Elevated plasma levels of the angiogenic factor Ang-2 correlated with poor prognosis in a larger series of CLL patients. Ang-2 levels may reflect crosstalk between CLL cells and their microenvironment, specifically related to angiogenesis and endothelial cells, and therefore emphasize the importance of angiogenesis in CLL.
In this issue of Blood, Maffei and colleagues report on the prognostic impact of plasma levels of Angiopoietin-2 (Ang-2) in patients with chronic lymphocytic leukemia (CLL).1 They measured Ang-2 plasma levels in 316 patients and examined the relationship between Ang-2, clinical outcome, and other prognostic markers. They found that high Ang-2 predicted for a shorter time to first treatment and overall survival. Also, significant associations were found between high levels of Ang-2 and advanced clinical stage, high β2 microglobulin, unmutated status of the immunoglobulin variable gene segments (IgVH), and cytogenetic risk factors.
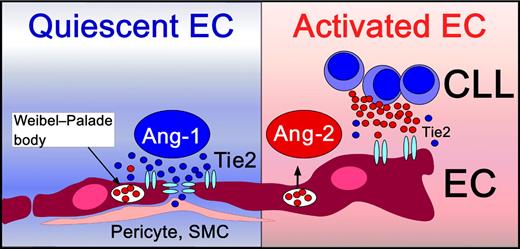
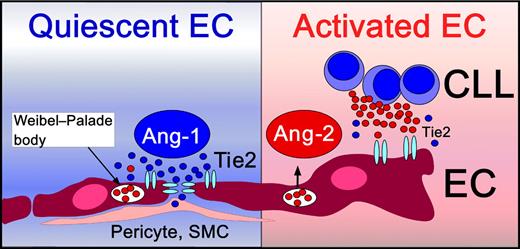
Angiopoietins are angiogenic factors essential for vascular development and maturation, and include Ang-1 and Ang-2, which both bind to the receptor tyrosine kinase Tie2, expressed on endothelial cells (ECs). Besides the well-studied vascular endothelial growth factor (VEGF) receptor system, the Tie receptors and their angiopoietin ligands constitute a second receptor tyrosine kinase system specifically regulating angiogenesis (reviewed in Augustin et al2 ). Constitutive activation of the Ang-1/Tie2 axis functions as a gatekeeper of quiescent ECs, promoting structural integrity of mature vessels, and protecting the endothelium from unwanted activation by cytokines (see figure, left box). Ang-2 is produced and stored in ECs, and becomes released after EC activation, leading to a transition from a quiescent to an activated EC phenotype (see figure). Ang-2 disrupts the Ang-1/Tie2 signaling by preventing Ang-1 from binding to Tie2, facilitating EC responsiveness to exogenous cytokines. Ang-2 is primarily produced by ECs, and its expression becomes induced by factors, such as hypoxia and VEGF.2 CLL cells also can secrete Ang-2 and VEGF and thereby promote angiogenesis in a paracrine fashion.3 This capacity of CLL and other leukemia cells to secrete angiogenic factors, and specifically Ang-2 and VEGF, indicates that CLL cells play a proactive role in modulating their microenvironment. Secretion of these factors could foster marrow neoangiogenesis and could explain the increased microvessel density seen in marrows from CLL patients.4
Angiogenesis, the development of new blood vessels from preexisting vessels, has long been accepted as a critical factor for progression of solid tumors, where vascular supply needs to keep up with tumor growth. The role of angiogenic factors in hematopoietic cancers has been more controversial from a conceptual standpoint, given that leukemia cells in the marrow presumably have a less restricted access to blood and its nutrients. However, the recent emphasis on the microenvironment's influence in leukemia progression and drug resistance is also nurturing the interest in angiogenesis and in interactions between leukemia and endothelial cells.5 Normal marrow hematopoiesis requires intimate contact of hematopoietic elements with a heterogeneous population of adherent cells collectively referred to as “stroma.” These accessory feeder cells form specialized “niches” that are close to the marrow vasculature (vascular niche) or to the endosteum (osteoblast niche). Leukemia cells usurp these niches, causing hematopoietic dysfunctions.6 Intimate contact between leukemia cells and ECs in marrow niches and the increased microvessel density in marrows infiltrated by CLL cells4 support the concept of crosstalk between CLL and ECs via the VEGF and the Ang-2/Tie2 axes, and indicate that such interactions are an integral part of the marrow micro-environment.
Quiescent endothelial cells (ECs) are in intimate contact with pericytes and smooth muscle cells (SMCs). Vascular stability and integrity is maintained by constitutive Ang-1/Tie2 signaling (left side). Ang-1 clusters Tie2 receptors at endothelial cell junctions.2 Upon activation, ECs normally release their endogenously stored Ang-2 pools from Weibel-Palade bodies. Ang-2 activates ECs by competition with the homeostatic Ang-1 for binding to their common EC receptor, Tie2. CLL cells also can release Ang-2 and thereby induce EC activation and angiogenesis.
Quiescent endothelial cells (ECs) are in intimate contact with pericytes and smooth muscle cells (SMCs). Vascular stability and integrity is maintained by constitutive Ang-1/Tie2 signaling (left side). Ang-1 clusters Tie2 receptors at endothelial cell junctions.2 Upon activation, ECs normally release their endogenously stored Ang-2 pools from Weibel-Palade bodies. Ang-2 activates ECs by competition with the homeostatic Ang-1 for binding to their common EC receptor, Tie2. CLL cells also can release Ang-2 and thereby induce EC activation and angiogenesis.
Besides the fascinating insights into the role of Ang-2 in CLL pathophysiology, Maffei et al emphasize that Ang-2 is an independent prognostic marker and could potentially become clinically useful for risk assessment. The large number of other prognostic factors in CLL, and the fact that none of these established prognostic markers has yet changed our clinical practice (observation in early disease stages, treatment in advanced stages) raise the question whether and why we need yet another prognostic marker in CLL. Early intervention studies in CLL patients have not been beneficial in the past, and a trial by the German CLL study group (CLL7) in which high-risk patients are randomized to receive FCR chemo-immunotherapy either at an early stage or at an advanced stage is ongoing, with data from the trial not yet available. Several other angiogenic factors, such as VEGF, basic fibroblast growth factor (bFGF), and Ang-2 in smaller series have previously been identified as prognostic factors in CLL, but never became widely used. Possible overlap with other angiogenesis-related markers and unique features of Ang-2 needs to be better defined in future studies to establish a solid rationale for measuring Ang-2 in CLL. On a more technical note: the cut-off for Ang-2 of 2459 pg/mL to distinguish high- versus low-risk patients seems impractical and should be rounded for future studies. Overall, Ang-2 is likely to be primarily used in correlative research studies in the near future. However, this may change once we better understand the source(s) and regulation of Ang-2 expression in CLL and/or once we have drugs available to therapeutically target this axis.
What makes Ang-2 different from other prognostic markers is the fact that Ang-2 is related to angiogenesis and probably reflects complex interactions between CLL cells and their microenvironment. In contrast, the most prominent other prognostic markers in CLL, the IgVH mutation status, cytogenetic abnormalities, and expression of CD38 and zeta-associated protein of 70-kD (ZAP-70), use intrinsic features of the leukemia cells as prognostic indicators. This raises question about Ang-2 regulation and the source(s) of elevated Ang-2 levels in patients with higher-risk CLL. The authors state that their prior work3 and the immunohistochemistry data in this paper1 suggest that CLL cells are the principal source of elevated Ang-2 levels in CLL patients. Surprisingly and in contrast to patients with multiple myeloma, the authors did not find any correlation between the degree of marrow infiltration and Ang-2 levels, nor did they find any changes of Ang-2 levels in patients undergoing treatment, indicating that the disease burden may have no impact on Ang-2 levels. Studies about the regulation of Ang-2 expression by CLL cells, for example, the effect of coculture of CLL cells with endothelium on Ang-2 expression, may help to clarify this important issue. As such, this paper encourages more exploration of function and significance of angiogenic factors in CLL. Such studies will help to determine whether or not Ang-2 will play in the premier league of prognostic markers in CLL.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■