Abstract
We present results from a prospective, multicenter, open-label, single-arm study evaluating response of cardiac and liver iron to deferasirox therapy for 18 months. Twenty-eight patients with abnormal T2* and normal left ventricular ejection fraction were enrolled from 4 US centers. All patients initially received deferasirox doses of 30 to 40 mg/kg per day. Patients were severely iron overloaded: mean liver iron concentration (LIC) 20.3 mg Fe/g dry weight, serum ferritin 4417 ng/mL, and cardiac T2* 8.6 ms. In the intent-to-treat population, 48% reached the primary endpoint (cardiac T2* improvement at 18 months, P = not significant). There were 2 deaths: 1 from congestive heart failure and 1 from sepsis. In the 22 patients completing the trial, LIC and cardiac T2* improvements were 16% (P = .06) and 14% (P = .07), respectively. Cardiac T2* improvement (13 patients) was predicted by initial LIC, final LIC, and percentage LIC change, but not initial cardiac T2*. Cardiac iron improved 24% in patients having LIC in the lower 2 quartiles and worsened 8.7% in patients having LIC in the upper 2 quartiles. Left ventricular ejection fraction was unchanged at all time points. Monotherapy with deferasirox was effective in patients with mild to moderate iron stores but failed to remove cardiac iron in patients with severe hepatic iron burdens. This study was registered at www.clinicaltrials.gov as #NCT00447694.
Introduction
Iron-induced cardiac failure and arrhythmia are responsible for as many as 71% of deaths in β-thalassemia major.1-3 Patient survival has steadily improved with birth cohort as patients begin to receive appropriate chelation therapy from an early age; however, cardiac disease remains the cause of death in two-thirds of patients.1 Until recently, deferoxamine (DFO) was the only approved iron chelator in North America. DFO administration requires routine subcutaneous or intravenous administration, 5 to 7 days per week, making it onerous for patients. Inadequate compliance with DFO is common and dramatically reduces survival.2-5
The oral iron chelator, deferasirox, was approved in November 2005 with the hope of improving patient compliance and quality of life. Numerous well-controlled clinical trials have demonstrated the efficacy of once-daily oral deferasirox in controlling liver iron concentration (LIC).6-11 Deferasirox is preferred by patients,12 improves compliance,13 has a 12- to 16-hour half-life,14,15 and is routinely prescribed 7 days per week. As a result, deferasirox chronically suppresses labile plasma iron levels better than intermittent, subcutaneous DFO therapy.16,17
Unbound iron or labile plasma iron is the main form of iron that enters cardiac myocytes. Thus, it would be anticipated that chronic labile plasma iron suppression would be efficacious in preventing cardiac iron overload. For cardiac iron clearance, myocyte penetration by the chelator is beneficial as well. In vitro studies reveal the ability of deferasirox to enter myocytes, bind labile iron, suppress reactive oxygen species, and restore myocyte function.18,19 Animal studies also demonstrate that deferasirox can access and lower cardiac iron stores.20,21 Human data supporting the cardiac efficacy of deferasirox are emerging, with recent international studies suggesting cardiac T2* improvement rates of 1.4% per month.22,23
In this manuscript, we present data from the first study in the U.S. thalassemia population designed to evaluate the efficacy of deferasirox on cardiac iron concentration. This trial was an investigator-initiated study designed before Food and Drug Administration approval of the drug and had its first patient visit in December 2005. Primary endpoints for the study were cardiac T2*, LIC, and serum ferritin over 18 months of treatment.
Methods
Study protocol
This prospective, open-label, single-arm, multicenter trial was conducted to study the cardiac chelation efficacy of deferasirox over 18 months of treatment at 4 centers in the United States. Male or female patients (≥ 10 years of age) with β-thalassemia receiving chronic transfusion therapy (> 8 transfusions/year) and a minimum of 100 lifetime red blood cell transfusions were screened for magnetic resonance imaging (MRI) evidence of cardiac iron (T2* < 20 ms) and normal left ventricular ejection fraction (LVEF) more than or equal to 56%, rounded to the nearest integer. Patients with overt cardiac dysfunction were excluded because continuous DFO or combined DFO and deferiprone are standard of care for such patients. Cardiac iron levels vary inversely with cardiac T2*, and values less than 20 ms indicate detectable cardiac iron.24,25 The prevalence of cardiac dysfunction and the risk of subsequent symptomatic heart failure increase dramatically as cardiac T2* decreases below 10 ms.24,26 Therefore, the patients in this trial were currently asymptomatic from cardiac perspective but were at high risk for cardiovascular complications during the study interval.
For safety considerations, patients were required to meet the criteria of serum ferritin levels of more than 1000 ng/mL or LIC of more than 2 mg Fe/g dry weight (dw) as confirmed by liver R2 and R2* measurements.27 Prior iron chelation therapy was allowed with 1-day washout immediately before the first dose of study drug. All the patients were treated at a starting dose of 30 mg/kg per day. The dose was escalated to a maximum of 40 mg/kg per day if there was less than 25% improvement in cardiac T2* compared with baseline values (provided LIC was > 3 mg Fe/g dw); dose escalation was considered after the MRI evaluation at both the 6- and 12-month time points. Patients on deferasirox treatment before study entry were allowed to continue their prestudy dose if it was more than 30 mg/kg per day (2 patients). Patients who were already on 40 mg/kg per day and had less than 25% improvement in T2* were encouraged to divide the 40-mg/kg dosage into morning and evening doses of 20 mg/kg to improve homogeneity of absorption. Improved cardiac efficacy of divided dosing has been demonstrated in animals.20 Patients who were unable or unwilling to comply with twice a day dosing remained on study. Dose reductions were performed because of side effects or because of persistent serum ferritin values less than 1000 ng/mL according to the clinical judgment of the individual site investigators. Drug adherence was monitored by pill counts, but incomplete site documentation precluded usable quantification. This study was approved by the Committee on Clinical Investigation at Children's Hospital Los Angeles. All patients (or parents/guardians) provided written informed consent, and the study was conducted in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki, and institutional ethics policies.
Study assessments
The primary endpoint of the trial was to evaluate changes in cardiac iron (MRI T2*) over 18 months. All MRI assessments, including LIC (measured by MRI R2 and R2*), cardiac T2*, and LVEF, were assessed every 6 months. Patients would be discontinued from the trial if their T2* decreased more than 50% or LVEF declined to less than 53%. For safety, patients were monitored on an ongoing basis with serum ferritin, serum chemistry, and complete blood count with differential, monitored at least once a month using local laboratories. In addition, recording and monitoring of all adverse events (AEs), their severity, and dose adjustments were performed regularly.
MRI for LIC and cardiac T2*
MRI was performed on 3 1.5 Tesla General Electric scanners and one Philips scanner. Liver iron was estimated from liver R2 and R2* measurements in 3 centers and magnetic susceptometry in another. Details on MRI image acquisition and cross-validation may be found in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical methods
All patients who registered for the trial and took at least one dose of medication were included in the intent-to-treat (ITT) population. Continuous metrics of chelator efficacy are based on subjects who completed 18 months of treatment. Results are mean plus or minus SE and/or ranges (mean [range]). All continuous parameters were analyzed using paired 2-tailed Student t test, using a P value of .05 as the threshold of statistical significance.
Because cardiac T2* is reciprocally proportional to cardiac iron,25,28 we linearized the relationship by log transformation of cardiac T2* before T test calculation. Aggregate cardiac T2* data are thus reported in terms of geometric mean values with SE calculated from the log-transformed T2* values. The relationship between cardiac and liver iron stores was assessed by linear regression analysis. We used cardiac R2* (equal to 1000/T2*) in the regression analysis because cardiac R2* is linearly proportional to cardiac iron. Percentage cardiac R2* change over 18 months was compared with initial LIC, final LIC, and the percentage change in LIC.
Cardiac T2* values at 18 months were deemed “responsive” to therapy if they increased more than the 14.7% (MRI limits of agreement) from baseline. All statistical analyses were performed using SAS, Version 8.2 or JMP 5.1.
Results
Forty-five patients underwent screening and 28 patients were enrolled at 4 U.S. centers. Seventeen patients failed screening: 14 because they lacked MRI-detectable cardiac iron or exhibited abnormal ejection fraction, and 3 were disqualified by medical history, abnormal physical examination, or screening laboratories. Six patients did not complete the trial; one withdrew consent before starting the drug (leaving 27 patients in the ITT population), and 5 were discontinued because of AEs or abnormal monitoring laboratory value. Data from 5 patients receiving drug are included in the baseline statistics and ITT metrics but excluded from correlation analyses.
All patients had received more than 150 units of lifetime blood exposure and had a history of DFO usage. A total of 17 of 27 patients had switched to deferasirox at the time of enrollment, and the remaining 10 were on DFO. Mean exposure to deferasirox before enrollment was 1.3 years. Baseline characteristics of the ITT population are shown in Table 1. Patients were severely iron overloaded with an average LIC of 20.3 mg Fe/g dw, serum ferritin of 4417 ng/mL, and T2* of 8.6 ms (geometric mean). There was a 2.4:1 female-to-male gender disparity but no significant differences in baseline iron metrics or response to therapy. Cardiac ejection fraction was normal in all subjects but one; this patient was mistakenly enrolled and later removed. Mean prescribed dose at the start of study was 31 mg/kg per day (range, 30-40 mg/kg per day; median, 30 mg/kg per day), with 23 patients starting at 30 mg/kg per day. Dosage increased slightly throughout the study, reaching 31.3 mg/kg per day (range, 20-40 mg/kg per day; median, 30 mg/kg per day) at 6 months, 32.1 mg/kg per day (range, 20-40 mg/kg per day; median, 35 mg/kg per day) at 12 months, and 33.3 mg/kg per day (range, 20-40 mg/kg per day; median, 35 mg/kg per day) at 18 months.
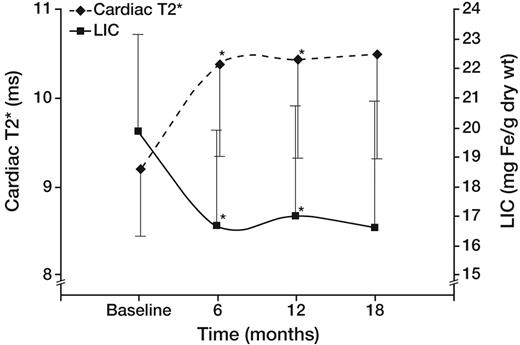
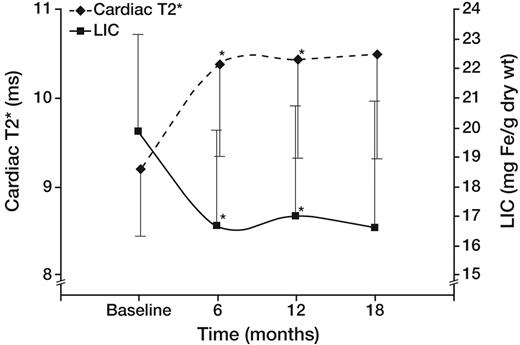
In the ITT cohort, 13 of 27 patients reached the primary endpoint (> 14.7% improvement in cardiac T2*). The cardiac T2* (geometric mean ± SE) in patients completing 18 months of therapy was 9.2 plus or minus 0.8 ms at baseline and 10.5 plus or minus 0.9 ms at 18 months (P = not significant). Figure 1 demonstrates the temporal response of liver iron and cardiac T2* (geometric mean) in all patients completing the study. T2* and LIC were significantly improved at 6 and 12 months, but changes were no longer significant at 18 months (P = .07 and .06, respectively).
Effect of deferasirox on cardiac T2* (♦ represents left axis; geometric mean) and LIC (■ represents right axis) in the 22 patients completing 18 months of therapy. Cardiac T2* (♦ represents left axis; geometric mean) and LIC (■ represents right axis) improved rapidly by 6 months and remained stable thereafter. Differences were significant at 6 and 12 months, but not at 18 months (P = .07).
Effect of deferasirox on cardiac T2* (♦ represents left axis; geometric mean) and LIC (■ represents right axis) in the 22 patients completing 18 months of therapy. Cardiac T2* (♦ represents left axis; geometric mean) and LIC (■ represents right axis) improved rapidly by 6 months and remained stable thereafter. Differences were significant at 6 and 12 months, but not at 18 months (P = .07).
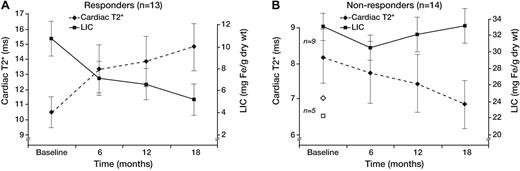
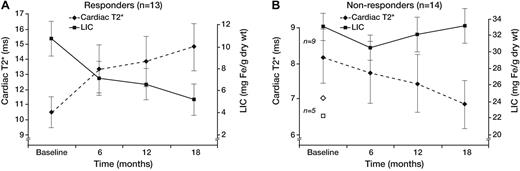
The apparent “plateau” in cardiac efficacy observed across the whole study population (Figure 1) was artifactual and actually represented combinations of patients who steadily improved or who steadily worsened (Figure 2). Responders (13 of 27 patients, Figure 2A) progressively improved cardiac and liver iron burden throughout the study (43.3% ± 18.8% and 53.9% ± 27.7%, respectively), demonstrating reciprocal changes in cardiac T2* and LIC. In contrast, nonresponders (14 of 27, Figure 2B) failed to lower their LIC at any time point, and cardiac T2* steadily worsened (−15.2% ± 17.8%, respectively). Not surprisingly, responders had significantly lower hepatic (10.7 ± 5.8 vs 29.2 ± 4.5 mg/g dry weight, P < .001) and ferritin (3005 ± 575 vs 5885 ± 3348 ng/mL, P = .03) iron burdens; cardiac T2* was also higher but did not reach statistical significance (geometric mean, 10.4 ± 1.1 vs 7.4 ± 1.1 ms, P = .09). Ejection fraction was similar at baseline in responders and nonresponders (62.1% ± 4.8% vs 62.4% ± 4.0%), and no significant difference was observed at the end of study as well (62.7% ± 5.6% vs 62.1% ± 5.9%). Three patients transiently decreased their LVEF to less than 56% during the study but finished with LVEF in the normal range. One patient had his LVEF decline from 56.9% to 53.4% at 18 months; T2* decreased from 7 to 4.3 ms over the same interval.
Cardiac T2* and LIC time course. Patients responsive (A) and nonresponsive (B) to therapy. Same format as Figure 1. The 5 patients who did not complete the trial (◇ represents T2*; and □, LIC) were classified as nonresponders. Baseline T2* and LIC levels for these patients were 6.5 ± 2.4 ms and 22.3 ± 8.3 mg Fe/g dw. Error bars were suppressed for graph clarity.
Cardiac T2* and LIC time course. Patients responsive (A) and nonresponsive (B) to therapy. Same format as Figure 1. The 5 patients who did not complete the trial (◇ represents T2*; and □, LIC) were classified as nonresponders. Baseline T2* and LIC levels for these patients were 6.5 ± 2.4 ms and 22.3 ± 8.3 mg Fe/g dw. Error bars were suppressed for graph clarity.
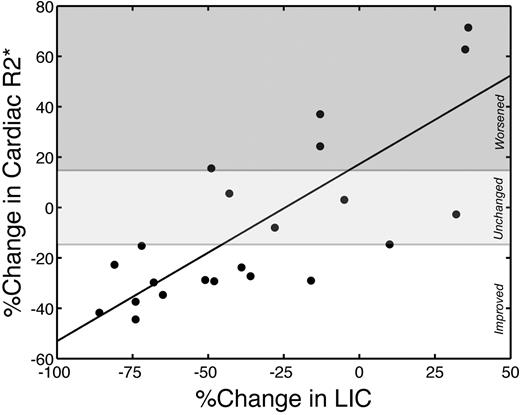
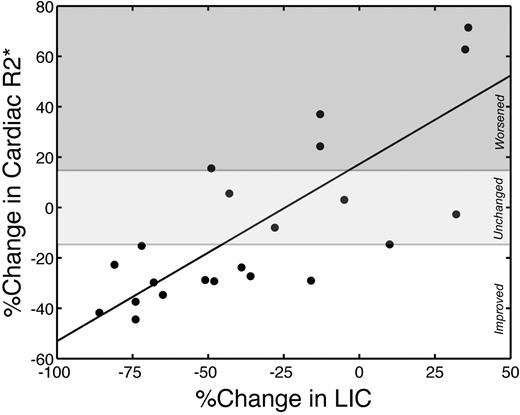
Change in cardiac iron (R2*) correlated (r = 0.78; P < .001) with changes in LIC (Figure 3). On average, each percentage decrease in LIC translated to a 0.70% improvement in cardiac R2*. All patients who lowered their LIC by more than 50% improved their cardiac iron. No patient with worsening LIC improved their cardiac iron. Patients who lowered their LIC between 0% and 50% over 18 months had variable cardiac response, indicating that hepatic responsiveness was necessary but not sufficient to guarantee cardiac improvement.
Changes in cardiac R2* are linearly proportional to changes in LIC. r = 0.78; P < .001. Cardiac R2* and LIC are expressed as percentage change over the study interval. The white region represents significant cardiac iron clearance; light gray region, no statistically significant individual change; and dark gray region, significant cardiac iron accumulation.
Changes in cardiac R2* are linearly proportional to changes in LIC. r = 0.78; P < .001. Cardiac R2* and LIC are expressed as percentage change over the study interval. The white region represents significant cardiac iron clearance; light gray region, no statistically significant individual change; and dark gray region, significant cardiac iron accumulation.
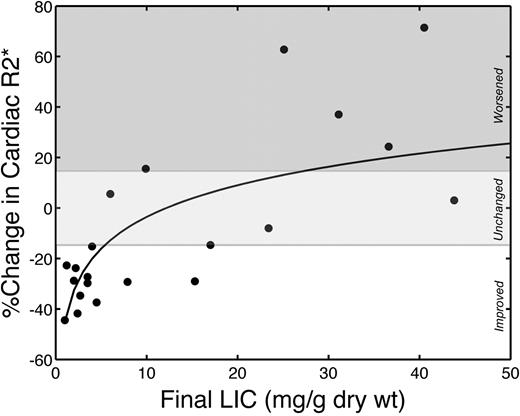
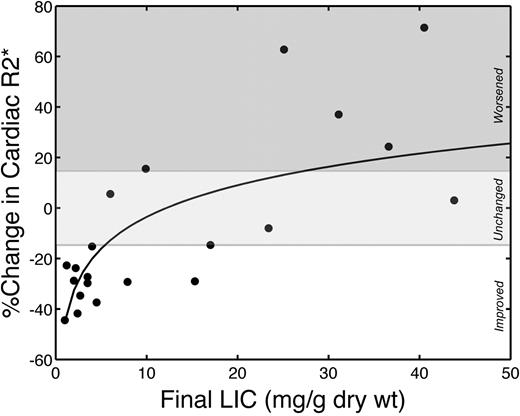
Cardiac iron improvement increased sharply as LIC normalized. Figure 4 demonstrates percentage improvement in cardiac R2* as a function of final LIC. The relationship is linear with respect to the log of LIC (r = 0.70; P < .001), with cardiac iron elimination rate increasing dramatically as LIC approaches normal levels. All patients with final LIC less than 5 mg Fe/g dw improved their cardiac R2* and had significantly better cardiac iron clearance (41.9% ± 9.8% improvement vs 11.3% ± 32.7% worsening, P < .001) than patients having higher final liver iron concentrations.
Cardiac R2* changes were logarithmically proportional to final LIC. r = 0.70; P < .001. Drug efficacy appears to increase sharply as LIC approaches the normal range; all patients with final LIC less than 5 mg improved their cardiac iron. Solid line represents a linear fit to log-transformed LIC values. The white region represents significant cardiac iron clearance; light gray region, no statistically significant individual change; and dark gray region, significant cardiac iron accumulation.
Cardiac R2* changes were logarithmically proportional to final LIC. r = 0.70; P < .001. Drug efficacy appears to increase sharply as LIC approaches the normal range; all patients with final LIC less than 5 mg improved their cardiac iron. Solid line represents a linear fit to log-transformed LIC values. The white region represents significant cardiac iron clearance; light gray region, no statistically significant individual change; and dark gray region, significant cardiac iron accumulation.
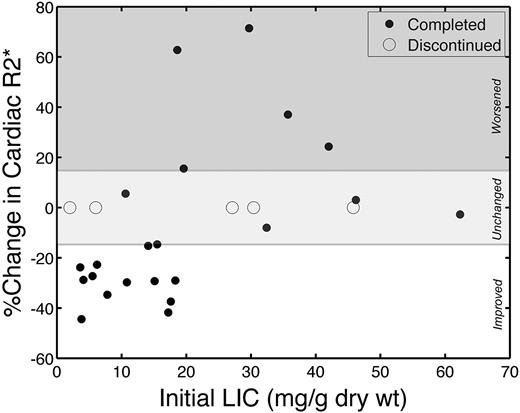
Although change in LIC appears to be a useful metric for judging cardiac response to deferasirox, it is desirable to know, a priori, whether monotherapy with deferasirox is appropriate treatment in a patient with asymptomatic cardiac iron burden. Figure 5 demonstrates the magnitude of cardiac iron (R2*) clearance as a function of initial LIC; open circles denote patients who were unable to complete the study, and mean time on trial was 155 plus or minus 90.8 days (range, 34-268 days). Ten of 11 patients in the lower 2 quartiles for LIC significantly improved their cardiac iron compared with only 3 of 11 patients with greater initial liver iron (P = .002).
Cardiac iron removal varied with initial LIC. Solid symbols represent patients who completed the study; and open symbols, patients who were discontinued. The discontinued patients had little time to change cardiac T2* values.
Cardiac iron removal varied with initial LIC. Solid symbols represent patients who completed the study; and open symbols, patients who were discontinued. The discontinued patients had little time to change cardiac T2* values.
Surprisingly, initial cardiac iron was a weak predictor of cardiac iron clearance. Initial cardiac T2* (and R2*) were not significantly different in nonresponders, nor was there a linear relationship between the change in cardiac R2* and initial cardiac R2* (P = .872). None of the 4 patients with the lowest T2* values (1.8-6 ms) improved their cardiac iron, but 3 of those patients also had LIC levels in the upper 2 quartiles. Using initial values of serum ferritin, annual transfusion burden, cardiac R2*, and LIC in multivariate linear regression, only initial LIC was retained as a significant predictor of cardiac R2* changes over 18 months.
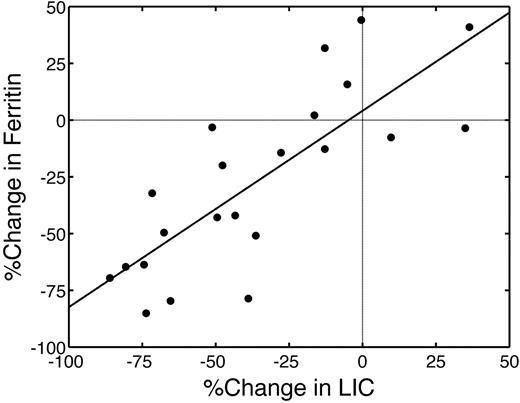
Whereas LIC was a good predictor of drug response in this trial, serum ferritin is the sole metric for monitoring deferasirox efficacy in most of the world. Thus, it is important to determine how well serum ferritin reflects liver and cardiac iron stores. Initial serum ferritin was correlated with initial LIC (r = .79; P < .001; not shown). Percentage changes in serum ferritin over 18 months were also correlated with percentage changes in LIC (r = .79; P < .001, Figure 6); 15 of 22 patients decreased both their LIC and serum ferritin over 18 months of therapy (Figure 6 lower left quadrant). Cardiac R2* changes tracked serum ferritin differences (r = 0.63, P < .002; slope = 0.52), similar to changes in LIC (not shown). Initial serum ferritin also predicted 18-month cardiac response. Ten of 11 patients with baseline ferritin values in the lower 2 quartiles had good cardiac response to deferasirox compared with 3 of 11 patients having higher serum ferritin values (P < .002).
Percentage change in serum ferritin was linearly proportional to percentage change in LIC. r = .79, P < .001. Only 15 of 22 patients simultaneously reduced their ferritin and LIC metrics.
Percentage change in serum ferritin was linearly proportional to percentage change in LIC. r = .79, P < .001. Only 15 of 22 patients simultaneously reduced their ferritin and LIC metrics.
Mean transfusional iron burden was 0.56 plus or minus 0.19 (0.35-1.11 mg/kg per day). It did not correlate with baseline iron stores or with ferritin, hepatic, or cardiac responses to therapy.
The AE profile was comparable with prior clinical trials of deferasirox. The most common serious AEs included abdominal pain (n = 4; 14.8%), fever (n = 4; 14.8%), vomiting (n = 3; 11.1%), dehydration (n = 2; 7.4%), hypotension (n = 2; 7.4%), and death (n = 2; 7.4%). Only 2 of these events (one abdominal pain and one vomiting) were attributed to study drug. A total of 183 AEs were reported, of which 42 were considered to be related to study drug (Table 2). Reasons for study withdrawal were varied. One withdrew consent before taking study medication and another because of rash 1 month into the trial. One patient was discontinued by the site investigator after 6 months of therapy because of complete, overt noncompliance with study medication. One patient was an unrecognized screen failure (LVEF 51%) who was discontinued by the site investigator after 12 months of therapy; LVEF was stable, and there were no AEs. Two patients discontinued because of serious AEs and ultimately died. The first patient enrolled with markedly elevated baseline cardiac iron (T2* = 1.8 ms), developed prerenal azotemia, and died secondarily to congestive heart failure before the 6-month time point; the patient had received deferasirox for less than 3 months. The second patient presented to a community hospital with high fever, tachycardia, hypotension, cough, and abdominal pain. The patient was admitted to the hospital and died approximately 2 months later from multiorgan failure. Sepsis was the presumptive diagnosis of the attending intensivist, but no infectious agent was identified. Cardiac ultrasound on hospital day 11 demonstrated an ejection fraction of 74%, making primary cardiac decompensation unlikely.
Discussion
In this high-risk study population (mean initial T2*, 8.6 ms), 13 of 22 (59%) patients showed significant cardiac T2* improvement over 18 months of deferasirox therapy; success rate was 48% in the ITT population. The most striking observation was the clear demarcation between responders and nonresponders. Any medication will have its share of patients who respond well or respond poorly, but determinants of that response are often complex interactions of genetic polymorphisms and patient environment, making it nearly impossible to predict responsiveness a priori. In contrast, initial liver iron level and serum ferritin were highly significant and clinically relevant predictors of cardiac response to deferasirox therapy.
The most logical explanation for this observation is that LIC and ferritin were simply surrogates for drug compliance, one of the most powerful predictors of survival.2,4 Although compliance metrics were included in the study design, incomplete documentation prevented accurate independent assessment of this hypothesis. Even with perfect compliance (monitored administration), some patients respond poorly to deferasirox because they have decreased drug bioavailability.29 Although deferasirox absorption is relatively uniform at doses of 10 and 20 mg/kg per dose, deferasirox absorption is known to be quite variable at higher doses.15 Nonetheless, decreased bioavailability cannot be invoked for nearly 50% of the study population, nor does it appear to correlate with iron load.29 The lack of hepatic efficacy (Figure 2B solid line) represents the strongest evidence that noncompliance was the major factor in the nonresponders because high-dose deferasirox successfully reduced liver iron and serum ferritin in other heavily iron overload patient cohorts.22,23,30,31
However, high initial iron burden may represent more than just a compliance surrogate. Although the cardiac nonresponders failed to improve their liver iron concentration, they did at least achieve neutral liver iron balance (at their elevated baseline levels). In contrast, their cardiac T2* values steadily worsened over time (Figure 2, right panel, dotted line), suggesting that the high iron burden placed them at increased prospective risk of cardiac iron accumulation, even with sufficient chelation to prevent worsening of hepatic iron. One possible explanation is that the high dose of deferasirox, 40 mg/kg per day in all the nonresponders, was sufficient to balance liver iron even with several missed doses per week. In this scenario, long periods of unprotected exposure to nontransferrin bound iron could have predisposed to increased cardiac iron uptake. With DFO, the number of infusions per week is the strongest marker of cardiac protection.2 Similar results might also be expected with deferasirox. In addition, the labile iron pool increases sharply at high liver iron concentration.32 Thus, the penalty for chelator noncompliance is almost certainly worse in patients with higher total body iron burdens. The relationship between liver iron and prospective cardiac risk is a complicated function of chelator, chelator history, dose, pattern of use, and compliance, but the observations in this trial are consistent with prior observations.4,32,33
Independent of the mechanism, however, this relationship may be a valuable clinical guide for tailoring chelation therapy. Deferasirox monotherapy effectively reduced cardiac iron in patients with mild to moderate LIC. However, patients having severe hepatic iron overload may warrant more aggressive therapy if the therapeutic goal is rapid cardiac iron clearance, the patient already has severe cardiac iron deposition, the patient is known to be noncompliant, or facilities for monitoring cardiac iron and cardiac function are limited or unavailable.
Cardiac iron clearance was also a powerful function of final LIC, sharply increasing as LIC approached normal levels. We noted a similar relationship in our observational longitudinal trial.34 The simplest explanation is that iron chelation with deferasirox is a competitive process, with cardiac effectiveness improving once liver iron is depleted. Regardless of the mechanism, this observation should provoke reevaluation of chelation endpoints, particularly with recent work suggesting endocrine benefits of stricter iron control.35 Increased toxicity was not observed in the 7 patients having final serum ferritin values less than 1000 (3 values < 500), but the safety of deferasirox at near-normal iron levels is an emerging question.
Clinically, these observations reinforce the need to track and control total body iron concentrations. Based on these data, it is unlikely that deferasirox will remove cardiac iron if it fails to control LIC. Some patients even failed to decrease cardiac iron despite significant improvements in LIC (up to 50% reduction). Nevertheless, the data suggest that aggressive and persistent control of somatic iron stores will improve cardiac iron. Both LIC and serum ferritin were predictive of responsiveness. This observation is critically important because serum ferritin is the only iron-monitoring tool available to most thalassemia practitioners worldwide.
Mean transfusion burden in this study was quite high (0.56 mg/kg per day), placing it well into the top quintile of thalassemia major patients,36 representing selection bias related to the presence of cardiac iron loading. Not surprisingly, the median dose at study completion was 35 mg, even accounting for patients who were dose-reduced because of serum ferritin values less than 1000. Our study was not adequately powered to observe any relationship between transfusional rate, organ iron loading, and chelator response.
Prior studies using deferiprone, either alone or in combination with DFO, were able to demonstrate LVEF improvement in patients thought to have normal cardiac function.37,38 In the present study, we were unable to demonstrate functional improvement despite lowering cardiac iron. Similar findings were recently described by Pennell et al.23 Nonetheless, preservation of cardiac function over 18 months in such a high-risk group is important, given that the prospective risk of symptomatic heart failure over a 1-year period is 50% for a T2* less than 6 ms, 30% for T2* between 6 and 8 ms, and 14% for T2* between 8 and 10 ms.26
One limitation of this study is that approximately two-thirds of patients entering the trial were already taking deferasirox, creating a potential selection bias toward patients who respond well to the drug. However, the magnitude of this effect is probably low because most U.S. patients switched to deferasirox after its regulatory approval. Prior deferasirox treatment could also partially explain the predictive ability of initial liver iron; patients who absorb deferasirox poorly would probably start the trial with a higher initial liver iron. A second limitation is that patients with heavy cardiac iron burdens but low iron stores (LIC < 2 mg Fe/g dw or serum ferritin levels of < 1000 ng/mL) were excluded; whether deferasirox can safely reduce cardiac iron in such patients remains to be determined. Finally, the small sample size and open-label design may make this study vulnerable to selection and population biases; this was partially tempered by multicenter participation and ethnically diverse populations.
In conclusion, deferasirox monotherapy using doses of between 30 and 40 mg/kg per day lowered cardiac iron in patients with moderate cardiac and liver iron burden, but not in patients with severe hepatic siderosis. Near normalization of LIC levels appeared to markedly improve cardiac iron clearance with deferasirox. One cardiac death occurred during the study in a patient with a starting T2* of 1.8 ms. Cardiac function otherwise remained stable throughout the study, despite high a priori probability of developing congestive heart failure, but no trend toward functional improvement was observed. Deferasirox had a favorable toxicity profile, even at doses of between 30 and 40 mg/mg per day and at the low LIC experienced by some patients. Taken as a whole, these data support a role for deferasirox (30-40 mg/kg per day) in the management of patients with moderate cardiac and hepatic siderosis, with normal cardiac function. More aggressive therapy is warranted for patients with severe combined hepatic and cardiac siderosis or patients with ventricular dysfunction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anne Nord, Susan Carson, Kelly Verel, Dena Fasheh, Elizabeth Evans, Jacqueline Madden, Dr Matthew Cham, and Matthew Herz for their help in patient recruitment and management; Dr Kevin Mennitt, Barbara Bettencourt, and Dr Cindy Rigsby for their assistance in MRI measurements; and Dr Ghulam Warsi for statistical assistance.
This work was supported by Novartis Pharmaceuticals Corp.
Authorship
Contribution: J.C.W. and T.D.C. designed the experiment, collected data, analyzed and interpreted the data, and wrote the manuscript; A.T., P.H., and P.G. collected data and wrote the manuscript; B.P.K., C.P., and T.G. designed the experiment, analyzed and interpreted data, and wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.C.W., T.D.C., P.H., A.T., and P.G. have received other research funding from Novartis. A.T. has received research funding from Baxter. P.G. attended a speakers' bureau. B.P.K., T.G., and C.P. are employees of the study sponsor, Novartis Pharmaceuticals Corp.
Correspondence: John C. Wood, Children's Hospital of Los Angeles, Mailstop 34, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: JWood@chla.usc.edu.