001. ≠Significant difference (P < .05) with cDCs with KLH. #Significant difference (P < .05) with cDCs without KLH.
Vaccine PGE2 DCs have a high stimulatory capacity. (A) The profile of cytokines secreted by PBLs on contact with allogeneic cDCs, vaccine DCs, and vaccine PGE2 DCs was measured by cytokine bead array. The graph represents the fold change in cytokine production of vaccine DCs and vaccine PGE2 DCs relative to cDCs of 3 different donors. The table presents the mean concentration (picograms per milliliter) of each cytokine in absolute numbers for all conditions. (B) IFN-γ production by PBLs cocultured with vaccine DCs or vaccine PGE2 DCs in the absence ( ) or presence (
) or presence ( ) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.
) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.  represents DCs loaded with KLH; and
represents DCs loaded with KLH; and  represents DCs without KLH. *P < .05. ***P < .001. ≠Significant difference (P < .05) with cDCs with KLH. #Significant difference (P < .05) with cDCs without KLH.
represents DCs without KLH. *P < .05. ***P < .001. ≠Significant difference (P < .05) with cDCs with KLH. #Significant difference (P < .05) with cDCs without KLH.
We also investigated whether vaccine DCs are capable of stimulating antigen-specific T cells, which was demonstrated by KLH-specific proliferation of PBLs isolated from patients who had been previously vaccinated with KLH-loaded DCs. Figure 5C demonstrates that vaccine DCs and vaccine PGE2 DCs are able to induce KLH-specific T-cell proliferation. Interestingly, PBLs from these patients showed already a higher proliferation in cocultures with vaccine DCs and vaccine PGE2 DCs cultured without KLH, which may be directed against one of the vaccines used for DC maturation.
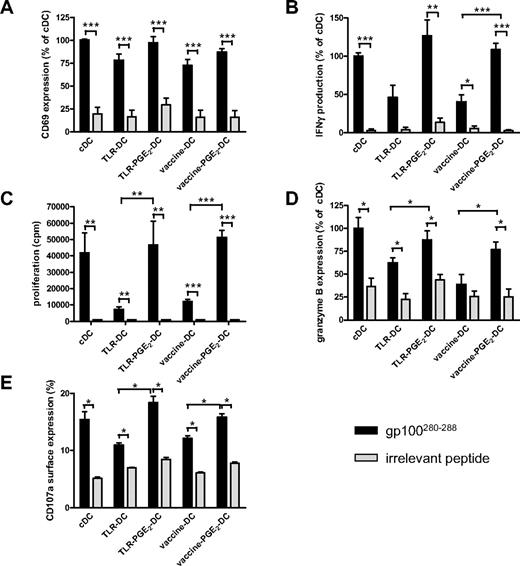
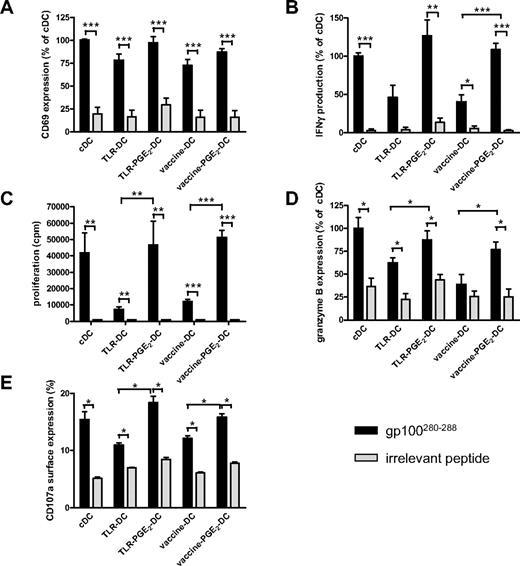
Besides skewing CD4 T cells toward a Th1 cytokine profile, IL-12p70 is involved in the activation and induction of CD8+ T cells with CTL activity.8,9 To study whether vaccine PGE2 DCs are capable of inducing effector T cells, differently matured, peptide-loaded DCs were coincubated with autologous naive CD8+CD45RA+ T cells expressing gp100:280-288–specific T-cell receptor-α and -β. All differently matured DCs induced peptide-specific T-cell activation, as demonstrated by CD69 expression and IFN-γ production after 16 hours of coincubation of DCs and CD8+CD45RA+ T cells (Figure 6A and B, respectively). Interestingly, naive CD8+ T cells activated by DCs matured in the presence of PGE2 (PGE2 DCs: cDCs, TLR PGE2 DCs, vaccine PGE2 DCs) produced higher levels of IFN-γ than T cells activated by DCs matured in the absence of PGE2 (Figure 6B). Similarly, PGE2 DCs induced stronger antigen-specific CD8+ T-cell proliferation compared with DCs matured in the absence of PGE2 (Figure 6C). Moreover, only T cells incubated for 5 days with peptide-loaded PGE2 DCs express the effector T-cell marker granzyme B (Figure 6D), suggesting that naive CD8+ T cells incubated with PGE2 DCs, including vaccine PGE2 DCs, acquire CTL function. To study whether vaccine PGE2 DCs induce CTL with the capacity to kill target cells, we have transfected effector CTLs with mRNA encoding the gp100:280 T-cell receptor and measured CD107a surface expression after stimulation with differently matured gp100:280-loaded DCs. CD107a surface expression is a measure for degranulation of cytotoxic granules and correlates with target killing by CTLs.30 Like granzyme B expression, antigen-specific CD107a surface expression on CD8+ T cells was induced by all differently matured DCs; however, PGE2 DCs induced stronger CD107a surface expression than DCs matured in the absence of PGE2 (Figure 6E).
Vaccine PGE2 DCs induce antigen-specific effector CD8+ T cells. Naive CD45RA+CD8+ T cells specific for gp100:280-288 (50 000 per well) were cocultured with autologous cDCs, TLR DCs, TLR PGE2 DCs, vaccine DCs, or vaccine PGE2 DCs (7000 per well) that were loaded with either gp100:280-288 or an irrelevant peptide. After 16 hours, antigen-specific activation of CD8+ T cells was analyzed by measurement of CD69 surface expression (A) and secretion of IFN-γ in the supernatant (B). T-cell proliferation was analyzed by 3H-thymidine incorporation after 4 days (C). Granzyme B expression was measured by intracellular FACS staining after 5 days (D). Antigen-specific degranulation was measured by CD107a surface expression on gp100;280 TCR-expressing PBLs cocultured for 5 hours with differently matured DCs in the presence of Golgi-stop and PE-Cy5–labeled anti-CD107a (E).  represents DCs loaded with specific peptide (gp100:280-288); and
represents DCs loaded with specific peptide (gp100:280-288); and  represents DCs loaded with irrelevant peptide. (A-B,D) Mean ± SEM values of the relative mean fluorescence intensity (A,D) or relative IFN-γ production (B) compared with cDCs of 3 (B) or 4 (A,D) independent experiments performed with cells from different donors; 100% corresponds to 40% ± 27% CD69-positive cells (A), 834 ± 697 pg/mL IFN-γ (B), and a mean fluorescence intensity of 238 ± 363 (D). Panel C shows mean ± SEM of 1 representative experiment of 4 performed. (E) Mean ± SEM values of the percentage of cells expressing CD107a. *P < .05. **P < .01. ***P < .001.
represents DCs loaded with irrelevant peptide. (A-B,D) Mean ± SEM values of the relative mean fluorescence intensity (A,D) or relative IFN-γ production (B) compared with cDCs of 3 (B) or 4 (A,D) independent experiments performed with cells from different donors; 100% corresponds to 40% ± 27% CD69-positive cells (A), 834 ± 697 pg/mL IFN-γ (B), and a mean fluorescence intensity of 238 ± 363 (D). Panel C shows mean ± SEM of 1 representative experiment of 4 performed. (E) Mean ± SEM values of the percentage of cells expressing CD107a. *P < .05. **P < .01. ***P < .001.
Vaccine PGE2 DCs induce antigen-specific effector CD8+ T cells. Naive CD45RA+CD8+ T cells specific for gp100:280-288 (50 000 per well) were cocultured with autologous cDCs, TLR DCs, TLR PGE2 DCs, vaccine DCs, or vaccine PGE2 DCs (7000 per well) that were loaded with either gp100:280-288 or an irrelevant peptide. After 16 hours, antigen-specific activation of CD8+ T cells was analyzed by measurement of CD69 surface expression (A) and secretion of IFN-γ in the supernatant (B). T-cell proliferation was analyzed by 3H-thymidine incorporation after 4 days (C). Granzyme B expression was measured by intracellular FACS staining after 5 days (D). Antigen-specific degranulation was measured by CD107a surface expression on gp100;280 TCR-expressing PBLs cocultured for 5 hours with differently matured DCs in the presence of Golgi-stop and PE-Cy5–labeled anti-CD107a (E).  represents DCs loaded with specific peptide (gp100:280-288); and
represents DCs loaded with specific peptide (gp100:280-288); and  represents DCs loaded with irrelevant peptide. (A-B,D) Mean ± SEM values of the relative mean fluorescence intensity (A,D) or relative IFN-γ production (B) compared with cDCs of 3 (B) or 4 (A,D) independent experiments performed with cells from different donors; 100% corresponds to 40% ± 27% CD69-positive cells (A), 834 ± 697 pg/mL IFN-γ (B), and a mean fluorescence intensity of 238 ± 363 (D). Panel C shows mean ± SEM of 1 representative experiment of 4 performed. (E) Mean ± SEM values of the percentage of cells expressing CD107a. *P < .05. **P < .01. ***P < .001.
represents DCs loaded with irrelevant peptide. (A-B,D) Mean ± SEM values of the relative mean fluorescence intensity (A,D) or relative IFN-γ production (B) compared with cDCs of 3 (B) or 4 (A,D) independent experiments performed with cells from different donors; 100% corresponds to 40% ± 27% CD69-positive cells (A), 834 ± 697 pg/mL IFN-γ (B), and a mean fluorescence intensity of 238 ± 363 (D). Panel C shows mean ± SEM of 1 representative experiment of 4 performed. (E) Mean ± SEM values of the percentage of cells expressing CD107a. *P < .05. **P < .01. ***P < .001.
Overall, these data show that vaccine PGE2 DCs can stimulate both CD4+ T helper cells and CD8+ CTLs in an antigen-specific manner.
Vaccine PGE2 DCs can efficiently be electroporated with mRNA encoding tumor antigens
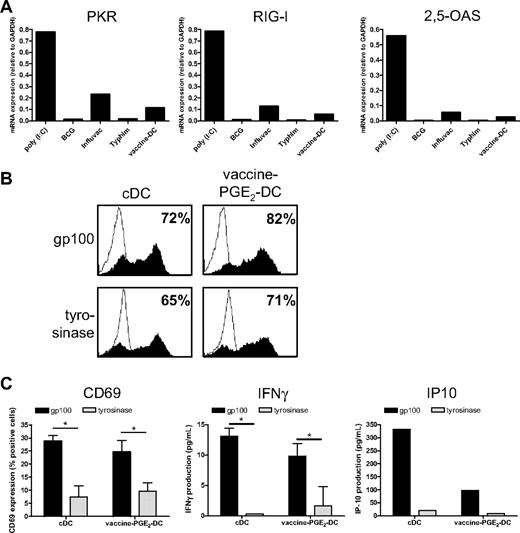
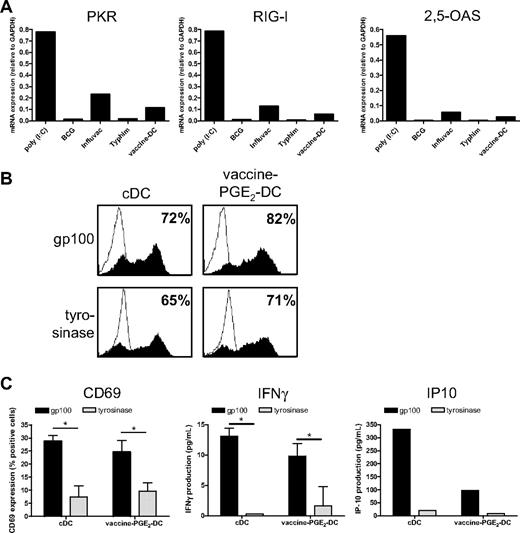
A crucial aspect of DC-based immunotherapy is the method of antigen delivery to DCs. We previously showed that maturation with the TLR3 ligand poly(I:C) hampers protein expression of tumor antigen-mRNA after electroporation because of increased expression of the viral sensor RIG-I and the effector molecules PKR and 2,5-OAS.22 Therefore, we examined whether vaccines also provoke the expression of these proteins. We measured gene expression of PKR, RIG-I, and 2,5-OAS in DCs matured with poly(I:C), which has been shown to induce the expression of these genes, the single vaccines BCG, Influvac, and Typhim, or with a cocktail of these vaccines. poly(I:C) strongly increased the expression of PKR, RIG-I, and 2,5-OAS (Figure 7A). Influvac induced low mRNA expression of these molecules, whereas BCG and Typhim had no effect. The vaccine cocktail induced expression of PKR, RIG-I, and 2,5-OAS, which was, however, very low.
Vaccine-PGE2 DCs do not express viral sensors and effector molecules and express and present tumor antigens after mRNA electroporation. (A) DCs were matured for 48 hours with the conventional cytokine cocktail (cDCs), combined vaccines (BCG, Typhim, and Influvac; vaccine DCs), poly(I:C), or with separate preventive vaccines. mRNA levels of PKR, RIG-I, and 2,5-OAS were determined using quantitative PCR 48 hours after maturation. (B) DCs were matured for 48 hours with the conventional cytokine cocktail or combined vaccines (BCG, Typhim, and Influvac; vaccine DCs) with PGE2. DCs were electroporated with mRNA encoding gp100 or tyrosinase. After 4 hours, gp100 or tyrosinase protein expression was determined by FACS analysis. Filled curves represent staining with specific antibody; and thin-lined curves represent the isotype control. Numbers indicate percentage of cells expressing the antigen. Data are a representative experiment of 3 performed. Average antigen expression (mean ± SEM) of 3 experiments was 73 ± 7 for gp100 and 76 ± 3 for tyrosinase on vaccine PGE2 DCs, and 65 ± 7 for gp100 and 73 ± 4 for tyrosinase on cDCs. (C) A total of 50 000 gp100:280-specific CD8+ T cells were coincubated with 7000 cDCs or vaccine PGE2 DCs 4 hours after electroporation with gp100 mRNA or tyrosinase mRNA as a control, and T-cell activation was analyzed by measurement of CD69 surface expression (left), IFN-γ production (middle), and IP-10 production (right). Data are mean ± SEM of 1 representative experiment of 3 performed in triplicate (CD69 and IFN-γ) or 1 representative experiment (IP-10). *P < .05.
Vaccine-PGE2 DCs do not express viral sensors and effector molecules and express and present tumor antigens after mRNA electroporation. (A) DCs were matured for 48 hours with the conventional cytokine cocktail (cDCs), combined vaccines (BCG, Typhim, and Influvac; vaccine DCs), poly(I:C), or with separate preventive vaccines. mRNA levels of PKR, RIG-I, and 2,5-OAS were determined using quantitative PCR 48 hours after maturation. (B) DCs were matured for 48 hours with the conventional cytokine cocktail or combined vaccines (BCG, Typhim, and Influvac; vaccine DCs) with PGE2. DCs were electroporated with mRNA encoding gp100 or tyrosinase. After 4 hours, gp100 or tyrosinase protein expression was determined by FACS analysis. Filled curves represent staining with specific antibody; and thin-lined curves represent the isotype control. Numbers indicate percentage of cells expressing the antigen. Data are a representative experiment of 3 performed. Average antigen expression (mean ± SEM) of 3 experiments was 73 ± 7 for gp100 and 76 ± 3 for tyrosinase on vaccine PGE2 DCs, and 65 ± 7 for gp100 and 73 ± 4 for tyrosinase on cDCs. (C) A total of 50 000 gp100:280-specific CD8+ T cells were coincubated with 7000 cDCs or vaccine PGE2 DCs 4 hours after electroporation with gp100 mRNA or tyrosinase mRNA as a control, and T-cell activation was analyzed by measurement of CD69 surface expression (left), IFN-γ production (middle), and IP-10 production (right). Data are mean ± SEM of 1 representative experiment of 3 performed in triplicate (CD69 and IFN-γ) or 1 representative experiment (IP-10). *P < .05.
Because vaccines did not induce strong expression of viral sensors and effector molecules, we next investigated whether vaccine DCs can be efficiently electroporated with mRNA encoding the tumor antigens gp100 and tyrosinase, which we use for DC vaccination of melanoma patients.36 cDCs and vaccine PGE2 DCs were electroporated with gp100- or tyrosinase-mRNA, and protein expression was analyzed using flow cytometry 4 hours after electroporation. Electroporation of both cDCs and vaccine PGE2 DCs was very effective, as indicated by high expression of gp100 and tyrosinase (Figure 7B). To examine whether DCs electroporated with gp100-encoding mRNA can present gp100-derived epitopes to T cells, we incubated cDCs and vaccine PGE2 DCs, 4 hours after electroporation with gp100 mRNA or control mRNA (tyrosinase), with gp100:280-specific CD8+ T cells. CD69 expression, IFN-γ production, and IP-10 production by gp100:280 specific CD8+ T cells were induced by gp100 mRNA–electroporated cDCs and vaccine PGE2 DCs, but not by tyrosinase mRNA–electroporated DCs (Figure 7C). Together, these data show that gp100 mRNA–electroporated vaccine PGE2 DCs efficiently process the gp100 protein and present the processed peptides in major histocompatibility complex class I to specific T cells.
Discussion
In this study, we identified commonly used preventive vaccines as an alternative source of clinical-grade TLR ligands. We demonstrate that these vaccines induce DC maturation, yielding clinical-grade DCs that have high expression of maturation markers and high production of the Th1-polarizing cytokine IL-12. Addition of PGE2 to the vaccine cocktail gave rise to migratory DCs that are potent inducers of an MLR and antigen-specific T-cell activation. Importantly, vaccine DCs also express and present tumor antigens after electroporation with mRNA encoding these antigens.
Several PAMPs that are used as immunomodulators have been shown to induce DC maturation.14,37,38 Our difficulties in obtaining these products for clinical studies and for the production of DC vaccines prompted us to search for alternatives. We here show that various preventive vaccines contain different TLR ligands and stimulate DCs. In line with previous studies in human blood DCs39,40 and murine DCs,41,42 we found that BCG activates human TLR2 and induces moDC maturation and IL-12 production. Because mycobacterial antigens are also recognized by other pathogen recognition receptors, including the C-type lectins DC–specific intercellular adhesion molecule-3–grabbing nonintegrin (SIGN) and dectin-1,32,43 it is probable that these pattern recognition receptors are also involved in DC activation by BCG and may act synergistically with TLR2 in our DC cultures. Act-HIB contains TLR4 ligands and enhances CD80 expression on moDCs. Infanrix activated both TLR2 and TLR4, thus making it a promising candidate for DC maturation. However, Infanrix was too toxic for the DCs to be used in our studies. Although BMR activated TLR5 on transfected HEK293 cells, it did not induce a mature phenotype or IL-12 production when added to our DC cultures. Previous studies describing TLR5 expression on human moDCs are inconsistent.18 Conceivably, TLR5 expression on our moDCs is either absent or too low to induce effective DC maturation. For Influvac and Typhim, we did not find any ligands of extracellular TLRs using the TLR ligand screening. However, Typhim enhanced the expression of maturation markers on moDCs and induced IL-12p70 production. Influvac induced some up-regulation of mRNA expression of viral sensors and effector molecules, which is mediated by TLR3 ligation,21 indicating that DCs are activated by Influvac as well. Moreover, our data show that stimulation with a cocktail of BCG, Influvac, and Typhim gives rise to fully mature DCs that produce large amounts of IL-12p70, suggesting that these 3 vaccines have a synergistic effect on DC maturation. Possibly, Influvac and Typhim activate intracellular TLRs, which could not be tested with the HEK293 system, or Influvac and Typhim act on distinct receptors on DCs that operate in synergism with TLRs, thus enhancing DC activation.33,34
For efficient antigen presentation to T cells, DCs used for vaccination of cancer patients need to migrate from the injection site to T-cell areas of regional lymph nodes. Our data show that, compared with cDCs, vaccine DCs have a strongly reduced migratory capacity, as we previously demonstrated for TLR DCs matured with poly(I:C) and R848.12 DCs homing to the lymph nodes are guided by chemokines CCL19 and CCL21, ligands for the chemokine receptor CCR7. The presence of CCR7 on the DC cell surface is induced by PGE2 and is essential for DC migration to the lymph node.44-46 In line with our previous studies,12 addition of PGE2 to the maturation cocktail enhanced surface expression of CCR7 and partially restored the migratory capacity of vaccine DCs. Although the migratory capacity of vaccine PGE2 DCs was not as high as that of cDCs, vaccine PGE2 DCs migrate efficiently compared with both imDCs and vaccine DCs that do not migrate at all.47
Although PGE2 restores the motility of vaccine-matured DCs, its presence during DC maturation reduced IL-12p70 production, as shown before.45,46,48 However, in our studies, secreted levels are still 100-fold higher than that of cDCs. And in line with previous studies,12,49 our data show that the levels produced by vaccine PGE2 DCs are sufficient to induce IFN-γ– and TNF-α–secreting Th1 cells, which produce only low levels of IL-4 and IL-5. Together, our data show that, although they produce reduced levels of IL-12p70 compared with vaccine DCs, vaccine PGE2 DCs have the capacity to induce antigen-specific T-cell activation and Th1 responses. Based on IL-12 production during maturation and the induction of Th1 responses, we expected that TLR DCs and vaccine DCs would induce stronger CTL responses than cDCs. Unexpectedly, stimulating naive CD8+ T cells by cDCs, vaccine PGE2 DCs and TLR PGE2 DCs resulted in equal levels of effector markers. We hypothesize that a mature phenotype is more important in the direct activation of CD8+ T cells in the assays used here than cytokines produced by the DCs. The elevated levels of IL-12 produced by vaccine PGE2 DCs and TLR PGE2 DCs may have a more indirect effect on CD8+ T cells by Th1 induction. Indeed, the neutralization of IL-12 in a CD4-dependent allogeneic response significantly reduced IFN-γ production.
An important aspect of DC vaccination is the method of antigen loading of the DCs. To date, most clinical studies use DCs loaded with tumor lysates or tumor peptides.3,50 Alternatively, DCs can be loaded with RNA coding for tumor antigens, preferentially via electroporation because this method is not considered gene therapy and can therefore be used to generate clinical-grade DC vaccines. We and others recently showed that electroporation of DCs matured with the TLR3 ligand poly(I:C) is very ineffective. This is caused by the induction of an antiviral state in the DCs by poly(I:C). poly(I:C) is synthetic dsRNA that triggers the expression of viral sensors and effector molecules,22,38 which may lead to RNA degradation or inhibition of protein synthesis, thus hampering effective protein expression after RNA electroporation. We here demonstrate that, in contrast to poly(I:C), the vaccine cocktail does not trigger the expression of viral sensors and effector molecules up to a level that RNA electroporation is reduced. Interestingly, Influvac enhanced the expression of RIG-I, PKR, and 2,5-OAS to some extent, despite negative results in the TLR ligand screening. However, the vaccine cocktail did not suppress protein expression after mRNA electroporation, demonstrating that maturation with a BCG, Typhim, and Influvac yields TLR-matured DCs that can be electroporated very efficiently.
A potential disadvantage to the use of vaccines for DC maturation may be that high amounts of antigens derived from the vaccines can mask weakly immunogenic tumor antigens and thus hamper antitumor responses. Using PBLs and DCs of patients who had previously been vaccinated with KLH-loaded DCs, we showed that vaccine DCs and vaccine PGE2 DCs also induce some T-cell proliferation in the absence of KLH, which may be a specific response to one of the vaccines present in the maturation cocktail. However, we also show that vaccine DCs and vaccine PGE2 DCs can induce KLH-specific immune responses. Moreover, vaccine PGE2 DCs loaded with tumor peptides or mRNA are able to activate gp100 specific CD8+ T cells, suggesting that vaccine DCs can induce antitumor responses despite the presence of vaccine-derived antigens. As with KLH, responses to the vaccines may even provide T-cell help and thus facilitate antitumor responses.51
In conclusion, we present here a new source of clinical-grade TLR ligands: commercially available vaccines. We introduce a new clinical-grade maturation cocktail consisting of a mixture of preventive vaccines and PGE2 that yields clinically applicable Th1-inducing TLR ligand-matured DCs. Clinical studies are now being performed to investigate whether vaccine-matured DCs improve antitumor responses in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Meeusen-Scharenborg, Annemiek de Boer, and Mandy van de Rakt for technical support.
This work was supported by the Dutch Cancer Society (grants KWF 2003-2917, KWF 2004-3126, and KWF 2004-3127), The Netherlands Organization for Scientific Research (grant VIDI 91776363), the TIL Foundation, the NOTK Foundation, and the European Union (Cancerimmunotherapy and DC-Thera).
Authorship
Contribution: G.S. designed and performed research, analyzed data, and wrote the paper; D.B.-R. contributed to experimental design and performed research; D.S., A.J.A.L., and M.v.H.-K. performed research; N.S. contributed vital reagents; C.J.A.P. contributed to experimental design; C.G.F. and G.J.A. contributed to experimental design and writing of the paper; and I.J.M.d.V. supervised the study and contributed to experimental design and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. Jolanda M. de Vries, Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: j.devries@ncmls.ru.nl.


 ) or presence (
) or presence ( ) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.
) of neutralizing anti–IL-12 antibody. The graph represents the mean ± SD from 2 experiments (with cells from different donors) of relative IFN-γ production compared with control vaccine DCs (100% corresponds to 48 ± 5 pg/mL). *P < .05. (C) KLH-specific proliferation of PBLs from a patient vaccinated with KLH-loaded DCs. PBLs were cocultured with autologous DCs matured with the cytokine cocktail, vaccines, or vaccines with PGE2 with or without KLH. Proliferation was measured by incorporation of tritiated thymidine. The graph represents mean ± SEM counts per minute relative to cDCs + KLH (100% corresponds to 1201 ± 818 cpm) of 3 experiments with different donors, performed in triplicate.