Abstract
The hypothesis that bone marrow–derived, circulating endothelial cells incorporate into tumor blood vessels is unresolved. We have measured the numbers of bone marrow–derived versus resident endothelial cells in spontaneous prostate cancers during different stages of tumor progression and in age-matched normal prostates. Bone marrow–derived endothelial cells were rare in dysplasia and in well differentiated cancers representing between 0 and 0.04% of the total tumor mass. Instead, approximately 99% of all tumor-associated bone marrow–derived cells were CD45+ hematopoietic cells, including GR-1+, F4-80+, and CD11b+ myeloid cells. Similar to peripheral blood mononuclear cells, these tumor-associated myeloid cells expressed matrix metalloproteinases (MMPs), consistent with their proposed catalytic role during tumor angiogenesis. Furthermore, freshly isolated CD11b+ cells stimulated tumor endothelial cell cord formation by 10-fold in an in vitro angiogenesis assay. The bone marrow is, therefore, a reservoir for cells that augment tumor angiogenesis, but the tumor endothelium is derived primarily from the local environment.
Introduction
Bone marrow has been proposed as a depot for tumor endothelial cells (TECs).1 However, there is controversy surrounding the temporal and quantitative aspects of bone marrow–derived endothelial cell (BMDEC) recruitment in different tumors.2,3 There are reported differences in BMDEC numbers depending on tumor grade4 and between humans and mice.5 Adding to these uncertainties is a lack of standardization in the methodology and markers used to identify and quantify BMDECs.6 For example, hematopoietic stem cells (HSCs) and bona fide endothelial cells (ECs) share common markers (eg, CD31, CD34, and VEGFR-2), creating confusion over their identity.7
In the present study, we have quantified BMDECs from spontaneous tumors at different stages of progression using transgenic adenocarcinoma of the mouse prostate (TRAMP) mice.8 Our results show a paucity of BMDECs in both tumors and normal prostates. On the other hand, marrow–derived CD45+ myelomonocytic cells were recruited in substantial numbers to tumors where they reside in a perivascular position in vivo and stimulate angiogenesis in vitro.
Methods
Methods are described in the online data supplement (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animal procedures were approved by the Animal Care and Use Committee of Children's Hospital, Harvard Medical School.
Results and discussion
We first established the threshold for detecting rare cells in vivo by titrating increasing numbers of CD31+ TECs9 with 1 × 106 CD31− TRAMP C1 tumor cells. Fluorescence-activated cell sorting (FACS) showed a linear relationship between numbers of TECs added and the percentage of TECs detected (r2 = 0.99; Figure 1A). We could detect as few as 100 CD31+ TECs per 1 million CD31− tumor cells, approximately 0.01% of the total cellular pool. Thus, values approximating 0.01% were considered the technical detection limit using this methodology.
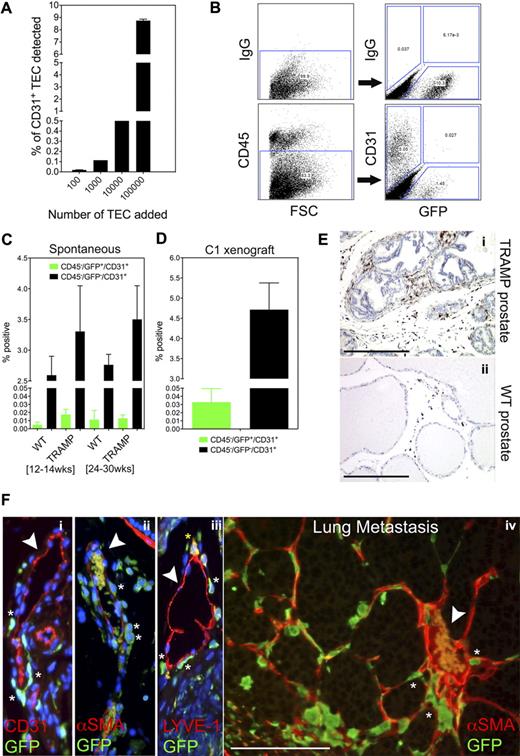
In vivo enumeration of bone marrow–derived endothelial cells in TRAMP prostate tumors. (A) Increasing concentrations of isolated TECs were spiked into 1 × 106 CD31− prostate cancer cells (TRAMP C1). CD31+ TECs were analyzed using FACS. The minimum number of TECs that could be detected was approximately 100 per 106 tumor cells, or approximately 0.01% of the total cellular pool. (B) FACS analysis of a well-differentiated prostate tumor in TRAMP (24 weeks of age) after bone marrow ablation/engraftment at 8 weeks of age. One-half of each tumor was used for histology and grading; the other half was used for FACS analysis. To enumerate BMDECs, live cells were selected from the forward scatter/side scatter (FSC/SSC) plot. FSC (x-axis) is in the linear scale and all fluorophores are in the log scale. CD45+ hematopoietic cells were gated out and the GFP+/CD31+ population was quantified. Resident or non–bone marrow–derived ECs were defined as CD45−/GFP−/CD31+. A third population (∼ 1%-2%) which was CD45−/GFP+/CD31− may be bone marrow mesenchymal stem cells. (C) Multiple prostates from wild-type mice and TRAMP mice were analyzed by FACS: WT, 12 to 14 weeks (n = 5); TRAMP, 12 to 14 weeks (n = 5); WT, 24 to 30 weeks (n = 4); TRAMP, 24 to 30 weeks (n = 5). Data were analyzed by analysis of variance (ANOVA) followed by Tukey multiple comparison test. There was no statistical difference in numbers of BMDECs between both groups and both time points. (D) The TRAMP C1 cell line was implanted subcutaneously in C57BL/6J mice 4 weeks after bone marrow ablation/engraftment. After 28 days, individual tumors (n = 5) were analyzed by FACS as described above. (E) Paraffin sections of either prostate tumors (i) or normal prostate (ii) were stained with GFP antibodies. GFP was detected using a horseradish peroxidase–coupled secondary antibody (brown staining). Nuclei were counterstained with hematoxylin (blue). Most GFP+ cells had a spherical appearance reminiscent of mononuclear cells, while others were larger and spindle-shaped. Scale bars represent 0.5 mm. (F) A well-differentiated prostate tumor stained with GFP antibodies in addition to antibodies for the blood vessel marker CD31 (i), the pericyte marker αSMA (ii), and the lymphatic endothelial cell marker LYVE-1 (iii). White arrows point to blood vessels. Asterisks mark perivascular GFP+ cells and the yellow asterisk marks a LYVE-1+/GFP+ perivascular cell. Note that erythrocytes autofluoresce within the lumen of some blood vessels. Nuclei were counterstained with DAPI (blue, i-iii). A perivascular positioning of GFP+ cells was also observed in a spontaneous lung metastasis in TRAMP mice (iv). The arrow points to a large, erythrocyte-filled tumor blood vessel surrounded by GFP+ marrow–derived cells (no counter stain was used). Scale bar represents 100 μm.
In vivo enumeration of bone marrow–derived endothelial cells in TRAMP prostate tumors. (A) Increasing concentrations of isolated TECs were spiked into 1 × 106 CD31− prostate cancer cells (TRAMP C1). CD31+ TECs were analyzed using FACS. The minimum number of TECs that could be detected was approximately 100 per 106 tumor cells, or approximately 0.01% of the total cellular pool. (B) FACS analysis of a well-differentiated prostate tumor in TRAMP (24 weeks of age) after bone marrow ablation/engraftment at 8 weeks of age. One-half of each tumor was used for histology and grading; the other half was used for FACS analysis. To enumerate BMDECs, live cells were selected from the forward scatter/side scatter (FSC/SSC) plot. FSC (x-axis) is in the linear scale and all fluorophores are in the log scale. CD45+ hematopoietic cells were gated out and the GFP+/CD31+ population was quantified. Resident or non–bone marrow–derived ECs were defined as CD45−/GFP−/CD31+. A third population (∼ 1%-2%) which was CD45−/GFP+/CD31− may be bone marrow mesenchymal stem cells. (C) Multiple prostates from wild-type mice and TRAMP mice were analyzed by FACS: WT, 12 to 14 weeks (n = 5); TRAMP, 12 to 14 weeks (n = 5); WT, 24 to 30 weeks (n = 4); TRAMP, 24 to 30 weeks (n = 5). Data were analyzed by analysis of variance (ANOVA) followed by Tukey multiple comparison test. There was no statistical difference in numbers of BMDECs between both groups and both time points. (D) The TRAMP C1 cell line was implanted subcutaneously in C57BL/6J mice 4 weeks after bone marrow ablation/engraftment. After 28 days, individual tumors (n = 5) were analyzed by FACS as described above. (E) Paraffin sections of either prostate tumors (i) or normal prostate (ii) were stained with GFP antibodies. GFP was detected using a horseradish peroxidase–coupled secondary antibody (brown staining). Nuclei were counterstained with hematoxylin (blue). Most GFP+ cells had a spherical appearance reminiscent of mononuclear cells, while others were larger and spindle-shaped. Scale bars represent 0.5 mm. (F) A well-differentiated prostate tumor stained with GFP antibodies in addition to antibodies for the blood vessel marker CD31 (i), the pericyte marker αSMA (ii), and the lymphatic endothelial cell marker LYVE-1 (iii). White arrows point to blood vessels. Asterisks mark perivascular GFP+ cells and the yellow asterisk marks a LYVE-1+/GFP+ perivascular cell. Note that erythrocytes autofluoresce within the lumen of some blood vessels. Nuclei were counterstained with DAPI (blue, i-iii). A perivascular positioning of GFP+ cells was also observed in a spontaneous lung metastasis in TRAMP mice (iv). The arrow points to a large, erythrocyte-filled tumor blood vessel surrounded by GFP+ marrow–derived cells (no counter stain was used). Scale bar represents 100 μm.
To enumerate BMDECs in tumors or normal prostates, mice were lethally irradiated followed by bone marrow reconstitution with green fluorescent protein (GFP)+ marrow. Using FACS, CD45+ hematopoietic cells were gated out and the GFP+/CD31+ and GFP−/CD31+ populations were analyzed. Figure 1B shows the 3 CD45− populations detected in a well differentiated tumor: GFP−/CD31+ (5.05%), GFP+/CD31+ (0.027%), and GFP+/CD31− (1.45%). Multiple mice analyzed in this way showed that average numbers of CD45−/GFP+/CD31+ were unchanged irrespective of tumor stage (Figure 1C). Moreover, the range of CD45−/GFP+/CD31+ ECs detected in all tissues (normal prostate or prostate tumors) was only 0 to 0.04% (∼ 0.4% of all ECs) and thus barely within the limits of our established threshold. These results contrast another study where BMDEC values as high as 14% for poorly differentiated cancers and 2% for well differentiated cancers were reported.4 Similarly, Spring et al reported stage-dependent increases in BMDECs in ALB-Tag liver tumors and RIP-Tag pancreatic tumors with values approaching 38% of the total TEC pool.10 However, Wickersheim et al found no evidence for BMDECs in B16 melanoma, Lewis lung carcinoma, and TRAMP tumors,11 and another study reported no evidence of BMDECs in multiple tumor models using both bone marrow transplants in irradiated recipients and parabiotic mice in a nonmyeloablative setting.3 These inconsistencies in BMDEC numbers between different laboratories are likely related to the methodology for quantifying BMDECs (FACS versus immunohistochemistry) and a consensus for what constitutes a bona fide BMDEC in tumors is still being debated.12
In contrast to BMDECs, we could readily detect resident ECs (CD45−/GFP−/CD31+) in prostate tumors and their numbers increased by 30% relative to normal prostates in both early (12-14 weeks) and late cancers (24-30 weeks; Figure 1C). Similar results were obtained using TRAMP C1 subcutaneous xenografts although the numbers of CD45−/GFP+/CD31+ ECs were on average almost 2-fold higher than that in spontaneous tumors (Figure 1D).
The GFP+ cells infiltrating these tumors averaged between 10% and 25% of the total tumor mass and were qualitatively increased in tumors versus normal prostate (Figure 1Ei-ii). Colocalizing GFP with the vessel markers CD31 (endothelium), α smooth muscle actin (αSMA; pericytes), and lymphatic vessel endothelial receptor 1 (LYVE-1; lymphatics) showed numerous perivascular GFP+ cells, but none were positioned at the luminal surface of blood vessels in either prostate tumors (Figure 1Fi-iii; supplemental Figure 1Ai-ii) or spontaneous lung metastases (Figure 1Fiv). Occasional perivascular and intraluminal GFP+ cells were also LYVE-1+ consistent with LYVE-1 expression in a subpopulation of tumor-infiltrating macrophages.13,14
To determine the phenotype of the GFP+ bone marrow–derived cells (BMDCs) we used FACS. From the GFP+ cellular pool, approximately 99% were CD45+ hematopoietic cells and 30%-40% of these expressed the monocyte/macrophage markers CD11b/F4-80 and the granulocyte marker GR-1 (Figure 2A-B). The remaining GFP+ cells that were negative for myelomonocytic markers (∼ 60%) are presumed to be lymphocytes or perhaps fibrocytes.15 Immunohistochemistry using double labeling with GFP and CD11b antibodies confirmed the identity of marrow–derived myeloid cells in TRAMP tumors in vivo (Figure 2C).
In vivo and in vitro characterization of bone marrow–derived cells in TRAMP prostate tumors. (A) FACS analysis of spontaneous prostate tumors in TRAMP mice (24-30 weeks of age). Representative plots of GFP+ cells retrieved from tumors costained with the indicated antibodies is shown. (B) Quantitative analysis of hematopoietic/myeloid cells in TRAMP prostate tumors (n = 3). Approximately 99% of all BMDC are CD45+ hematopoietic cells. (C) Sections of well-differentiated prostate tumors were stained with GFP and CD11b antibodies (i). Yellow cells indicate the merged coexpression. Nuclei were counterstained with DAPI (blue). Scale bar represents 100 μm. (D) After bone marrow ablation/engraftment, collagenase-digested prostate tumors were plated into tissue-culture dishes. Numerous GFP+ colonies appeared after approximately 1 week in culture from which spindle-shaped GFP+ cells emerged (i). Scale bar represents 0.5 mm. After 2 weeks, cells were treated with trypsin, and tightly adhered, trypsin-resistant cells TD-CFUs were analyzed by qRT-PCR. Similar to PBM cells, TD-CFUs were enriched in expression of CD45, CD11b, F4-80, and GR-1 but not VE-Cadherin (ii). Also by qRT-PCR, MMP2 is predominately expressed by TECs, while PBM cells and TD-CFUs express both MMP9 and MMP2 (iii). (E) TECs formed branching, cord-like structures when plated at low densities and admixed with freshly isolated PBM cells on thin matrigel layers. PBM cells, which appear as small, refractive cells in the high-powered magnification, closely aligned with these branching structures along the TEC surface (i). At right, the same field is shown where TECs fluoresce in red and PBM cells from GFP+ donor mice fluoresce in green (ii). Scale bars represent 50 μm. (F) PBM cells addition increased TEC branching when cells were plated in low serum and at low density on matrigel. The addition of TECs only resulted in few branching structures (i) while TECs cocultured with PBM cells resulted in TEC branching and tube formation (ii). PBM cells addition increased TEC branching 10-fold when averaged from 3 independent observations (iii). Data were evaluated by Student t test and the P value is indicated on the graph. Scale bars represent 0.5 mm.
In vivo and in vitro characterization of bone marrow–derived cells in TRAMP prostate tumors. (A) FACS analysis of spontaneous prostate tumors in TRAMP mice (24-30 weeks of age). Representative plots of GFP+ cells retrieved from tumors costained with the indicated antibodies is shown. (B) Quantitative analysis of hematopoietic/myeloid cells in TRAMP prostate tumors (n = 3). Approximately 99% of all BMDC are CD45+ hematopoietic cells. (C) Sections of well-differentiated prostate tumors were stained with GFP and CD11b antibodies (i). Yellow cells indicate the merged coexpression. Nuclei were counterstained with DAPI (blue). Scale bar represents 100 μm. (D) After bone marrow ablation/engraftment, collagenase-digested prostate tumors were plated into tissue-culture dishes. Numerous GFP+ colonies appeared after approximately 1 week in culture from which spindle-shaped GFP+ cells emerged (i). Scale bar represents 0.5 mm. After 2 weeks, cells were treated with trypsin, and tightly adhered, trypsin-resistant cells TD-CFUs were analyzed by qRT-PCR. Similar to PBM cells, TD-CFUs were enriched in expression of CD45, CD11b, F4-80, and GR-1 but not VE-Cadherin (ii). Also by qRT-PCR, MMP2 is predominately expressed by TECs, while PBM cells and TD-CFUs express both MMP9 and MMP2 (iii). (E) TECs formed branching, cord-like structures when plated at low densities and admixed with freshly isolated PBM cells on thin matrigel layers. PBM cells, which appear as small, refractive cells in the high-powered magnification, closely aligned with these branching structures along the TEC surface (i). At right, the same field is shown where TECs fluoresce in red and PBM cells from GFP+ donor mice fluoresce in green (ii). Scale bars represent 50 μm. (F) PBM cells addition increased TEC branching when cells were plated in low serum and at low density on matrigel. The addition of TECs only resulted in few branching structures (i) while TECs cocultured with PBM cells resulted in TEC branching and tube formation (ii). PBM cells addition increased TEC branching 10-fold when averaged from 3 independent observations (iii). Data were evaluated by Student t test and the P value is indicated on the graph. Scale bars represent 0.5 mm.
Collagenase-digested tumors plated into tissue culture dishes produced numerous GFP+ tumor-derived colony-forming units (TD-CFUs); however, none of these colonies had the morphology of ECs. Instead, TD-CFUs closely resembled previously described CFU-EC, which were proven to be formed by hematopoietic cells and not by ECs (Figure 2Di).16 Some TD-CFUs showed overlapping expression of CD11b/CD11c (dendritic cells and/or inflammatory monocytes) and occasional TD-CFUs were GR-1+/Ly-6G+ (true granulocytes), but the majority of TD-CFUs were Ly-6G−, which is more indicative of monocytes and not neutrophils (supplemental Figure 1B). The predominance of monocytes may be because TD-CFUs were placed in culture before FACS analysis and it is uncertain if neutrophils would thrive in the culture conditions. Thus, bone marrow–derived cells in these tumors are characterized by overlapping expression of common myeloid surface markers that may differ depending on tumor stage and type.17,18 We purified RNA from TD-CFUs and performed quantitative reverse transcription polymerase chain reaction (qRT-PCR). As expected, TD-CFUs showed enriched expression for the myeloid markers CD45, CD11b, F4-80, and GR-1, similar to peripheral blood mononuclear (PBM) cells that served as a positive control (Figure 2Dii). In contrast to TECs, TD-CFUs did not express any transcripts for the EC marker VE-Cadherin, further ruling out the possibility that TD-CFUs are ECs. The bone marrow origin of TD-CFUs was confirmed by expression of GFP using semiquantitative RT-PCR (supplemental Figure 1C). Of note, TECs strongly expressed mRNAs for MMP2 but not MMP9, whereas PBM cells predominately expressed MMP9 and to a lesser extent MMP2 (Figure 2Diii). The enzymatic activity was confirmed by zymography (supplemental Figure 1D). TD-CFUs also expressed both MMP9 and MMP2 mRNAs. In all 3 cell types, transcripts for MMP3 were similar but MMP10 was undetectable. Thus, TECs and circulating myelomonocytic cells may complement one another during tumor angiogenesis by a reciprocal expression of some MMPs.19,20
We hypothesized that PBM cells would augment in vitro cord formation when admixed with TEC cultures through a complementary expression of proangiogenic factors.21 Indeed, freshly isolated PBM cells closely aligned with TECs in matrigel (Figure 2Ei-ii) and PBM cells addition resulted in a 10-fold increase in TEC branching structures (Figures 2Fi-iii and S1E). Thus, our results are in good agreement with in vivo models whereby tumors actively recruit myeloid cells needed for a postulated angiogenic switch.22,23 While tumor cells are thought to be the predominant source of chemotactic factors for monocytes and neutrophils, another possibility is that chronically inflamed TECs may stimulate myeloid cell tropism and trafficking through ‘angiocrine’ expression of chemokines and cellular adhesion molecules, respectively.24 Indeed, we have found overexpression of both adhesion molecules and chemotactic factors in isolated cultures of TECs (A.C.D., unpublished data, January 2010) consistent with the view that a unique vascular niche in tumors conscripts multiple host-derived proangiogenic cell types. Taken together, our results indicate that BMDCs in TRAMP tumors are rarely, if ever, ECs. Instead, the majority of BMDCs are CD45+ hematopoietic cells that closely associate with tumor blood vessels, express proangiogenic factors, and can stimulate angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kristen Johnson for excellent assistance with the figures.
This work was supported by grants from the National Cancer Institute of the National Institutes of Health (CA140708 to A.C.D.; CA45548 and CA037392 to M.K.; and CA4554818 to M.A.M.).
National Institutes of Health
Authorship
Contribution: A.C.D. designed and carried out the experiments, interpreted the data, and wrote the manuscript; T.U. aided with bone marrow transplantations and edited the manuscript; J.M.M.-M. provided reagents, assisted with experimental design, and edited the manuscript; S.-C.S. performed qRT-PCR; A.C. performed gelatin zymography; M.A.M. provided reagents, interpreted and quantified gelatin zymograms, and edited the manuscript; and M.K. provided reagents and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Andrew C. Dudley, Harvard Medical School and Children's Hospital Boston, 300 Longwood Ave, Boston, MA 02115; e-mail: andrew.dudley@childrens.harvard.edu.