Abstract
The molecular basis of lymphangiogenesis remains incompletely characterized. Here, we document a novel role for the PDZ domain-containing scaffold protein synectin in lymphangiogenesis using genetic studies in zebrafish and tadpoles. In zebrafish, the thoracic duct arises from parachordal lymphangioblast cells, which in turn derive from secondary lymphangiogenic sprouts from the posterior cardinal vein. Morpholino knockdown of synectin in zebrafish impaired formation of the thoracic duct, due to selective defects in lymphangiogenic but not angiogenic sprouting. Synectin genetically interacted with Vegfr3 and neuropilin-2a in regulating lymphangiogenesis. Silencing of synectin in tadpoles caused lymphatic defects due to an underdevelopment and impaired migration of Prox-1+ lymphatic endothelial cells. Molecular analysis further revealed that synectin regulated Sox18-induced expression of Prox-1 and vascular endothelial growth factor C–induced migration of lymphatic endothelial cells in vitro. These findings reveal a novel role for synectin in lymphatic development.
Introduction
The lymphatic vasculature regulates interstitial fluid homeostasis, fat resorption, immune defense, inflammation and cancer metastasis. The molecular basis of the lymphatic development remains incompletely understood.1 Lymphatic endothelial cells (LECs) derive from venous blood endothelial cells (BECs).2-5 Sox186 and Prox-1,2-5 as well as miRNAs,7 play a key role in this process. Intriguingly, despite their venous origin, formation of lymphatics relies in part on signals that participate in arterial development. For instance, the forkhead transcription factors Foxc1/2 are required for arterial specification and sprouting of LECs.8,9 In addition, the PDZ interaction site in EphrinB2, itself a marker of arterial endothelial cells (ECs), is essential for lymphatic development.10 Similarly, Dll4/Notch signaling regulates arterial and lymphatic development.11
Recently, we identified the PDZ domain–containing scaffold protein synectin (GIPC1) as a regulator of arterial but not venous growth,12 at least in part through control of Vegfa signaling.13 Prompted by the finding that similar factors control arterial and lymphatic development, we studied whether synectin also regulated lymphatic development. Although PDZ domain–dependent signaling is crucial for lymphatic development,10 a role for synectin in lymphatic development has not been documented yet. We therefore explored a role of synectin in this process using zebrafish and Xenopus tadpole models.
Methods
Zebrafish analysis
Fli1:eGFPy1,14 Gata-1:DsRed15 zebrafish and PLCγy1016 zebrafish were maintained under standard conditions. All morpholinos were previously reported and purchased from Gene Tools (supplemental Table 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Different doses of morpholinos were injected into single- to 4-cell stage embryos, as previously described.12 Phenotyping is described in supplemental material. All animal experimentation was approved by the Katholieke Universiteit Leuven institutional ethical committee.
Xenopus analysis
The generation and characterization of transgenic Flk1:eGFP Xenopus laevis frogs will be reported elsewhere (manuscript in preparation). Eggs were obtained by natural mating of hormonally induced Flk1:eGFP females and wild-type males and injected with different doses of synectin-specific or control morpholinos (Gene Tools, supplemental Table 1) into the 2-cell stage.17 Nonoverlapping translational blocking synectin morpholinos were designed based on published GenBank Xenopus laevis sequences (NM_001088594) and morpholino efficiency was tested using an in vitro luciferase reporter assay17 and immunoblotting (supplemental Methods). Tadpoles were kept in tadpole growth medium (0.1 × MMR) at 18°C until gastrulation was completed and from then on at 22°C.17 Phenotyping is described in supplemental Methods.
Immunohistochemistry and in situ hybridization
Fli1:eGFPy1 zebrafish embryos were fixed overnight in 4% paraformaldehyde/PBS at 4°C and processed for paraffin- or cryosectioning. Sections (7 μm) were stained using a polyclonal chicken anti–human synectin antibody (Abcam) and a rabbit anti-GFP antibody (Torrey-Pines Biolabs), which were then detected using biotinylated anti-chicken (Abcam) and Alexa Fluor 488–conjugated anti-rabbit secondary antibodies, respectively. Sections were mounted with Vectashield DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories).
For Xenopus in situ hybridizations, embryos were fixed in Memfa fixative and whole-mount in situ hybridization using antisense probes for Prox-117 and synectin (primer sequence, supplemental Table 1) was performed as described.17 Analysis of Prox-1–stained areas in the tadpole tail was performed as reported (supplemental Methods).17 For zebrafish whole-mount in situ hybridization, dechorionated embryos were fixed overnight in 4% paraformaldehyde at 4°C. In situ hybridization was performed as described,12 using antisense probes for EphrinB2a,12 Gridlock (Grl),18 EphB4,19 Flt4,12 and Tie-2.20 Both sections and whole-mounts were visualized on a Zeiss Axioplan 2 imaging microscope.
Cell culture experiments
Human microvascular lung endothelial cells (HMVEC-Lly; Lonza, Invitrogen) and adult dermal LECs (HMVEC-dLyAd; Lonza, Invitrogen), telomerase transfected dermal LECs (hTERT-HDLECs21 ), immortalized (i) LECs, and human umbilical vein ECs (Lonza, Invitrogen) were grown in endothelial growth medium (EGM)-2–MV medium (Lonza, Invitrogen) at 37°C. Human dermal LEC (HDLEC) cells were obtained from Promocell and cultured in endothelial medium provided by the supplier. Synectin expression was evaluated by quantitative reverse-transcription polymerase chain reaction (qRT-PCR; supplemental Table 1 primer sequences). The LEC spheroid and Sox18-mediated lymphatic reprogramming assays are described in supplemental Methods.
Statistical analysis
Absolute values were used to calculate mean ± SEM. Significance levels were calculated by unpaired Student t test, univariate, or multivariate analysis with the treatment groups as fixed factor and experiment as covariate. To determine the penetrance of the zebrafish and tadpole phenotypes, we counted the number of embryos, exhibiting the (different severities of) morphant phenotype. We used χ2 analysis to determine whether the severity distribution differed between treatment groups.
Results
Synectin is expressed in LECs in zebrafish
Because the expression of synectin in lymphatics has not been documented yet, we analyzed its expression pattern during lymphangiogenesis in zebrafish embryos. For reasons of clarity, we will briefly explain first how lymphatics develop in zebrafish (supplemental Figure 1).11,20,22-25 The thoracic duct (TD) is the first perfused lymphatic, located between the dorsal aorta (DA) and posterior cardinal vein (PCV). TD development initiates between 30 and 50 hours postfertilization (hpf) when secondary sprouts branch off dorsally from the PCV. Half of these sprouts are angiogenic, as they connect to primary (arterial) intersomitic vessels (aISVs), which thereby become venous ISVs (vISVs). The other half of these secondary sprouts are lymphangiogenic, as they migrate dorsally to the horizontal myoseptum, where they form (by 36-60 hpf) a transient, nonlumenized string of parachordal lymphangioblast (PL) cells, termed so because they are precursors of LECs forming the TD. Beyond 60 hpf, PL cells navigate ventrally and dorsally alongside aISVs, with ventral sprouts migrating to their location between the DA and PCV to fuse and establish the TD (3–6 days postfertilization [dpf]).
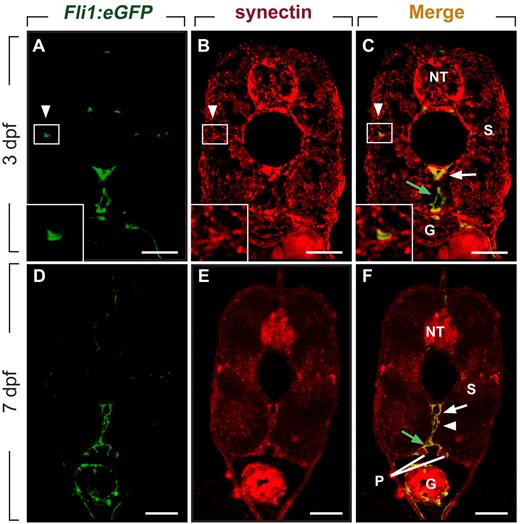
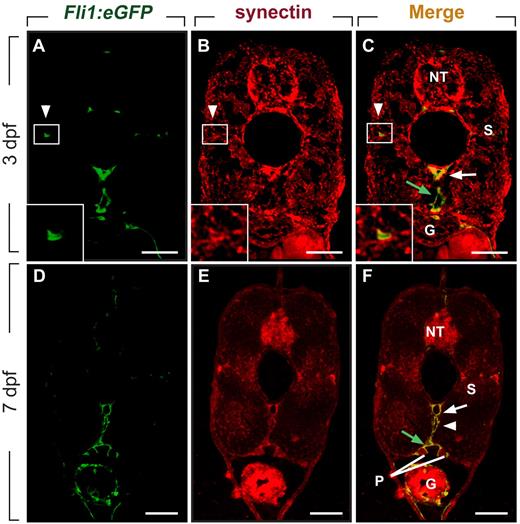
We and others previously reported that synectin is expressed in the PCV when secondary lymphangiogenic sprouts form,12,26 but did not characterize synectin expression in lymphatic vessels. When using Fli1:eGFPy1 embryos, which express enhanced green fluorescent protein (eGFP) in blood and lymph vessels,14,25 we found that synectin was expressed in the DA, PCV, PL cells, and TD (Figure 1A-F) by double immunostaining for GFP and synectin in 3- and 7-dpf embryos. Furthermore, in agreement with previous findings,12,26 synectin was detectable in the head (not shown) and in the neural tube, pronephric duct, somites, and gut (Figure 1A-F). Widespread expression of synectin has been observed in human, mouse, and Xenopus tissues.27-29 Of note, synectin was also detectable in primary and immortalized human LECs (supplemental Figure 2), consistent with previous observations.30
Synectin is expressed in lymphatic vessels in zebrafish embryos. Scale bars represent 10 μm in panels A-F. In all panels, the dorsal side of the embryo is at the top of the figure. G indicates gut; NT, neural tube; P, pronephric duct; and S, somite. (A-C) Transverse sections through the trunk of a 3-dpf wild-type Fli1:eGFPy1 zebrafish embryo, stained by immunohistochemistry using a polyclonal anti–human synectin antibody, and by DAPI nuclear stain, revealing prominent synectin expression in the NT, G, S, dorsal aorta (DA, white arrow), posterior cardinal vein (PCV, green arrow) and parachordal lymphangioblast (PL) cells (white arrowhead). Insets show magnification of the boxed areas (PL cells) in each panel. (D-F) Using the same staining procedure on transverse sections through the rostral trunk of a 7 dpf wild-type Fli1:eGFPy1 zebrafish embryo, similar widespread synectin expression in the NT, G, S, P, DA (white arrow), PCV (green arrow), and thoracic duct (white arrowhead) was observed.
Synectin is expressed in lymphatic vessels in zebrafish embryos. Scale bars represent 10 μm in panels A-F. In all panels, the dorsal side of the embryo is at the top of the figure. G indicates gut; NT, neural tube; P, pronephric duct; and S, somite. (A-C) Transverse sections through the trunk of a 3-dpf wild-type Fli1:eGFPy1 zebrafish embryo, stained by immunohistochemistry using a polyclonal anti–human synectin antibody, and by DAPI nuclear stain, revealing prominent synectin expression in the NT, G, S, dorsal aorta (DA, white arrow), posterior cardinal vein (PCV, green arrow) and parachordal lymphangioblast (PL) cells (white arrowhead). Insets show magnification of the boxed areas (PL cells) in each panel. (D-F) Using the same staining procedure on transverse sections through the rostral trunk of a 7 dpf wild-type Fli1:eGFPy1 zebrafish embryo, similar widespread synectin expression in the NT, G, S, P, DA (white arrow), PCV (green arrow), and thoracic duct (white arrowhead) was observed.
Incomplete silencing of synectin in zebrafish does not affect angiogenesis
To study the role of synectin in lymphangiogenesis, we silenced its expression (synectinKD) in Fli1:eGFPy1 zebrafish embryos using previously characterized SynATG1 and SynATG2 morpholinos.12 As synectin regulates angiogenesis, we first determined a range of submaximal morpholino doses, which only minimally affected blood vessel development, to avoid that angiogenic defects secondarily caused lymphatic defects. At a dose of 9 ng SynATG1 and 6 ng SynATG2 per embryo, only 7% of synectinKD embryos displayed subtle vascular defects, including a slightly thinner DA and a few malformed ISVs (Figure 2A-B and supplemental Table 2). The remainder of the blood vasculature in the head, trunk, tail and subintestinal vessels developed normally, had a normal size and shape, and exhibited comparable branching and density (supplemental Figure 3). Blood flow in large axial vessels and ISVs was normal in 2-dpf synectinKDGata-1:DsRed embryos, harboring DsRed+ erythrocytes15 (Figure 2C-D). Furthermore, arterio-venous differentiation of large axial vessels and primary ISVs was normal in synectinKD embryos, as evidenced by in situ hybridization for arterial and venous markers at 28 hpf (Figure 2E-L). Only morphant embryos with normal trunk circulation and body size and without developmental delay, tissue malformations, edema, or toxic signs were analyzed. (We refer to synectinKD embryos without mentioning that silencing was incomplete.)
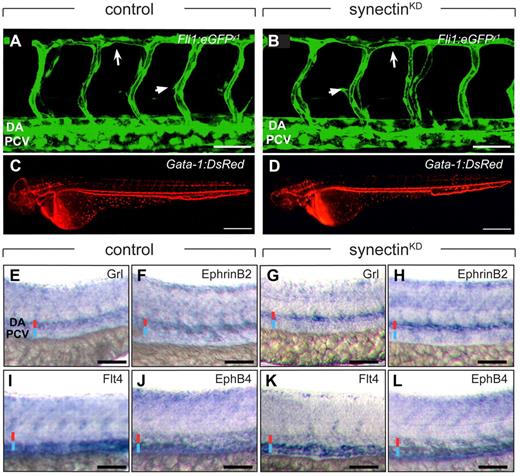
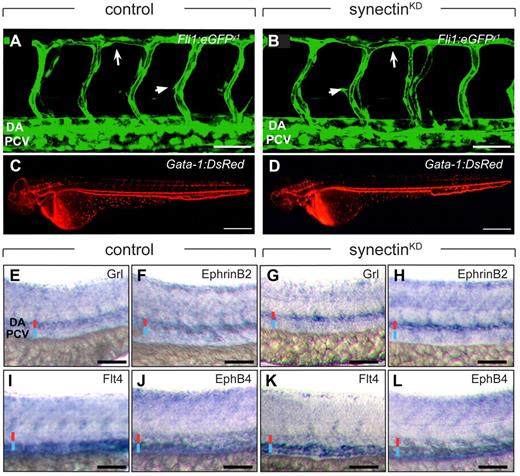
Normal blood vessel development in synectinKD embryos. In all panels, head faces left and dorsal is up. Scale bars represent 50 μm in panels A-B and E-L, and 150 μm in panels C-D. DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; and PCV, posterior cardinal vein. (A-B) Confocal side views of the trunk of 48-hpf control (A) and synectinKD (B) Fli1:eGFPy1 zebrafish embryos, showing normal vascular morphogenesis in the morphant embryo. Large arrow indicates the DLAV while the small arrow indicates an intersegmental vessel. (C-D) Macroscopic side views of 48-hpf control (C) and synectinKD (D) Gata-1:DsRed embryos expressing DsRed specifically in erythrocytes. SynectinKD embryos show a normal distribution of erythrocytes throughout the body, indicating a fully functional circulation. (E-L) Bright-field side views of the trunk of 28-hpf control (E-F,I-J) and synectinKD (G-H,K-L) embryos stained by whole-mount in situ hybridization for a panel of arterial (Grl, E,G; EphrinB2a, F,H) and venous markers (Flt4, I,K; EphB4, J,L). No difference in expression of any of these markers could be observed in the synectinKD embryos, indicating that arterio-venous differentiation had occurred normally in these embryos. Red and blue lines denote DA and PCV, respectively.
Normal blood vessel development in synectinKD embryos. In all panels, head faces left and dorsal is up. Scale bars represent 50 μm in panels A-B and E-L, and 150 μm in panels C-D. DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; and PCV, posterior cardinal vein. (A-B) Confocal side views of the trunk of 48-hpf control (A) and synectinKD (B) Fli1:eGFPy1 zebrafish embryos, showing normal vascular morphogenesis in the morphant embryo. Large arrow indicates the DLAV while the small arrow indicates an intersegmental vessel. (C-D) Macroscopic side views of 48-hpf control (C) and synectinKD (D) Gata-1:DsRed embryos expressing DsRed specifically in erythrocytes. SynectinKD embryos show a normal distribution of erythrocytes throughout the body, indicating a fully functional circulation. (E-L) Bright-field side views of the trunk of 28-hpf control (E-F,I-J) and synectinKD (G-H,K-L) embryos stained by whole-mount in situ hybridization for a panel of arterial (Grl, E,G; EphrinB2a, F,H) and venous markers (Flt4, I,K; EphB4, J,L). No difference in expression of any of these markers could be observed in the synectinKD embryos, indicating that arterio-venous differentiation had occurred normally in these embryos. Red and blue lines denote DA and PCV, respectively.
Silencing of synectin in zebrafish impairs TD formation
We studied TD formation by measuring its length at 7 dpf, when this vessel was completely formed in control embryos, using previous methods.11 Briefly, the TD length was measured in 10 somites and expressed as percentage of this trunk fragment (supplemental Figure 4).11 Because the penetrance of the lymphatic phenotype was variable (supplemental Note 1), we counted the fraction of embryos with severe, intermediate, or subtle lymphatic defects. For SynATG1 and SynATG2, the severity and penetrance of the knockdown phenotypes were dose-dependent (supplemental Tables 3–4); for reasons of brevity, only the most penetrant phenotype is shown (9 ng of SynATG1). In addition, lymphatic development in zebrafish occurs in a metameric pattern, since lymphangiogenic sprouts, and hence the PL and TD, form, statistically, at every second unilateral somite segment, giving rise to discontinuous TD fragments that fuse with each other to establish a continuous perfused TD (supplemental Figure 1).11
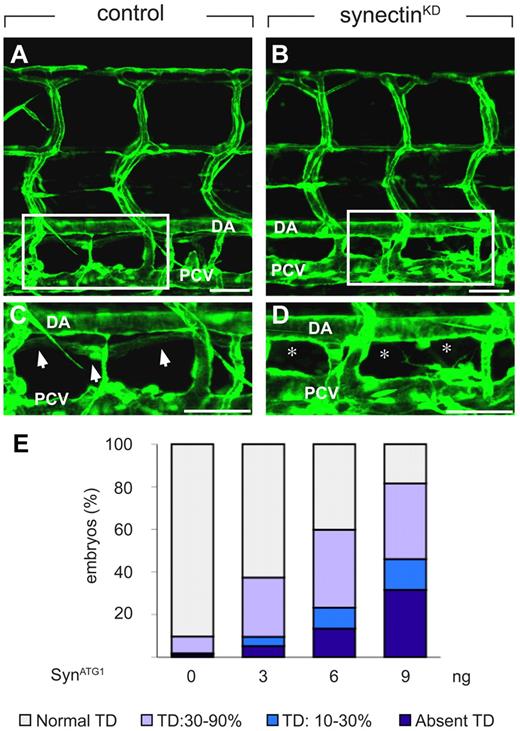
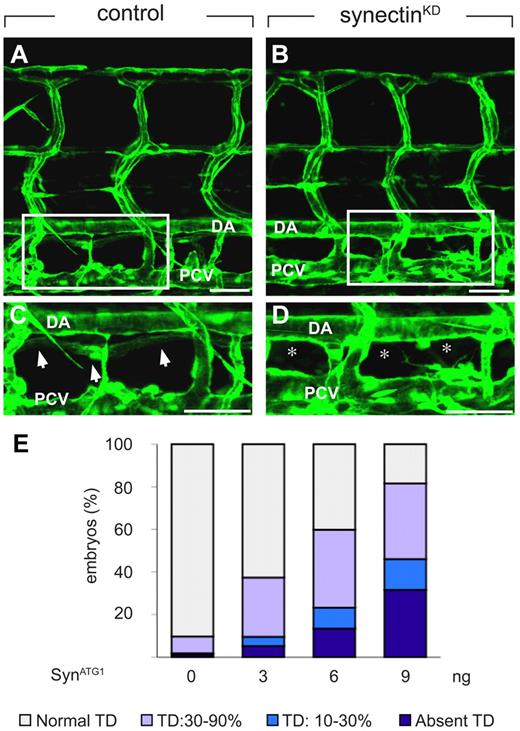
In control embryos, injected with a 5-base-pair mismatch morpholino (SynCtr), the TD developed as a continuous lymphatic by 7 dpf (Figure 3A,C,E and supplemental Table 3). In contrast, 32% of synectinKD embryos completely lacked a TD by 7 dpf (“severe” defects; Figure 3B,D-E and supplemental Table 3). Follow-up studies further revealed that TD development was not rescued at 10 dpf (supplemental Figure 3E-F), indicating that lymphatic formation was aborted and not merely delayed in these synectinKD embryos. In another 14% of morphants, TD rudiments developed over only 1 to 3 somites into an incomplete string of disconnected segments (“intermediate” defects; Figure 3E and supplemental Table 3). Incomplete TD formation over 30%-90% of its normal length was further observed in 36% of synectinKD embryos (“subtle” defects; Figure 3E and supplemental Table 3), while TD development was normal in the remainder of the morphant embryos. Because the TD develops in a metameric pattern (see above), the finding that the TD formed over for instance only 20% of its length implies that TD development was aborted in the 8 other of the 10 somites analyzed. Thus, silencing of synectin aborted lymphatic development completely in all somites in a third of all morphants, and partially in a fraction of the somites in another 50% of the morphants.
Knockdown of synectin disrupts thoracic duct formation in zebrafish. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 100 μm in panels A-B and 50 μm in panels C-D. DA indicates dorsal aorta; and PCV, posterior cardinal vein. (A-D) Confocal images of GFP+ vessels in the trunk of 7-dpf Fli1:eGFPy1 zebrafish embryos, showing the formation of a normal lymphatic thoracic duct (TD) in the control embryo (A,C) but not in the synectinKD embryo (B,D). Panels C and D represent close-ups of the boxed areas in panels A and B, between the dorsal aorta (DA) and posterior cardinal vein (PCV). In these latter panels, arrows highlight the TD, while asterisks denote absence of TD. (E) Quantification of the TD formation defects after injection of SynATG1 at 7 dpf. Percentages of embryos displaying complete lack of TD, TD formation over 10%-30% or 30%-90% of its normal length, and a normal TD are represented for each treatment group (see also supplemental Table 3). We quantitatively analyzed TD formation by scoring its presence in 10 consecutive somite segments (from somite 5 to somite 15).
Knockdown of synectin disrupts thoracic duct formation in zebrafish. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 100 μm in panels A-B and 50 μm in panels C-D. DA indicates dorsal aorta; and PCV, posterior cardinal vein. (A-D) Confocal images of GFP+ vessels in the trunk of 7-dpf Fli1:eGFPy1 zebrafish embryos, showing the formation of a normal lymphatic thoracic duct (TD) in the control embryo (A,C) but not in the synectinKD embryo (B,D). Panels C and D represent close-ups of the boxed areas in panels A and B, between the dorsal aorta (DA) and posterior cardinal vein (PCV). In these latter panels, arrows highlight the TD, while asterisks denote absence of TD. (E) Quantification of the TD formation defects after injection of SynATG1 at 7 dpf. Percentages of embryos displaying complete lack of TD, TD formation over 10%-30% or 30%-90% of its normal length, and a normal TD are represented for each treatment group (see also supplemental Table 3). We quantitatively analyzed TD formation by scoring its presence in 10 consecutive somite segments (from somite 5 to somite 15).
Knockdown of synectin impairs early lymphatic development in zebrafish
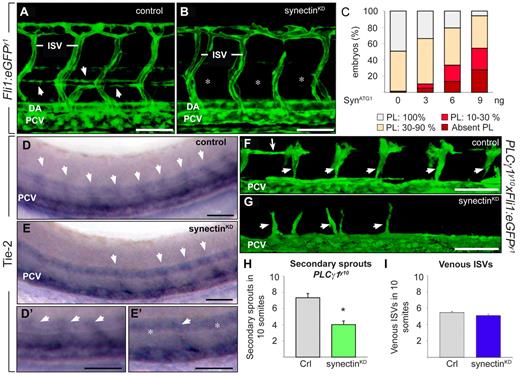
We then explored whether the TD defect in synectinKD morphants was related to a defect in PL formation by quantifying its length in 10 somites in Fli1:eGFPy1 embryos at 60 hpf (using a similar method as used for the TD). In control embryos, PL cells were detected in nearly every somite segment (Figure 4A,C and supplemental Table 4). By contrast, the PL was absent in 28% of synectinKD embryos (Figure 4B-C and supplemental Table 4) or formed over only 10%-30% of its normal length in another 27% of morphants (Figure 4C and supplemental Table 4). An additional 40% of synectinKD embryos displayed PL cells in only 3 to 9 somites, while in the remaining 5%, the PL string developed normally. The close correlation between TD and PL defects suggests that silencing of synectin impaired early lymphatic formation.
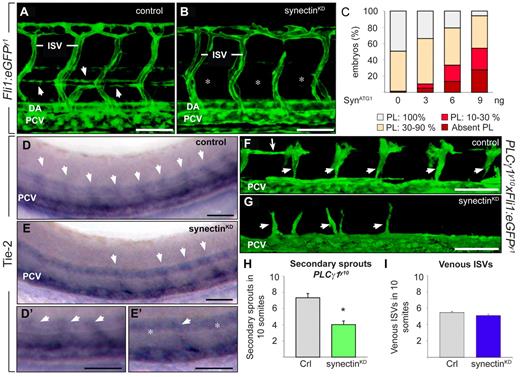
Knockdown of synectin impairs early lymphatic development in zebrafish. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 50 μm. DA indicates dorsal aorta; ISV, intersomitic vessel; and PCV, posterior cardinal vein. (A-B) Confocal images of 60-hpf control (A) and synectinKD (B) Fli1:eGFPy1 embryos revealing a reduced formation of the parachordal lymphangioblast (PL) string (arrows in A) upon synectin knockdown. Asterisks in panel B denote absence of PL cells. (C) Quantification of the PL cells in control and synectinKDFli1:eGFPy1 zebrafish embryos at 60 hpf. The percentages of embryos lacking all PL cells and displaying PL string formation over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group (see also supplemental Table 4). Formation of the PL was scored per somite in 10 consecutive somites between somites 5 and 15. (D-E) Whole-mount in situ hybridization of 48-hpf control (D) and synectinKD (E) embryos for Tie-2, labeling all secondary sprouts (arrows) emerging from the PCV. In synectinKD embryos the number of secondary sprouts was markedly reduced, by approximately 50%. Panels D′ and E′ are magnifications of panel D and E, respectively. In synectinKD embryos somites lacking a secondary sprout are indicated with an asterisk. (F-G) Confocal images of 48-hpf Fli1:eGFPy1xPLCγ1y10 control (F) and synectinKD(G) embryos showing a reduction of secondary sprouts upon synectin knockdown. Small arrows indicate unilateral secondary sprouts; long arrow denotes PL cells. (H) Quantification of the number of unilateral secondary sprouts in a 10-somite region of 48-hpf Fli1:eGFPy1xPLCγ1y10 embryos confirmed a significant reduction of nearly 50% in secondary sprouts budding from the PCV upon synectin knockdown. (I) Quantification of the fraction of venous ISVs, identified by their connection to the PCV upon confocal screening, in a 10-somite region in 4-dpf Fli1:eGFPy1 embryos revealed a normal ratio of venous ISVs upon synectin knockdown. Error bars in panels H-I represent SEM; *P < .05 by univariate analysis.
Knockdown of synectin impairs early lymphatic development in zebrafish. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 50 μm. DA indicates dorsal aorta; ISV, intersomitic vessel; and PCV, posterior cardinal vein. (A-B) Confocal images of 60-hpf control (A) and synectinKD (B) Fli1:eGFPy1 embryos revealing a reduced formation of the parachordal lymphangioblast (PL) string (arrows in A) upon synectin knockdown. Asterisks in panel B denote absence of PL cells. (C) Quantification of the PL cells in control and synectinKDFli1:eGFPy1 zebrafish embryos at 60 hpf. The percentages of embryos lacking all PL cells and displaying PL string formation over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group (see also supplemental Table 4). Formation of the PL was scored per somite in 10 consecutive somites between somites 5 and 15. (D-E) Whole-mount in situ hybridization of 48-hpf control (D) and synectinKD (E) embryos for Tie-2, labeling all secondary sprouts (arrows) emerging from the PCV. In synectinKD embryos the number of secondary sprouts was markedly reduced, by approximately 50%. Panels D′ and E′ are magnifications of panel D and E, respectively. In synectinKD embryos somites lacking a secondary sprout are indicated with an asterisk. (F-G) Confocal images of 48-hpf Fli1:eGFPy1xPLCγ1y10 control (F) and synectinKD(G) embryos showing a reduction of secondary sprouts upon synectin knockdown. Small arrows indicate unilateral secondary sprouts; long arrow denotes PL cells. (H) Quantification of the number of unilateral secondary sprouts in a 10-somite region of 48-hpf Fli1:eGFPy1xPLCγ1y10 embryos confirmed a significant reduction of nearly 50% in secondary sprouts budding from the PCV upon synectin knockdown. (I) Quantification of the fraction of venous ISVs, identified by their connection to the PCV upon confocal screening, in a 10-somite region in 4-dpf Fli1:eGFPy1 embryos revealed a normal ratio of venous ISVs upon synectin knockdown. Error bars in panels H-I represent SEM; *P < .05 by univariate analysis.
Knockdown of synectin impairs secondary sprout formation
We then evaluated whether absence of PL cells resulted from lymphangiogenic sprout defects. In case lymphangiogenic but not angiogenic sprout formation would be defective, we expected that 50% of all secondary sprouts would be affected/absent. Several assays were used to address this issue. First, we used whole-mount in situ staining for Tie-2 to identify both angiogenic and lymphangiogenic secondary sprouts at 48 hpf. In 81% of control embryos (n = 66), 8 to 10 secondary sprouts developed in the 10 unilateral somites analyzed, (ie, in nearly every somite [Figure 4D-D′]); in another 17%, 6 to 8 secondary sprouts developed, while very rarely (in 2%), only half the number of secondary (4 to 5) sprouts formed. By contrast, in 22% of synectinKD embryos, only half of the normal number of secondary sprouts formed (n = 74; P < .001; Figure 4E-E′), while in another 41%, only 6 to 8 Tie-2+ sprouts developed (N = 74; P < .001 vs control), while the remainder fraction displayed no secondary sprouting defects. Notably, comparable fractions of morphant embryos suffered severe secondary sprout defects, and lacked a PL and TD, suggesting that the latter and former processes were linked.
Second, we used the genetic PLCγ1y10 model to visualize secondary sprouts in isolation of primary ISVs. Indeed, in these mutant embryos, aISVs fail to form, while secondary sprouts emanate normally.16 Synectin silencing in PLCγ1y10xFli1:eGFPy1 embryos showed that only half the normal number of secondary sprouts developed by 48 hpf (sprouts in 10 somites: 7.3 ± 0.9 in control vs 4.0 ± 0.8 in synectinKD; N = 53-57 embryos, P < .01; Figure 4F-H). Thus, also with this method, approximately 50% fewer secondary sprouts branched off in synectinKD embryos.
Silencing of synectin does not alter secondary angiogenic sprouting
We then assessed whether synectin silencing impaired lymphangiogenic sprouting using an indirect method (ie, by counting the number of ISVs that were connected to the PCV [vISVs]), as the latter are established when angiogenic secondary sprouts connect to primary ISVs. Because a comparable fraction of lymphangiogenic and angiogenic sprouts normally emanates from the PCV, a normal number of vIVSs, in combination with a reduced total number of secondary sprouts, would be evidence for defective formation of lymphangiogenic sprouts. In control 4 dpf Fli1:eGFPy1 embryos (N = 29), 55% of the ISVs were connected to the PCV and thus venous, while the remaining fraction was connected to the DA and hence arterial (Figure 4I). Notably, in the most severely affected synectinKD embryos, lacking the PL completely or forming only a partial PL in 30% of the somites (N = 18), half of the ISVs was still connected to the PCV (Figure 4I), showing that angiogenic sprouts normally emanated from the PCV and implying that lymphangiogenic sprouting was impaired in synectinKD embryos. As a lymphangiogenic sprouting defect might reflect a defect in migration and/or emergence of the lymphatic lineage, but the dynamics of Prox-1 expression during lymphatic differentiation can be better visualized in frog than zebrafish embryos, we resorted to tadpoles, a previously validated model to study lymphangiogenesis,17 to dissect the underlying mechanism in more detail.
Knockdown of synectin impairs lymphangiogenesis in tadpoles
We first analyzed synectin expression in tadpoles. In agreement with previous findings,28 whole-mount in situ hybridization at stage 35-40 revealed expression of synectin in the neural tube, eye, brain, otic vesicle, somites, and branchial arches, as well as at locations of the DA, PCV, and dorsal longitudinal anastomosing vessel (DLAV), where the ventral caudal lymph vessel (VCLV) and dorsal caudal lymph vessel (DCLV) develop in the frog embryo (not shown and supplemental Figure 5A-F). We also used a novel ‘LEC labeling’ technique in transgenic Flk1:eGFP frogs, that express eGFP in blood and lymph vessels (generation of this line will be reported elsewhere). Upon intracardial injection and extravasation from blood vessels, tetramethyl rhodamine isothiocyanate (TRITC)-dextran is selectively taken up by LECs, allowing isolation of doubly labeled yellow LECs and singly labeled green BECs by flow cytometry. RT-PCR showed that sorted BECs and LECs expressed synectin (synectin/105 β-actin mRNA copies: 874 ± 625 for BECs vs 1415 ± 822 for LECs; N = 4).
We then evaluated whether silencing of synectin impaired lymphatic development. In tadpoles, BECs from the PCV, expressing Prox-1, migrate ventrally to form the VCLV, or dorsally to the level of the DLAV to establish the DCLV.17 To knockdown synectin, Flk1:eGFP tadpoles were injected with the nonoverlapping morpholinos xSynATG1 or xSynATG2, targeting the ATG of the Xenopus laevis synectin mRNA. As both morpholinos blocked translation (supplemental Figure 6) and caused indistinguishable phenotypes (supplemental Table 5), only results for xSynATG1 are shown. We first determined a range of submaximal morpholino doses, that minimally affected vascular development. Screening of GFP+ tadpoles at stage 45 (5 dpf) showed that, at 30 ng morpholino per embryo, 17% of GFP+ tadpoles had only subtle vascular defects (reduced complexity of capillary network interconnecting ISVs; Figure 5A-B and supplemental Table 5). In line herewith, blood flow in axial vessels was normal in most tadpoles (supplemental Table 5).
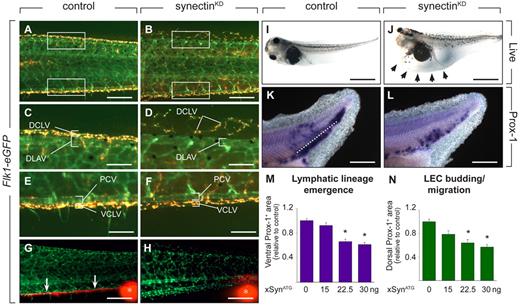
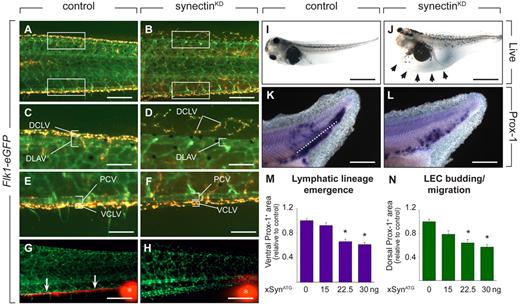
Synectin knockdown impairs lymphatics in tadpoles. In all panels, the head of the tadpoles faces left and dorsal is up. Scale bars represent 100 μm in panels A-B and K-L; 50 μm in panels C-F; 150 μm in panels G-H; and 400 μm in panels I-J. DCLV indicates dorsal caudal lymph vessel; DLAV, dorsal anastomosing longitudinal vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. (A-F) Fluorescent analysis of blood and lymph vessels in stage 47 transgenic Flk1:eGFP tadpoles. Lymph vessels are labeled with TRITC-dextran that was intracardially injected at stage 45 and taken up by LECs after extravasation from the blood vessels. Hence, GFP+ BECs can be easily distinguished from doubly labeled (yellow) LECs. Areas in the trunk corresponding to the boxed areas in panels A or B are shown in panels C-F. In control tadpoles, both the DCLV (C) and the VCLV (E) developed normally. Injection of 30 ng of synectin morpholino resulted in a hypoplastic, disorganized, and discontinuous DCLV (D), while the VCLV (F) had a grossly normal appearance. (G-H) Lymphangiography of stage 45 Flk1:eGFP tadpoles, revealing dysfunction of the VCLV after synectin knockdown (H) in contrast to normal controls (G). Whereas in control injected embryos, the dye is drained normally in a rostral direction (white arrows in G), no dye uptake was observed in the VCLV in synectinKD tadpoles. White asterisks indicate injection site of the fluorescent dye. (I-J) Bright-field pictures of live embryos at stage 45 (5 dpf), showing lymphedema (arrows) in a synectinKD tadpole (J) compared with a control tadpole (I). (K-L) Whole mount Prox-1 in situ hybridization of stage 35/36 tadpoles, revealing the presence of fewer Prox-1+ LECs in the area ventrally to the dorsal margin of the endoderm (reflecting lymphatic lineage emergence) and in the area dorsally to this margin (reflecting LEC budding/migration) in the posterior trunk of synectinKD-morphant tadpoles (L) compared control (K) tadpoles. Dotted line: dorsal margin of the endoderm. (M-N) Morphometric measurement revealed a dose-dependent decrease of the Prox-1+ area both ventrally (M) and dorsally (N) of the dorsal margin of the endoderm in the trunk of Prox-1–stained synectinKD compared with control tadpoles at stage 35/36, indicating that lymphatic lineage development and migration is affected upon synectin knockdown. Values expressed relative to control. *P < .001 by multivariate analysis.
Synectin knockdown impairs lymphatics in tadpoles. In all panels, the head of the tadpoles faces left and dorsal is up. Scale bars represent 100 μm in panels A-B and K-L; 50 μm in panels C-F; 150 μm in panels G-H; and 400 μm in panels I-J. DCLV indicates dorsal caudal lymph vessel; DLAV, dorsal anastomosing longitudinal vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. (A-F) Fluorescent analysis of blood and lymph vessels in stage 47 transgenic Flk1:eGFP tadpoles. Lymph vessels are labeled with TRITC-dextran that was intracardially injected at stage 45 and taken up by LECs after extravasation from the blood vessels. Hence, GFP+ BECs can be easily distinguished from doubly labeled (yellow) LECs. Areas in the trunk corresponding to the boxed areas in panels A or B are shown in panels C-F. In control tadpoles, both the DCLV (C) and the VCLV (E) developed normally. Injection of 30 ng of synectin morpholino resulted in a hypoplastic, disorganized, and discontinuous DCLV (D), while the VCLV (F) had a grossly normal appearance. (G-H) Lymphangiography of stage 45 Flk1:eGFP tadpoles, revealing dysfunction of the VCLV after synectin knockdown (H) in contrast to normal controls (G). Whereas in control injected embryos, the dye is drained normally in a rostral direction (white arrows in G), no dye uptake was observed in the VCLV in synectinKD tadpoles. White asterisks indicate injection site of the fluorescent dye. (I-J) Bright-field pictures of live embryos at stage 45 (5 dpf), showing lymphedema (arrows) in a synectinKD tadpole (J) compared with a control tadpole (I). (K-L) Whole mount Prox-1 in situ hybridization of stage 35/36 tadpoles, revealing the presence of fewer Prox-1+ LECs in the area ventrally to the dorsal margin of the endoderm (reflecting lymphatic lineage emergence) and in the area dorsally to this margin (reflecting LEC budding/migration) in the posterior trunk of synectinKD-morphant tadpoles (L) compared control (K) tadpoles. Dotted line: dorsal margin of the endoderm. (M-N) Morphometric measurement revealed a dose-dependent decrease of the Prox-1+ area both ventrally (M) and dorsally (N) of the dorsal margin of the endoderm in the trunk of Prox-1–stained synectinKD compared with control tadpoles at stage 35/36, indicating that lymphatic lineage development and migration is affected upon synectin knockdown. Values expressed relative to control. *P < .001 by multivariate analysis.
To characterize lymphatic development, we focused on the formation of the VCLV and DCLV. LECs in Flk1:eGFP tadpoles were labeled with TRITC-dextran to distinguish them from GFP+ BECs. Imaging of control tadpoles at stage 47 revealed that the DCLV and VCLV formed a regular continuous vessel over its entire length (Figure 5A,C,E). By contrast, in 57% of synectinKD tadpoles, the DCLV was incompletely formed and dysmorphogenic, consisting of isolated LEC clusters, distributed discontinuously over the trunk (Figure 5B,D and supplemental Table 5). The VCLV appeared largely normal in most synectinKD tadpoles, at least morphologically, while other morphants showed an irregular structure (Figure 5B,F). Lymphangiography at stage 45 revealed that the DCLV and VCLV were functional in all control tadpoles (not shown and Figure 5G). When analyzing the DCLV in 15 synectinKD tadpoles, the dye was not drained at all or only over a short distance, indicating that this lymphatic was dysfunctional (not shown). Similarly, despite its apparent normal morphologic appearance, the VCLV failed drain the dye (Figure 5H). We previously noticed that the DCLV is more severely affected than the VCLV upon silencing of Vegfr3,17,31 Vegfc (unpublished) or Liprinβ-1,32 presumably because LECs, arising from the PCV, have to migrate further to form the distant DCLV than the nearby VCLV. In line with these findings, 32% of synectinKD tadpoles suffered lymphedema around the heart and gut (Figure 5I-J and supplemental Table 5). Thus, synectin also regulates lymphatic development in the tadpole model.
Synectin regulates lymphatic differentiation and migration in tadpoles
The tadpole model allows study of the effects on LEC differentiation and migration.17 To explore whether lymphatic differentiation or migration were impaired in synectinKD tadpoles, we stained stage 35/36 embryos by whole-mount in situ hybridization for Prox-1 to measure the accumulation of Prox-1+ cells in the ventral trunk as an index of lymphatic lineage emergence, and in the more dorsal trunk area as a measure of LEC migration using previously established methods.17 Morphometric quantification revealed a dose-dependent decrease of both Prox-1+ areas in synectinKD tadpoles (by 40% at the highest morpholino dose; Figure 5K-N). Thus, synectin regulates both lymphatic lineage formation and LEC migration in tadpoles.
Synectin genetically interacts with Vegfc/Vegfr3
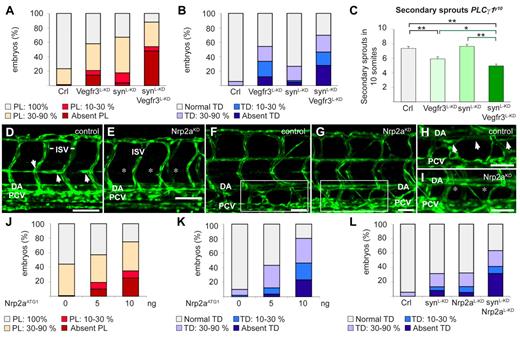
We then explored how synectin regulated LEC migration, using the Fli1:eGFPy1 zebrafish model. Among the possible interacting partners of synectin, we focused on neuropilin-2 (Nrp2), as it contains a PDZ-domain33 and regulates, as a coreceptor of Vegfr3,34,35 lymphatic sprouting in mice.36,37 Vegfc stimulates LEC migration through Vegfr3 signaling in mice,1,38,39 zebrafish20,40 and tadpoles.17,31 Because Vegfr3 is the signaling receptor of the Vegfr3/Nrp2 complex, and silencing of synectin phenocopies the lymphatic defects induced by silencing of Vegfc or Vegfr3 in zebrafish20,40 and tadpoles,17,31 we first assessed whether synectin and Vegfr3 genetically interacted, by testing whether a combination of low morpholino doses of synectin (2.5 ng/embryo) and Vegfr3 (1.25 ng/embryo), which by themselves induced only a minimal effect, caused a more significant lymphatic defect (the low morpholino doses were denoted by L-KD, as in synectinL-KD, to distinguish them from the dose used in the incomplete silencing studies). To silence Vegfr3, we used the previously characterized Vegfr3ATG1 morpholino.40 This analysis revealed that, compared with each single knockdown, the combined knockdown caused more severe defects in TD and PL formation in synectinL-KDxVegfr3L-KD morphants (P < .05 vs single knockdown; Figure 6A-B).
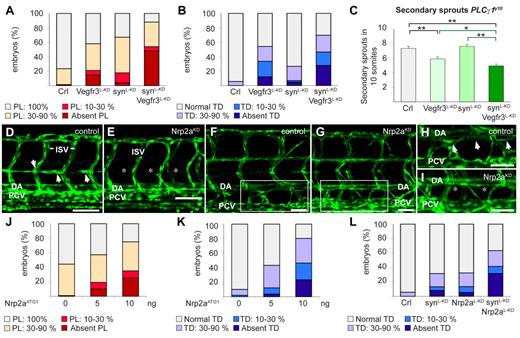
Synectin modulates Vegfr3 signaling in lymphatic development in vivo. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 50 μm in panels D-E and 100 μm in panels F-I. DA indicates dorsal aorta; ISV, intersomitic vessel; and PCV, posterior cardinal vein. (A) Quantification of parachordal lymphangioblast (PL) cell defects after injection of SynATG1 (2.5 ng; synL-KD; N = 79), Vegfr3ATG1 (1.25 ng; Vegfr3L-KD; N = 62) or both (N = 50) at 60 hpf. Percentages of embryos displaying complete lack of PL cells, PL string over 10%-30% or 30%-90% of its normal length and a normal PL string are shown for each group. Compared with single knockdown groups, the PL formation was more severely impaired in double morphants (P < .001). (B) Quantification of TD defects after injection of SynATG1 (2.5 ng; synL-KD; N = 101), Vegfr3ATG1 (1.25 ng; Vegfr3L-KD; N = 107) or both (N = 86) at 7 dpf. Percentages of embryos displaying complete lack of TD, TD formation over 10%-30% or 30%-90% of its normal length and a normal TD are represented for each treatment group. Compared with single knockdown groups, the TD was more severely impaired in double morphants (P < .001 vs synectinL-KD; P < .05 vs Vegfr3L-KD). (C) Quantification of the number of unilateral secondary sprouts in a 10-somite region of 48-hpf Fli1:eGFPy1xPLCγ1y10 embryos revealed that coknockdown of synectin and Vegfr3 significantly aggravated the secondary sprouting defects compared with single knockdown of either gene when using suboptimal doses of SynATG1 (2.5 ng; synL-KD) and Vegfr3ATG1 (2.5 ng; Vegfr3L-KD); (N = 45, 37, 48, and 63 for control, Vegfr3L-KD, synectinL-KD, and coknockdown, respectively; *P < .05; **P < .01; ***P < .001). (D-E) Confocal images of 60-hpf control (D) and Nrp2aKD (E) Fli1:eGFPy1 embryos revealing impaired formation of the PL string (arrows) upon Nrp2a knockdown. Asterisks denote absence of PL cells. (F-I) Confocal images of GFP+ vessels in the trunk of 7-dpf Fli1:eGFPy1 zebrafish embryos, showing formation of a normal TD in a control embryo (F,H) but not in a Nrp2aKD embryo (G,I). Panels H and I represent close-up magnifications of the boxed areas in panels F and G; arrows denote TD, asterisks denote absence of TD. (J) Quantification of PL cells in control and Nrp2aKDFli1:eGFPy1 zebrafish embryos at 60 hpf. The percentages of embryos lacking PL cells and displaying PL string over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group. Formation of the PL string was scored per somite in 10 consecutive somites between somite 5 and 15 (N = 106, 152, and 60 for 0, 5, and 10 ng of Nrp2aATG1, respectively). (K) Quantification of TD in control and Nrp2aKDFli1:eGFPy1 zebrafish embryos at 7 dpf. The percentages of embryos lacking TD and displaying TD formation over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group. Formation of the TD was scored per somite in 10 consecutive somites between somite 5 and 15 (N = 99, 164, and 56 for 0, 5, and 10 ng of Nrp2aATG1, respectively). (L) Quantification of TD formation after injection of Nrp2aATG1 (5 ng; Nrp2aL-KD; N = 74), SynATG1 (2.5 ng; synL-KD; N = 98) or both (N = 41) revealed that coknockdown impaired lymphatic development more severely than single synectinL-KD (P < .001) or Nrp2aL-KD (P < .001).
Synectin modulates Vegfr3 signaling in lymphatic development in vivo. In all panels, the head of the embryo faces left and dorsal is up. Scale bars represent 50 μm in panels D-E and 100 μm in panels F-I. DA indicates dorsal aorta; ISV, intersomitic vessel; and PCV, posterior cardinal vein. (A) Quantification of parachordal lymphangioblast (PL) cell defects after injection of SynATG1 (2.5 ng; synL-KD; N = 79), Vegfr3ATG1 (1.25 ng; Vegfr3L-KD; N = 62) or both (N = 50) at 60 hpf. Percentages of embryos displaying complete lack of PL cells, PL string over 10%-30% or 30%-90% of its normal length and a normal PL string are shown for each group. Compared with single knockdown groups, the PL formation was more severely impaired in double morphants (P < .001). (B) Quantification of TD defects after injection of SynATG1 (2.5 ng; synL-KD; N = 101), Vegfr3ATG1 (1.25 ng; Vegfr3L-KD; N = 107) or both (N = 86) at 7 dpf. Percentages of embryos displaying complete lack of TD, TD formation over 10%-30% or 30%-90% of its normal length and a normal TD are represented for each treatment group. Compared with single knockdown groups, the TD was more severely impaired in double morphants (P < .001 vs synectinL-KD; P < .05 vs Vegfr3L-KD). (C) Quantification of the number of unilateral secondary sprouts in a 10-somite region of 48-hpf Fli1:eGFPy1xPLCγ1y10 embryos revealed that coknockdown of synectin and Vegfr3 significantly aggravated the secondary sprouting defects compared with single knockdown of either gene when using suboptimal doses of SynATG1 (2.5 ng; synL-KD) and Vegfr3ATG1 (2.5 ng; Vegfr3L-KD); (N = 45, 37, 48, and 63 for control, Vegfr3L-KD, synectinL-KD, and coknockdown, respectively; *P < .05; **P < .01; ***P < .001). (D-E) Confocal images of 60-hpf control (D) and Nrp2aKD (E) Fli1:eGFPy1 embryos revealing impaired formation of the PL string (arrows) upon Nrp2a knockdown. Asterisks denote absence of PL cells. (F-I) Confocal images of GFP+ vessels in the trunk of 7-dpf Fli1:eGFPy1 zebrafish embryos, showing formation of a normal TD in a control embryo (F,H) but not in a Nrp2aKD embryo (G,I). Panels H and I represent close-up magnifications of the boxed areas in panels F and G; arrows denote TD, asterisks denote absence of TD. (J) Quantification of PL cells in control and Nrp2aKDFli1:eGFPy1 zebrafish embryos at 60 hpf. The percentages of embryos lacking PL cells and displaying PL string over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group. Formation of the PL string was scored per somite in 10 consecutive somites between somite 5 and 15 (N = 106, 152, and 60 for 0, 5, and 10 ng of Nrp2aATG1, respectively). (K) Quantification of TD in control and Nrp2aKDFli1:eGFPy1 zebrafish embryos at 7 dpf. The percentages of embryos lacking TD and displaying TD formation over 10%-30%, 30%-90%, and 100% of its normal length are indicated per treatment group. Formation of the TD was scored per somite in 10 consecutive somites between somite 5 and 15 (N = 99, 164, and 56 for 0, 5, and 10 ng of Nrp2aATG1, respectively). (L) Quantification of TD formation after injection of Nrp2aATG1 (5 ng; Nrp2aL-KD; N = 74), SynATG1 (2.5 ng; synL-KD; N = 98) or both (N = 41) revealed that coknockdown impaired lymphatic development more severely than single synectinL-KD (P < .001) or Nrp2aL-KD (P < .001).
We also assessed whether synectin genetically interacted with Vegfr3 in regulating lymphangiogenic sprouting in the PLCγy10 zebrafish model. A low SynATG1 dose did not reduce the number of secondary sprouts (7.4 ± 0.3 in control vs 7.7 ± 0.3 in SynL-KD; N = 49-62, P = .40; Figure 6C), while a low dose of Vegfr3ATG1 slightly inhibited secondary sprouting (5.9 ± 0.3 in Vegfr3L-KD; N = 49-62, P < .001; Figure 6C). However, the combination of both morpholinos impaired secondary sprouting significantly more (4.9 ± 0.2 in synectinL-KDxVegfr3L-KD; N = 70, P < .001 vs synectinL-KD; P < .05 vs Vegfr3L-KD; Figure 6C). We therefore assessed whether angiogenic or lymphangiogenic secondary sprouts were affected in synectinL-KDxVegfr3L-KD embryos, by counting the number of vISVs in 4 dpf Fli1:eGFPy1 embryos (for rationale, see above).
In line with reports that Vegfr3 regulates angiogenic secondary sprouting,40 the fraction of vISVs was reduced by a low dose of Vegfr3ATG1 but not of SynATG1 (% vISVs: 26 ± 12% in Vegfr3L-KD vs 52 ± 7% in synectinL-KD or 54 ± 9% in control; N = 17-29; P < .001). Notably, coknockdown of Vegfr3 and synectin did not further reduce the fraction of vISVs (% vISVs: 25 ± 12% in synectinL-KDxVegfr3L-KD; N = 27; P = .968 vs Vegfr3L-KD), indicating that angiogenic sprouting was not under the control of a synectin/Vegfr3 interaction. Thus, the normal number of angiogenic sprouts, in combination with the reduced total number of secondary sprouts and impaired formation of PL and TD, in synectinL-KDxVegfr3L-KD embryos indicates that the genetic synectin/Vegfr3 interaction did not control angiogenic but lymphangiogenic sprouting. Because other experiments revealed that Vegf/Vegfr2 signaling did not affect lymphatic development in zebrafish (supplemental Note 2), we did not explore an interaction with synectin.
Synectin genetically interacts with Nrp2a in vivo
We then analyzed whether synectin genetically interacted with Nrp2. We first examined whether the zebrafish Nrp2a and Nrp2b coorthologues regulated lymphatic development, using previously validated morpholinos for Nrp2a (Nrp2aATG1) and Nrp2b (Nrp2bATG1).41 Consistent with previous findings,42 a maximal dose of 10 ng Nrp2aATG1 caused hind brain edema and arteriovenous (AV) shunts in a small fraction of embryos, while a similar dose of Nrp2bATG1 induced cardiac edema formation and mild DA dysmorphogenesis in some morphants (not shown). As reported,42 these blood vessel phenotypes were confined to the extreme caudal region of the trunk (not shown); morphant embryos with edema or morphologic anomalies were excluded. The residual Nrp2aKD and Nrp2bKD embryos developed normally by 6 dpf with no or only minimal blood vessel or circulatory defects in the mid-trunk (where we scored lymphatic development; ie, more rostral than the caudal region in which AV shunts/DA defects were present; Figure 6E,G). While lymphatic development was normal in Nrp2bKD embryos (supplemental Figure 7A), Nrp2aKD dose-dependently caused PL and TD defects (Figure 6D-K).
We then analyzed whether synectin genetically interacted with Nrp2a. A low dose of Nrp2aATG1 (Nrp2aL-KD) or SynATG1 (SynL-KD) minimally affected TD formation (Figure 6L). In contrast, knockdown of both genes by combined injection of a low dose of both morpholinos severely impaired lymphatic development, as the TD was absent in 30% of synectinL-KDxNrp2aL-KD morphants (P < .001 vs single knockdown; Figure 6L). Similar findings were obtained when analyzing the PL (not shown).
Notably, however, Nrp2a knockdown did not affect secondary sprout formation in PLCγ1y10xFli1:eGFPy1 embryos (supplemental Figure 7B). Coknockdown analysis did also not reveal a genetic interaction between synectin and Nrp2a in secondary sprout formation (supplemental Figure 7E; see below for discussion). Thus, our findings are consistent with a model, whereby synectin genetically interacts with Vegfr3 and Nrp2a during lymphatic development, but only beyond the initial stage of lymphangiogenic sprouting.
Synectin mediates VEGFC/VEGFR3-driven LEC sprouting in vitro
Because synectin regulates LEC migration in tadpoles and genetically interacts with Vegfr3, known to stimulate LEC migration,1,31,39 we examined whether synectin knockdown inhibited sprouting of cultured HDLECs in response to vascular endothelial growth factor C (VEGFC). We silenced synectin expression in LECs using simultaneously 2 nonoverlapping synectin–specific siRNAs. qRT-PCR analysis showed that synectin mRNA levels in HDLECs were reduced by 80% (not shown). HDLECs treated with control or synectin-specific siRNA were aggregated as spheroids and lymphatic sprouts were visualized by CD31 immunostaining. VEGFC stimulated lymphatic sprouting in control spheroids (N = 17; Figure 7A,C,E), but synectinKD HDLECs formed substantially fewer sprouts in response to VEGFC (N = 26, P < .001; Figure 7B,D-E).
Synectin regulates Vegfc-driven lymphatic sprouting and Sox18-mediated lymphatic reprogramming in vitro. (A-D) CD31 immunostaining of untreated (A-B) or VEGFC-stimulated (C-D; 200 ng/mL) LEC spheroids in fibrin gels, revealing reduced VEGFC sprouting response by LECs transfected with synectin-specific (B,D; synectinKD) compared with control siRNA (A,C). Scale bar represents 100 μm. (E) Quantification of the sprout number per LEC spheroid, revealing that synectin knockdown impaired lymphatic sprouting in response to VEGFC (N = 17 for control; N = 26 for synectinKD), while baseline sprouting of control (N = 17) and synectinKD LECs (N = 35) was comparable. *P < .001 by univariate analysis. (F) RT-PCR of control and synectinKD H5V blood vascular endothelial cells (BECs), transfected with Sox18-virus (BECsSox18) or GFP-virus (BECsGFP), revealing that knockdown of synectin impaired up-regulation of Prox-1 in BECsSox18 without affecting Nrp1 expression. Results are represented as fold change vs control BECsGFP. Dashed line indicates baseline expression levels in BECsGFP. N = 3; *P < .01 by Student t test. Error bars represent SEM.
Synectin regulates Vegfc-driven lymphatic sprouting and Sox18-mediated lymphatic reprogramming in vitro. (A-D) CD31 immunostaining of untreated (A-B) or VEGFC-stimulated (C-D; 200 ng/mL) LEC spheroids in fibrin gels, revealing reduced VEGFC sprouting response by LECs transfected with synectin-specific (B,D; synectinKD) compared with control siRNA (A,C). Scale bar represents 100 μm. (E) Quantification of the sprout number per LEC spheroid, revealing that synectin knockdown impaired lymphatic sprouting in response to VEGFC (N = 17 for control; N = 26 for synectinKD), while baseline sprouting of control (N = 17) and synectinKD LECs (N = 35) was comparable. *P < .001 by univariate analysis. (F) RT-PCR of control and synectinKD H5V blood vascular endothelial cells (BECs), transfected with Sox18-virus (BECsSox18) or GFP-virus (BECsGFP), revealing that knockdown of synectin impaired up-regulation of Prox-1 in BECsSox18 without affecting Nrp1 expression. Results are represented as fold change vs control BECsGFP. Dashed line indicates baseline expression levels in BECsGFP. N = 3; *P < .01 by Student t test. Error bars represent SEM.
Initial evidence for a role of synectin lymphatic differentiation in vitro
The reduced number of Prox-1+ cells in the ventral region in synectinKD tadpoles suggested a role for synectin in lymphatic differentiation. In mice, Sox18 acts upstream of Prox-1 during this process and lymphatic reprogramming is mimicked in vitro by overexpression of Sox18 in venous ECs.3,6 We therefore overexpressed Sox18 in murine H5V BECs, transfected them with control or synectin-specific siRNA at day 0, and analyzed lymphatic marker expression on day 7, as reported.6 The siRNA transfection procedure was repeated on day 3 to maintain prolonged synectin silencing until 7 days, when synectin mRNA levels were reduced by 60% (not shown).
As reported,6 Prox-1 transcript levels were higher in BECs transfected with Sox18-lentivirus (BECssox18) than control GFP-virus (BECsGFP), when treated with control siRNA; expression of the blood vascular marker Nrp1 was not altered (Figure 7F). Upon synectin knockdown, induction of Prox-1, but not of Nrp1, expression in BECsSox18 was inhibited (Figure 7F). Knockdown of synectin did not alter gene expression in BECsGFP (relative mRNA levels vs control: 1.07 ± 0.03 for Sox18; 0.84 ± 0.12 for Prox1; and 0.97 ± 0.09 for Nrp1; N = 3; P > .05). Thus, silencing of synectin impaired Sox18-driven lymphatic reprogramming in vitro.
Discussion
The present study provides genetic evidence for a role of synectin in early lymphatic development. In zebrafish and frog embryos, knockdown of synectin dose-dependently impaired and, in the most severely affected embryos, even aborted lymphatic formation. Several lines of evidence suggest that TD defects in synectinKD zebrafish were due to defects in lymphangiogenic sprout formation from the venous system. First, lymphangiogenic sprouts that give rise to PL cells (ie, precursors of LECs forming the TD) were underdeveloped or, in the most severely affected morphants, did not form at all in synectinKD embryos. In the ccbe1, Vegfc, or Vegfr3 mutants, lack of formation of these lymphangiogenic sprouts also aborts further lymphatic development.20,40 Second, the fraction of synectinKD embryos without TD closely correlated with the fraction of morphants without lymphangiogenic sprouts and PL cells. Third, the missing secondary sprouts in synectinKD embryos were lymphangiogenic and not angiogenic, since these embryos developed at the expected ratio vISVs, the formation of which requires normal angiogenic secondary sprouting.
So far, silencing of only a few other genes (Ccbe1, Vegfc, or Vegfr3) is known to abort lymphangiogenic sprouting, the formation of the PL and TD.20,40 However, in these morphant embryos, branching of angiogenic sprouts from the PCV was also impaired.20 By contrast, in Dll4/Notch hypomorphants, the fraction of angiogenic sprouts is increased at the expense of lymphangiogenic sprouts.11 It is thus noteworthy that synectin silencing selectively impaired lymphangiogenic without affecting angiogenic secondary sprouting. The identification of synectin as the first selective regulator of lymphangiogenic sprouting suggests that formation of lymphangiogenic versus angiogenic sprouts is under distinct genetic control.
Synectin silencing inhibited migration of cultured LECs in response to VEGFC and reduced the dorsal Prox-1+ area, a parameter of LEC migration, in tadpoles. Similar LEC migration defects contribute to lymphatic impairment in tadpoles or mice lacking VEGFC or VEGFR3.1,17,31,38,39 Moreover, silencing of synectin in zebrafish aggravated the lymphatic defects, induced by silencing of Vegfr3 as well as of Nrp2a, the coreceptor of Vegfr3,34,35 that contains a PDZ-binding domain,33 through which synectin interacts with its partners.27 These data thus illustrate a genetic interaction of synectin with Vegfr3 as well as with Nrp2a. While Vegfr3/synectin participated already in lymphangiogenic sprouting, Nrp2a/synectin only regulated subsequent formation of the PL and TD. In mice, Nrp2 is also dispensable for initial lymphangiogenic budding from veins, but required for subsequent lymphatic development.36 Together, these genetic studies suggest a model whereby synectin modulates Vegfr3 signaling in lymphatic development in a Nrp2a-independent manner (during lymphangiogenic sprouting) or Nrp2a-dependent manner (PL and TD formation).
How synectin regulates Vegfr3 signaling is an intriguing question. During arterial development, synectin promotes intracellular Vegfr2 trafficking to early endosomes, required for downstream signaling, via Nrp1-dependent and -independent mechanisms.13,43 By analogy with Vegfr2/Nrp1, it is tempting to speculate that synectin might perhaps also participate in Vegfr3 endocytosis, at least partially, via an interaction with Nrp2. Supporting this model, Vegfr3 not only colocalizes with Nrp2 during endocytosis upon stimulation by Vegfc and Vegfd,35 but internalization is also required for proper Vegfr3 signaling.44 Because synectin modulates Vegfr3-driven lymphangiogenic but not angiogenic sprouting, it is tempting to speculate that synectin-mediated endocytosis regulates distinct Vegfr3 signaling pathways. Elucidation of the underlying molecular details will require future study.
Lymphatic defects were unlikely to be secondary to angiogenic alterations, as the PCV formed normally and expressed proper venous markers in synectinKD embryos, consistent with findings that synectin is dispensable for venous morphogenesis.12 In addition, the DA and primary aISVs formed normally and expressed arterial markers upon incomplete silencing of synectin. In fact, alterations in arterial identity or morphogenesis are unlikely to perturb lymphangiogenic sprouting or cause the type of lymphatic defects, observed upon synectin knockdown, since secondary sprouts and PL cells formed normally in PLCγ1y10 mutants, which even lack primary ISVs and exhibit an underdeveloped and improperly differentiated DA.16,25 In addition, we and others22 observed that lymphatic development is nearly normal upon silencing of Vegfaa- or Kdr-like, 2 well-established regulators of arterial morphogenesis and specification.45,46
A limitation of our study is that Prox-1 levels in the PCV at the time of lymphatic differentiation in zebrafish are below the threshold of detection (unpublished findings, P.C. and S.S.M.; personal communication, H. Gerhardt [London Research Institute, Cancer Research UK, London, United Kingdom] and F. Cotelli [University of Milan, Milan, Italy]), thereby precluding us from analyzing whether synectin silencing impairs LEC differentiation in zebrafish. We therefore used alternative models in vivo and in vitro. Indeed, our genetic analysis in tadpoles shows that silencing of synectin caused an underdevelopment of Prox-1+ cells at sites previously implicated in differentiation of LECs from venous ECs.17 In addition, silencing of synectin reduced lymphatic reprogramming of venous ECs in vitro upon overexpression of Sox18, an established regulator of lymphatic differentiation in mice.6 The role of Sox18 in lymphatic development in tadpoles and zebrafish has not yet been documented but, interestingly, silencing of Sox18 (in combination with Sox7) in zebrafish impaired venous ECs from which LECs differentiate.47-49 Notably, because PDZ-dependent binding has been reported for some of the SoxA family members,50 a possible interaction of synectin with Sox18 is an exciting possibility that remains to be further investigated.
In conclusion, synectin regulates lymphangiogenesis in zebrafish and tadpoles through genetic interactions with Vegfr3 and Nrp2a.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Brepoels, A. Carton, M. De Mol, E. Janssens, S. Louwette, A. Manderveld, M. Peeters, J. Souffreau, B. Tembuyser, A. Van den Eynde, A. Van Nuffelen, B. Vanwetswinkel, S. Verstraeten, S. Vinckier, and S. Wyns for technical assistance.
K.H., W.V., K.A., I.G., and F.D.S. are sponsored by a PhD grant of the Institute for the promotion of Innovation through Science and Technology (IWT) in Flanders (IWT-Vlaanderen), Belgium. E.G. and S.S.-M. are supported by the Royal Netherlands Academy of Arts and Sciences (KNAW). This work is supported by long-term structural Methusalem Funding by the Flemish Government to P.C., Research Foundation Flanders (FWO) Research Project Funding by the Flemish Government to P.C., Interuniversity Attraction Poles–Belgian Science Policy (IUAP P6/20) to M.D., and European Commission Lymphangiogenomics Consortium Funding (LSHG-CT-2004-503573) to P.C.
Authorship
Contribution: K.H., F.C., A.N., M.A., M.S., M.D., S.S.-M., E.D., K.A., and P.C. designed research; K.H., F.C., W.V., W.Z., I.G., F.O., A.N., F.D.S., E.G., K.A., and B.R. performed the experiments and analyzed the data; D.K. provided vital reagents; and K.H., F.C., M.D., and P.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Carmeliet, Vesalius Research Center, Vlaams Iustituut voor Biotechnologie, Katholieke Universiteit Leuven, Campus Gasthuisberg, Herestraat 49, B-3000, Belgium; e-mail: Peter.Carmeliet@vib-kuleuven.be.
References
Author notes
K.H. and F.C. contributed equally to this work.