Recent developments in the management of chronic lymphocytic leukemia (CLL) patients have made necessary the availability of dependable prognostic factors. We have developed a prognostic index derived from the multivariate analysis of 339 stage A patients at diagnosis, exhaustively studied for classical and recent predictive markers. Only 4 biologic parameters were found to be independent predictors of progression-free survival (PFS): serum thymidine kinase (sTK), lymphocytosis, β2-microglobulin, and CD38 expression. Two groups were distinguishable: cases with no or 1 risk factor (among whom 85% did not progress after 7 years), and cases with 2 or more factors showing a median PFS of 20 months. Finally, we propose an easy, fast, cost-effective strategy for a trustworthy prognostication in stage A patients, who currently represent more than 80% of the CLL population, allowing physicians to adapt follow-up individually.
Introduction
Within the past decade, treatment options in chronic lymphocytic leukemia (CLL) have moved from being palliative to potentially curative. The clinical staging systems developed by Rai et al1 and Binet et al2 define early-, intermediate-, and advanced-disease stages. Nonetheless, as substantial variability in the course of the disease is observed among patients at each stage, particularly stage A,3 there has been a sustained effort to identify reliable prognostic factors in CLL. However, the identification of high-risk (or low-risk) patients remains challenging.
Currently, up to 80% of newly diagnosed patients present with Binet stage A.4 Among them, identifying patients at high risk of progression and precisely evaluating time to progression are fundamental needs for both patients and physicians. For patients with indolent disease, number of follow-up visits may be reduced. Therefore, clinicians in charge of CLL patients would benefit from a simple prognostic index similar to the International Prognostic Index5 for aggressive non-Hodgkin lymphomas.
We exhaustively studied a cohort of 339 stage A CLL patients at diagnosis and determined both typical and specialized prognostic factors. The multivariate analysis allowed us to propose a prognostic score for an easy, fast, and effective assessment of the risk of progression in Binet stage A patients.
Methods
Patients
Over a period of 8 years, 339 patients diagnosed with CLL in 3 university hospitals were enrolled in the study. All cases were CD5+CD23+ and had an RMH-Matutes score ≥ 4. Patients were followed up from diagnosis until progression to Binet stage B/C. This study was approved by the institutional review board of Hôpital Avicenne.
IGHV gene mutational status
Cytogenetics/targeted chromosome alterations
The 4 relevant chromosomal abnormalities—del13q, trisomy12, del11q, and del17p—were investigated by interphasic fluorescence in situ hybridization (FISH) analysis.
sTK
Serum thymidine kinase (sTK) was investigated using a Profiligen TK-Rea kit (Diasorin).
CD38 expression by flow cytometry
CD38 expression was measured on fresh cells at diagnosis using PE-conjugated anti-CD38 antibody (Becton Dickinson). The results are reported as the percentage of CD19+CD38+ cells, with a threshold of 7%.
ZAP-70 expression
ZAP-70 expression was determined according to published recommendations8 using indirect staining with anti–ZAP-70 2F3.2 monoclonal antibody (mAb; Upstate Laboratories). The results are expressed both as percentage9 and as mean fluorescence intensity (MFI) ratio according to ZAP-70 expression in T cells.10
End points and statistical analysis
Progression-free survival (PFS) was defined as the interval from diagnosis to progression to stage B or C. The observation time was censored at the last follow-up date if no events occurred. Follow-up was updated to January 2010. Survival curves at this time were plotted using the Kaplan-Meier method, and comparisons were based on the 2-sided log-rank test. All tests were 2-sided, with a significance level of 0.05.
Results and discussion
Many published studies have been conducted either without considering simultaneously both routine and more recent prognostic factors,11,–13 or on cohorts including all stages of CLL, making the interpretation of disease progression difficult.14,,–17
In our cohort, median age was 64.8 years (range, 29-87 years), with a sex ratio of 1.5. Median follow-up time was 53.8 months (range 7.5-255 months). The median lymphocyte count was 13 × 103/mm3 (minimum [min]: 4; maximum [max]: 223). Our cohort included clinical Monoclonal B-Lymphocyrosis (MBL) patients18 (n = 55) to permit comparison with published prognostic studies, as none have excluded them. A sTK level > 10 UI/L was observed in 29% of the patients. Median β2-microglobulin was 2.4 (min: 0.9; max: 11.2). IGHV genes were found unmutated in 30% of the patients. ZAP-70 overexpression was observed in 34% of the cases, CD38 expression in 32%. FISH analysis revealed the expected frequency of the 4 abnormalities in stage A cases: < 5% 17p deletion, 6.5% 11q deletion, 11% trisomy 12, and 56% 13q deletion (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Median PFS was 112 months. The univariate analysis confirmed the prognostic importance of most of the biologic parameters (supplemental Table 2), that is, lymphocytosis (P < 10−4), CD38 expression (P < 10−4), β2-microglobulin (P < 10−4), sTK level (P < 10−4), mutational status (P < 10−4), ZAP-70 expression (P < 10−4), presence of an 11q deletion (P < 10−4), trisomy 12 (P = .01), and 17p deletion (P = .01). Only age, sex, and presence of a 13q deletion were not predictive of PFS.
Although ZAP-70 expression, IGHV mutational status, and FISH analysis were significant in univariate analysis, only 4 factors were found to be independent predictors of the PFS by multivariate analysis: sTK > 10 U (hazard ratio [HR] = 2.98; P < 10−4), lymphocytosis > 13 G/L (HR = 1.74; P = .01), β2-micro-globulin > 2.5 mg/L (HR = 3.17; P < 10−4), and CD38 > 7% (HR = 1.86, P = .01; Table 1). Internal validation was implemented using bootstrapping.19 The prominence of factors related to proliferation is in line with the key role of BCR signaling capacity and microenvironment in disease progression.6,20
As the 4 prognostic factors had independent prognostic weight, we combined these unfavorable factors. The cohort was split into 5 groups according to the number of unfavorable prognostic factors they exhibited. Those with 0, 1, 2, 3, and 4 risk factors accounted for 24%, 29%, 22%, 13% and 12% of the cohort, respectively. The PFS of these groups at 48 months were 95%, 83%, 57%, 14%, and 6%, respectively (supplemental Figure 1).
When considering the cases with 0 to 1 risk factors (group A) and comparing them to cases with 2, 3, and 4 risk factors (group B), we observed that > 85% of the group A patients had not progressed at 7 years, whereas the median PFS was reached at ∼ 20 months in group B (P < 10−4; Figure 1).
This discrimination is remarkably powerful compared with the ability of other prognostic factors, such as IGHV mutational status, to predict the rapidity of progression among stage A patients. Discrimination remains significant among mutated (P < .0001) or unmutated (P = .00179) cases (supplemental Figure 2). Even if most patients with unmutated IGHV genes are prone to ultimately progress, the median time to progression was 29.1 months in our cohort, in accordance with published data.21
Nonetheless, although the combination of these simple biomarkers accurately predicted PFS or treatment-free survival (TFS) at diagnosis, it did not allow us to draw any conclusion on their ability to predict survival or response to treatment. Moreover, it does not preclude the importance of detecting 17p deletion before therapy or the significance of more sophisticated prognostic factors such as ZAP-70 expression or mutational status in predicting response to therapy or overall survival.
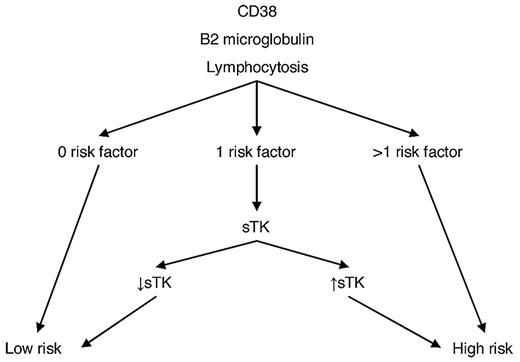
Therefore, we propose a cost-effective strategy for prognostication in stage A patients at diagnosis (Figure 2). Determination of lymphocyte count, β2-microglobulin and CD38 expression may be easily performed on a routine basis at diagnosis. sTK is less readily available, but according to our model, its determination may be restricted to the ∼ 29% of patients with only 1 of the other 3 risk factors.
In conclusion, these results may be translated into a practical approach for all stage A CLL patients at diagnosis, who cur-rently represent > 80% of the CLL population. The stratification into 2 groups allows physicians to adapt patient care individually. The number of visits of low-risk patients may be reduced and their follow-up shared with general practitioner. Conversely, high-risk patients are eligible for closer monitoring in reference centers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank R. Porcher, Inserm U717, Hôpital Saint Louis, for statistical advice.
Authorship
Contribution: R.L., V.E., F.B.-M., D.V., D.N., O.S., F.N., and F.D. performed research; H.M.-B., X.T., and F.A.-C. provided patient samples and clinical data; V.L. and S.K. performed statistical analysis; and R.L., V.L., and F.C. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Florence Ajchenbaum-Cymbalista, Laboratoire d'Hématologie, Hôpital Avicenne, 125, rue de Stalingrad, 93009 Bobigny, Cedex, France; e-mail: florence.cymbalista@avc.aphp.fr.