Abstract
Abstract 1910
The outcome of multiple myeloma (MM) patients has markedly improved in the last decade. Thus, overall response rates between 85%-95%, with 30%-50% complete remission (CR) rates are now being reported in young patients treated with novel agents plus high-dose therapy/autologous stem cell transplantation (HDT/ASCT). A similar scenario is also emerging in the elderly (non-transplant candidates) population. Accordingly, more sensitive techniques are needed to assess patients’ response; these may contribute to compare the efficacy of different treatment schemas, to monitor minimal residual disease (MRD) and for prognostication.
In the present study we have assessed the frequency and the prognostic value of IR by multiparameter flow cytometry in a total of 516 newly diagnosed MM patients included in three consecutive PETHEMA/GEM Spanish trials: two designed for transplant candidate patients - GEM 2000 (n=157) and GEM2005<65y (n=206) - and one for elderly patients - GEM2005>65y (n=153). The GEM2000 trial was based on 6 induction cycles of VBMCP/VBAD followed by HDT/ASCT; the GEM2005<65y included three arms with 6 cycles each (Thalidomide/Dexamethasone -TD-, Bortezomib/Thalidomide/Dexamethasone -VTD- and, VBMCP/VBAD with Bortezomib in the two final cycles -VBMCP/VBAD/Bortezomib) followed by HDT/ASCT; and the GEM2005>65y compared 6 cycles of Bortezomib/Melphalan/Prednisone -VMP- vs. Bortezomib/Thalidomide/Prednisone -VTP-. All three trials had in common that patients received 6 induction cycles and IR was evaluated at this time point. In addition, IR was assessed on day +100 after HDT/ASCT in the first two trials. Patients were defined to be in IR when myelomatous plasma cells (MM-PCs) were undetectable by MFC or when less than one phenotypically aberrant PC was detected among 104 cells analyzed. Patients were referred for MRD studies if they were mainly in CR or VGPR. The IR rates reported here were calculated on intention to treat analysis.
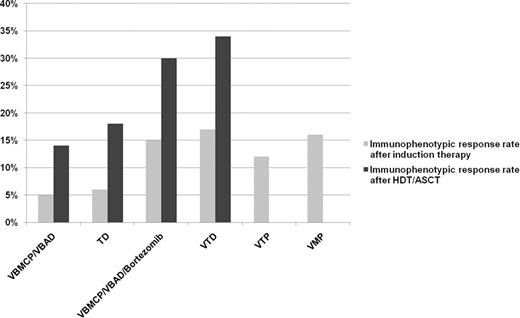
Figure 1 summarizes the IR rates after induction. The lowest IR rates corresponded to the VBMCP/VBAD and TD schemes (5% and 6%, respectively) while with the bortezomib-based regimens an approximately 3-fold increment in the IR rates was observed: VTP (12%), VBMCP/VBAD/Bortezomib (15%), VMP (16%) and VTD (17%). After HDT/ASCT, IR rates were found to be significantly increased (p<.001) in the GEM2000 protocol (14%) and in all arms of the GEM2005<65y trial: TD (18%), VBMCP/VBAD/Bortezomib (30%) and VTD (34%). Thus, a minimum 2-fold increment of IR rates was further achieved after HDT/ASCT. In addition, IR rates achieved after HDT/ASCT in patients included in all three arms of the GEM2005<65y trial were significantly superior (p≤.008) to cases treated according to the GEM2000 protocol, indicating that induction regimens with novel agents improved post-transplantation rates of IR. Moreover, bortezomib-based regimens vs. TD were associated with increased IR rates not only before but also after HDT/ACSCT (p=.06 and p=.02 for VBMCP/VBAD/Bortezomib and VTD, respectively).
We further compared the impact of achieving an IR after induction and at day+100 after HDT/ASCT in the progression-free (PFS) and overall survival (OS) within the three protocols. Patients in IR status after an induction regimen according to the GEM2000, GEM2005<65y and GEM2005>65y protocols showed significantly longer (p<.001) 3-year PFS rates (100%, 100% and 90%, respectively) compared to patients in a no-IR status (61%, 59% and 35%, respectively). Similarly, 3-year OS rates were significantly longer (p=.01) in IR vs. no-IR patients status (100%, 100% and 94% vs. 84%, 90% and 76% for the GEM2000, GEM2005<65y and GEM05>65y protocols, respectively). Likewise, an IR vs. no-IR status after HDT/ASCT in both the GEM2000 and GEM05<65y trials was also associated with significantly increased 3-year PFS (p<.001) and OS (p=.007) rates.
In summary, this study demonstrates that the achievement of an IR is a strong prognostic factor regardless of the type of treatment; thus, higher IR rates may help to identify optimal therapeutical schemes. In this sense, HDT/ASCT is able to markedly increase IR rates after induction even in the era of novel agents, and this translates into extended survival.
Off Label Use: VTP is not approved for the treatment of newly diagnosed myeloma patients and VT and VP are not approved for maintenance therapy. None of the combinations proposed, VBCMP/VBAD plus bortezomib, VT and VTD are approved as induction therapy in newly diagnosed myeloma patients. Mateos:Janssen Cilag: Honoraria; Celgene: Honoraria. Rosiñol:Janssen-Cilag: Honoraria; Celgene: Honoraria. Cibeira:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oriol:Janssen-Cilag: Honoraria; Celgene: Honoraria. de Arriba:Janssen-Cilag: Honoraria; Celgene: Honoraria. Palomera:Janssen Cilag: Honoraria. De La Rubia:Janssen-Cilag: Honoraria; Celgene: Honoraria. Díaz-Mediavilla:Janssen-Cilag: Honoraria; Celgene: Honoraria. Garcia-Laraña:Janssen Cilag: Honoraria; Celgene: Honoraria. Sureda:Janssen-Cilag: Honoraria; Celgene: Honoraria. Alegre:Janssen-Cilag: Honoraria; Celgene: Honoraria. Blade:Janssen cilag: Honoraria; Celgene: Honoraria. Lahuerta:Janssen-Cilag: Honoraria; Celgene: Honoraria. San Miguel:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.