Abstract
Hematogenous metastasis is promoted by interactions of tumor cells with leukocytes, platelets, and the endothelium in the local intravascular microenvironment. Here we show that the activation of the microvascular endothelium results in recruitment of monocytes to metastatic tumor cells and promotes the establishment of the metastatic microenvironment. This inflammatory-like endothelial response was observed in microvascular endothelial cells only. Microarray analysis of microvascular endothelial cells cocultured with tumor cells in the presence of leukocytes and platelets revealed a specific gene expression profile. Selectin-mediated interactions of tumor cells with platelets and leukocytes activated endothelial cells and induced production of C-C chemokine ligand 5 (CCL5). Inhibition of CCL5-dependent monocyte recruitment during the early phase of metastasis by a CCL5 receptor antagonist strongly reduced tumor cell survival and attenuated metastasis. Collectively, these findings demonstrate that the endothelial expression of CCL5 contributes to the formation of a permissive metastatic microenvironment.
Introduction
Metastasis remains the major cause for cancer-related death. Hematogenous metastasis comprises multiple steps including tumor cell dissemination through the circulation, arrest in the microvasculature, and ultimately colonization of distant organs.1,2 The known inefficiency of the metastatic process implies that tumor cells are very limited in ability to colonize and to grow in healthy tissues of distant organs.3 Thus, tumor cells survive and proliferate at distant sites only within a favorable microenvironment.3 Cell-cell interactions among tumor cells, platelets, leukocytes, and endothelial cells were shown to contribute to the enhanced tumor cell survival and to the facilitation of hematogenous metastasis.3,4 Platelet association with intravascular tumor cells enhances hematogenous dissemination and protects tumor cells from immune-mediated clearance.5,6 Activated platelets release cytokines and present surface molecules, which are able to activate endothelial cells.7 Several cell adhesion molecules including glycoprotein IIb/IIIa integrin and P-selectin are known to mediate platelet–tumor cell interactions and facilitate metastasis.8,9 In addition, activated platelets are commonly observed in the circulation of cancer patients.10
Although the contribution of leukocytes to the formation of primary tumors is well recognized, mechanisms by which leukocytes govern the process of metastasis remains poorly understood.11,12 Depending on the microenvironmental signals, leukocytes can either exert antitumoral activity or promote cancer progression.12 Tumor-associated myeloid cells including polymorphonuclear leukocytes (PMNs), monocytes, as well as differentiated macrophages were shown to facilitate immunosuppression, angiogenesis, tumor cell survival, and invasion and thereby contribute to metastatic dissemination.11,12 Previously, we showed that the absence of L-selectin results in an attenuation of tumor cell survival and metastasis, implicating leukocytes in the colonization process.13,14
Activation of the endothelium is observed both in advanced cancer patients and within the microenvironment of experimentally metastasizing tumor cells.15-17 Activated microvascular endothelial cells increase the expression of cell adhesion molecules and the production of chemokines, which leads to a recruitment of leukocytes, neutrophils, and monocytes.18 The activated state of endothelial cells is reflected in increased levels of soluble endothelial cell adhesion molecules, for example, E-selectin and vascular cell adhesion molecule 1 (VCAM-1), as found in the serum of colorectal cancer patients.17 In a mouse model of liver metastasis, sinusoidal microvascular endothelial cells were activated upon interaction of colorectal tumor cells with resident Kupffer cells.15 Inhibition of endothelial activation was shown to attenuate metastasis in several mouse models.4,16,19 By contrast, experimental activation of the endothelium by the administration of cytokines prior to the inoculation of tumor cells led to enhanced metastasis.20
Although the local importance of the endothelium in metastasis is increasingly accepted, its function in the creation of the metastatic microenvironment remains poorly understood. The present study was designed to analyze the function of the endothelium during the initial steps of metastasis. Here we report that interactions of tumor cells with platelets and leukocytes activate microvascular endothelial cells, resulting in up-regulation of endothelial C-C chemokine ligand 5 (CCL5) production and promotion of metastasis.
Methods
Cell culture
Human colorectal cancer cells LS180 and Caco-2 were grown in Dulbecco modified Eagle medium with 10% fetal calf serum (Invitrogen). HT-29 and Colo205 cells were grown in McCoy and RPMI 1640 medium containing 10% fetal calf serum (Invitrogen), respectively. MC-38GFP murine carcinoma cells were grown as described previously.13 Human lung microvascular endothelial cells (HMVECs) and human umbilical vein endothelial cells (HUVECs) were cultured on fibronectin-coated plates in endothelial cell growth medium–2 microvascular (EGM-2MV) or EGM-2 medium, respectively (Lonza).
Isolation of platelets and leukocytes
Human platelets were isolated as described previously.21 Briefly, 50 mL citrated blood from healthy volunteers was centrifuged at 200g. Platelets collected in the upper phase were washed in piperazine-1,4′bis(2-ethanesulfonic acid (PIPES) buffered saline (pH 6.8). Peripheral blood mononuclear cells were separated from PMNs and red blood cells on a Ficoll gradient (GE Health Services). PMNs were purified by dextran sedimentation, followed by lysis of red blood cells with PharmLyse (BD Biosciences). Monocytes were isolated from peripheral blood mononuclear cells using the monocyte isolation kit II (Miltenyi Biotec). The purity of blood elements reached more than 95% in all experiments as confirmed by Wright-Giemsa staining and flow cytometry.
Coculture and magnetic separation of endothelial cells
Adherent, quiescent HMVECs or HUVECs were cocultured with tumor cells (2 × 105 cells per milliliter), PMNs, monocytes, and platelets in EGM-2MV or EGM-2 cell medium, respectively. Leukocytes and platelets were added at similar ratios as found in the human blood (4 × 106 PMNs, 4 × 105 monocytes, and 150 × 106 platelets per milliliter). After 8 hours of coculture, adherent cells were detached and incubated with biotinylated anti-CD11b and anti–epithelial cell adhesion molecule (EpCAM) monoclonal antibodies (BD Biosciences and Neomarkers), followed by magnetic cell depletion using anti–biotin-beads (Miltenyi Biotec). The purity of blood elements reached more than 95% in all experiments as confirmed by Wright-Giemsa staining and flow cytometry (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
RNA isolation and microarray analysis
Total endothelial RNA was isolated using Tri-Reagent (Sigma-Aldrich). Biotinylated cRNA was hybridized to Affymetrix Human Genome U133 Plus 2.0 oligonucleotide microarrays and labeled according to the manufacturer's protocol (Affymetrix). Microarrays were scanned with the Affymetrix Scanner 3000 and intensities were MAS 5.0 normalized (Affymetrix). Determination of differential expression was performed with the GeneSpring software (Agilent). All microarray analysis has been deposited into the GEO public database under accession number GSE18113.22
Quantitative RT-PCR
After the removal of genomic DNA by DNAse digestion (Promega) single-stranded cDNA (QIAGEN) was prepared from total RNA. Primers for human CCL5, matrix metalloproteinase-1 (MMP1), MMP10, regulator of G-protein signaling 4 (RGS-4), versican, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), EpCAM, C-C chemokine receptor type 5 (CCR5), and murine CCL5 and GAPDH are listed in supplemental Table 1. Quantitative polymerase chain reaction (PCR) was performed in a MX-300P thermocycler (Stratagene) using a SYBR Green kit (Sigma-Aldrich) and relative changes were quantified.
Detection of CCL5 secretion
Endothelial cells were cocultured in different conditions for 8 hours. Washed, adherent cells were incubated in freshly added medium for another 12 hours. Protein concentration of CCL5 in the supernatant was analyzed with a cytometric bead array (CBA; BD Biosciences).
Detection of CCL5 in vivo
Lung cryosections from mice injected with MC-38GFP cells were incubated with an antimurine CCL5 antibody (R&D Systems), and anti–CD31-biotin–conjugated antibody (BD Biosciences). CCL5 staining was detected with donkey-anti–goat Ab conjugated with Alexa 568 (Invitrogen), and CD31+ endothelial cells were detected by streptavidin-Alexa 660 (Invitrogen). The presence of CCL5 in tissues was analyzed with a confocal microscope (SP5; Leica) and images were quantified with the Imaris software (Bitplane).
Endothelial cell activation
HMVECs or HUVECs were cocultured in different conditions for 8 hours. In some experiments, 10 μg/mL of a P-selectin blocking antibody (Syngenta) was added to the coculture or tumor cells were treated with O-sialoglycoproteinase (OSGPase; Cedarlane). After coculture, cells were detached with 2 mM ethylenediaminetetraacetic acid/phosphate-buffered saline (PBS) and incubated with an anti-CD105 (R&D Systems) and anti–E-selectin (BD Biosciences) antibodies, followed by flow cytometry (FACSCanto; BD Biosciences).
Chemotaxis and adhesion assays
HMVEC layers were washed twice after 12 hours of coculture. Transwell inserts (8 μM; BD Biosciences) containing 3 × 105 PKH2-labeled (Sigma-Aldrich) human monocytes were placed over the cocultured, washed HMVEC layers. Migrated PKH2-positive cells were counted in the lower chamber after 8 hours. In some experiments, 1 μg/mL of a CCL5 function-blocking antibody (2D5; BD Biosciences) or the corresponding isotype control (MOPC-21; BD Biosciences) was added. In others, Met-RANTES was added (2 μg/mL).23
Adhesion of PKH2-labeled monocytes (105) to the activated cocultured HMVECs was analyzed after 2 hours of incubation.
Nuclear translocation of p65
HMVECs and HUVECs were cocultured in different conditions. Cells were stained with anti-p65 and anti–CD31-fluorescein isothiocyanate (BD Biosciences) monoclonal antibodies in PBS containing 1% bovine serum albumin and 0.1% Triton X-100. Staining of p65 was detected with goat-anti–mouse Ab conjugated with Alexa 568 (Invitrogen). Slides were mounted with Prolong and analyzed by confocal microscopy (SP5; Leica; magnification 63×/1.30 NA oil objective). Measurements were normalized to the background signal of p65 nuclear localization in quiescent endothelial cells.
Experimental metastasis model
C57BL/6J mice were intravenously injected with murine MC-38GFP cells (3 × 105) and metastasis was evaluated by green fluorescent protein (GFP) measurement in lung homogenates after 21 days.13 Mice received 10 μg Met-RANTES intraperitoneally 6 hours before and 6, 18, 30, and 42 hours after the tumor cell injection.
Human LS180 cells (4.5 × 105) were intravenously injected in NMRI athymic mice that were treated with Met-RANTES during the first 48 hours. After 5 weeks, mice were killed and the extent of metastasis was determined by a quantitative reverse-transcription (RT)–PCR for detection of human-specific EpCAM transcripts isolated from murine lungs. Metastatic nodules were counted in lung sections stained with hematoxylin and eosin (Leica SP5; magnification 40×/0.75 NA air objective). All animal protocols were approved by the Zürich Kantonal committee.
Analysis of leukocyte–tumor cell association
Lung sections prepared from mice intravenously injected with MC-38GFP cells were stained with a F4/80 monoclonal antibody (AbD) detecting macrophages/monocytes. F4/80 staining was visualized by the secondary goat-anti–rat Ab conjugated with Alexa 568 (Invitrogen).
Statistics
Statistical analysis was performed with the GraphPad Prism software (Version 4.0) or Genespring GX software (Version 7.3; Agilent). All data are presented as mean plus or minus SEM and were analyzed by analysis of variance (ANOVA) with the posthoc Bonferonni multiple comparison test, unless specified differently.
Results
Tumor cells activate microvascular endothelial cells only in the presence of leukocytes and platelets
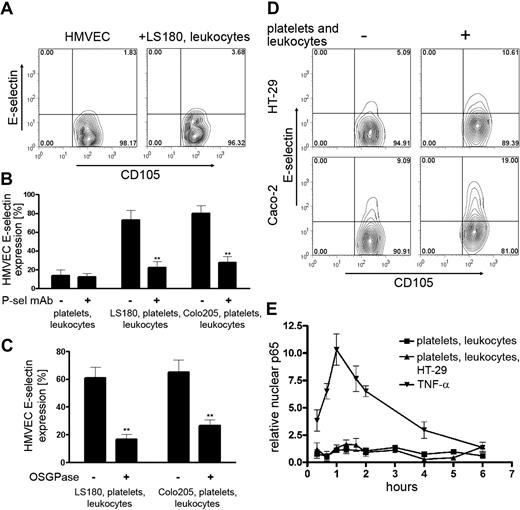
The assessment of the temporal and spatial molecular interactions causing the endothelial activation during metastatic initiation is technically difficult in vivo. We therefore developed an in vitro coculture system mimicking the hematogenous metastatic microenvironment. Human tumor cells, LS180 and Colo205, were cultured on monolayers of human microvascular endothelial cells (HMVECs) and the activation status of the endothelial cells was determined by E-selectin detection. Cell surface expression of E-selectin is associated with endothelial activation.15,18 Tumor cells alone did not induce expression of E-selectin on HMVECs (Figure 1A-B). We then hypothesized that leukocytes and platelets can contribute to the tumor cell–induced endothelial cell activation observed in vivo.4,15,16 We previously described the recruitment of innate immune cells, PMNs and monocytes, to metastasizing tumor cells.14 Therefore PMNs, monocytes, and platelets were added to the coculture of tumor cells on HMVECs. A strong induction of E-selectin expression on HMVECs was observed both with LS180 and Colo205 cells in this coculture condition (Figure 1A-B). Similarly, an enhanced expression of VCAM-1, an alternate activation marker of endothelial cells, was detected on HMVECs (supplemental Figure 2). In contrast, coculture of tumor cells individually with either platelets, PMNs, or monocytes caused no or minimal expression of E-selectin, further supporting the requirement of several blood elements for endothelial activation (supplemental Figure 2). Transcriptional up-regulation of proinflammatory markers, for example, E-selectin and VCAM-1, is known to be regulated by the nuclear factor κB (NF-κB) signaling pathway.18,24 We analyzed the activation of the NF-κB pathway by detection of p65 (subunit of NF-κB) in the nucleus of endothelial cells (Figure 1C-D). Stimulation of endothelial cells with tumor necrosis factor-α (TNFα) is known to induce nuclear localization of p65, and this activation was used as a positive control.18 Inflammatory-like activation of HMVECs was determined by p65 localization in the nucleus and could be confirmed only in coculture with LS-180 cells, leukocytes, and platelets.
Interactions of tumor cells with platelets and leukocytes activate HMVECs. (A) Human lung microvascular endothelial cells (HMVECs) were cocultured with LS180 or Colo205 cells in the absence or presence of polymorphonuclear leukocytes (PMNs), monocytes, and platelets. Representative flow cytometric data of E-selectin expression on HMVECs are shown. CD105 was used to identify HMVECs. (B) Statistical analysis of multiple coculture experiments (n = 4-6) measuring E-selectin expression on HMVECs. Percentage of E-selectin–positive HMVECs is presented (mean ± SEM). Statistical significance was determined by Bonferroni multiple comparison test. **P < .01; ***P < .001. (C) Representative fluorescence images of p65 localization in HMVECs. Nuclear localization of p65 was determined by colocalization (white) of p65 (red) with DAPI (4,6 diamidino-2-phenylindole [Sigma]; blue. HMVECs were detected by CD31 antibody (green). (D) Relative nuclear localization of p65 in HMVECs determined by Imaris software. Data are presented as mean ± SEM of 3 independent experiments.
Interactions of tumor cells with platelets and leukocytes activate HMVECs. (A) Human lung microvascular endothelial cells (HMVECs) were cocultured with LS180 or Colo205 cells in the absence or presence of polymorphonuclear leukocytes (PMNs), monocytes, and platelets. Representative flow cytometric data of E-selectin expression on HMVECs are shown. CD105 was used to identify HMVECs. (B) Statistical analysis of multiple coculture experiments (n = 4-6) measuring E-selectin expression on HMVECs. Percentage of E-selectin–positive HMVECs is presented (mean ± SEM). Statistical significance was determined by Bonferroni multiple comparison test. **P < .01; ***P < .001. (C) Representative fluorescence images of p65 localization in HMVECs. Nuclear localization of p65 was determined by colocalization (white) of p65 (red) with DAPI (4,6 diamidino-2-phenylindole [Sigma]; blue. HMVECs were detected by CD31 antibody (green). (D) Relative nuclear localization of p65 in HMVECs determined by Imaris software. Data are presented as mean ± SEM of 3 independent experiments.
Cell-cell interactions are responsible for the activation of microvascular endothelial cells
Tumor cell interactions with platelets and leukocytes (PMNs and monocytes) can be mediated by selectins.9,13,21,25 Furthermore, selectin-mediated interactions facilitate cell activation.26 Because the coculture of LS180 cells with leukocytes but without platelets did not induce E-selectin expression in HMVECs (Figure 2A), we hypothesized that P-selectin–mediated interactions of platelets with tumor cells are responsible for the observed activation of endothelial cells. Upon addition of a function-blocking P-selectin antibody to the coculture, minimal activation of endothelial cells could be detected (Figure 2B). To further confirm the role of selectins in the initiation of endothelial activation, we removed the selectin ligands on LS180 and Colo205 cells by treating with O-sialoglycoproteinase prior to coculturing on HMVECs with PMNs, monocytes, and platelets. Removal of carcinoma mucins by O-sialoglycoproteinase treatment led to a significant reduction of endothelial activation (Figure 2C). Finally, we analyzed HT-29 and Caco-2 tumor cells that are lacking ligands for L- and P-selectins.25 Whereas a coculture with only HT-29 or Caco-2 cells did not induce E-selectin expression on HMVECs, coincubation of these tumor cells together with leukocytes and platelets led to a minimal enhancement of E-selectin expression and no activation of the NF-κB pathway (Figure 2D-E). These findings indicate that selectin-mediated cell-cell interactions within the microenvironment of tumor cells mediate the activation of the endothelium.
Selectin ligands on tumor cells mediate the inflammatory response of HMVECs. (A) E-selectin expression by quiescent HMVECs (left) and by HMVECs cocultured with LS180, PMNs, and monocytes (right). (B) E-selectin expression on HMVECs after the coculture with blood elements and LS180 or Colo205 cells in the presence of a P-selectin function-blocking antibody (10 μg/mL). (C) E-selectin expression on HMVECs cocultured with blood elements and LS180 or Colo205 cells, treated with O-sialoglycoproteinase (OSGPase) or sham treated. Mean ± SEM. **P < .01 by 2-tailed Student t test. (D) Representative flow cytometric data of E-selectin expression on HMVECs cocultured with HT-29 or Caco-2 cells either in the absence or presence of blood elements (PMNs, monocytes, and platelets). (E) Relative nuclear translocation of p65 in HMVECs cocultured with HT-29 cells, leukocytes, and platelets. Stimulation with TNF-α was used as a positive control of endothelial activation. Mean ± SEM of 3 independent experiments.
Selectin ligands on tumor cells mediate the inflammatory response of HMVECs. (A) E-selectin expression by quiescent HMVECs (left) and by HMVECs cocultured with LS180, PMNs, and monocytes (right). (B) E-selectin expression on HMVECs after the coculture with blood elements and LS180 or Colo205 cells in the presence of a P-selectin function-blocking antibody (10 μg/mL). (C) E-selectin expression on HMVECs cocultured with blood elements and LS180 or Colo205 cells, treated with O-sialoglycoproteinase (OSGPase) or sham treated. Mean ± SEM. **P < .01 by 2-tailed Student t test. (D) Representative flow cytometric data of E-selectin expression on HMVECs cocultured with HT-29 or Caco-2 cells either in the absence or presence of blood elements (PMNs, monocytes, and platelets). (E) Relative nuclear translocation of p65 in HMVECs cocultured with HT-29 cells, leukocytes, and platelets. Stimulation with TNF-α was used as a positive control of endothelial activation. Mean ± SEM of 3 independent experiments.
Activated endothelial cells express genes associated with metastasis
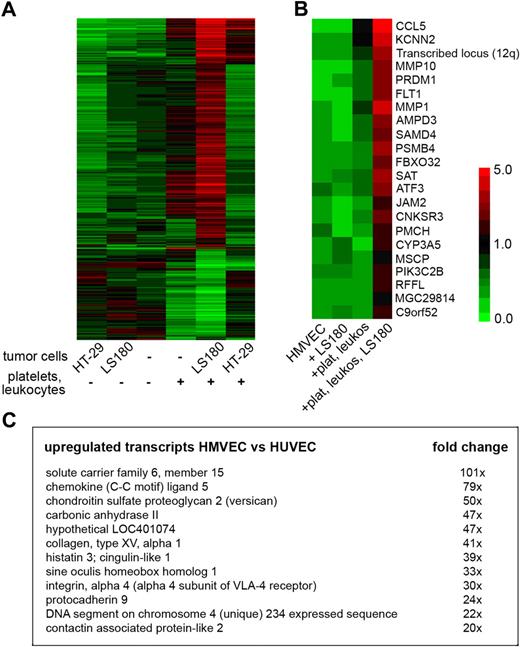
To characterize the endothelial response within a metastatic microenvironment, we analyzed the transcriptome of cocultured HMVECs using oligonucleotide microarrays (data deposited in GEO public database under the accession number GSE1811322 ). The comparison of HMVECs cocultured with tumor cells and blood elements (platelets, PMNs, and monocytes) with HMVECs cocultured only with blood elements led to the identification of several genes previously associated with cancer progression and metastasis (Figure 3A-B). Among others, an enhanced expression of matrix metalloproteinases MMP1 and MMP10 was detected (Figure 3B). These proteinases were previously shown to influence the primary tumor growth as well as the colonization of lungs.27,28 CCL5, a known inflammatory chemokine (also known as RANTES), was among the most highly induced genes (Figure 3B). The enhanced expression of identified genes was validated by quantitative RT-PCR analysis (supplemental Figure 3). CCL5 was previously shown to be implicated in the promotion of breast cancer metastasis.29 Detection of CCL5 was also found to be associated with cancer progression of different other carcinomas.30-32 We detected a more than 5-fold increase in CCL5 expression by HMVECs cocultured with LS180, leukocytes, and platelets for 8 hours compared with HMVECs cocultured with only leukocytes, and platelets. CCL5 was not detected in HMVECs alone or in HMVECs cocultured with only LS180 cells and was minimally induced upon incubation with platelets and leukocytes only (Figure 3B). Enhanced E-selectin expression detected by microarrays (supplemental Figure 4) confirmed the initial flow cytometric analysis (Figure 1, supplemental Figure 2).
Coculture of endothelial cells with tumor cells and blood elements induces expression of inflammation-associated genes including CCL5. (A) Expression map (red indicates strong expression; green, no expression) of genes that were significantly differentially expressed (P < .05, ANOVA, Benjamini-Hochberg posthoc test) in HMVECs cocultured as indicated. (B) Expression map of the highest up-regulated endothelial transcripts in HMVECs cocultured with blood elements and LS180 cells (+plat, leukos, LS180) compared with HMVECs cocultured only with leukocytes and platelets (+plat, leukos). Mean expression of 3 independent experiments is presented by the indicated color code (P < .05 by 2-tailed Student t test). (C) Fold changes of genes that were strongly induced in HMVECs cocultured with blood elements and LS180 cells compared with human umbilical vein endothelial cells (HUVECs) cocultured in the same condition. Genes that were differentially expressed between quiescent HMVECs and quiescent HUVECs were previously subtracted from the list (P < .05 by 2-tailed Student t test).
Coculture of endothelial cells with tumor cells and blood elements induces expression of inflammation-associated genes including CCL5. (A) Expression map (red indicates strong expression; green, no expression) of genes that were significantly differentially expressed (P < .05, ANOVA, Benjamini-Hochberg posthoc test) in HMVECs cocultured as indicated. (B) Expression map of the highest up-regulated endothelial transcripts in HMVECs cocultured with blood elements and LS180 cells (+plat, leukos, LS180) compared with HMVECs cocultured only with leukocytes and platelets (+plat, leukos). Mean expression of 3 independent experiments is presented by the indicated color code (P < .05 by 2-tailed Student t test). (C) Fold changes of genes that were strongly induced in HMVECs cocultured with blood elements and LS180 cells compared with human umbilical vein endothelial cells (HUVECs) cocultured in the same condition. Genes that were differentially expressed between quiescent HMVECs and quiescent HUVECs were previously subtracted from the list (P < .05 by 2-tailed Student t test).
By contrast, coculturing HMVECs with HT-29 cells, leukocytes, and platelets induced an entirely different expression profile than with LS180 cells (Figure 3A and supplemental Figure 5). These results were in agreement with previously observed differences in the endothelial cell activation (Figures 1A, 2D). Accordingly, CCL5 expression of HMVECs was only minimally induced in a coculture of HT-29 cells with blood elements (supplemental Figure 3). These results indicate that HT-29 and LS180 use different means to interact with their metastatic microenvironment. However, one gene in particular, the regulator of G-protein signaling 4 (RGS-4), was found to be strongly down-regulated in HMVECs cocultured with both tumor cell lines in the presence of blood elements. RGS-4 is an inhibitor of G-protein signaling and was identified among down-regulated genes in endothelial cells during angiogenesis.33 RGS-4 expression in HMVECs was slightly reduced when cocultured with leukocytes and platelets alone, but was practically absent in cocultures with LS180 or HT-29 cells (supplemental Figure 3).
Microvascular endothelial cells respond specifically to interactions with tumor cells
Endothelial cells are phenotypically heterogeneous depending on their location in the vascular tree.34 Interactions of tumor cells with the endothelium during metastatic colonization occur primarily on a microvascular level.1,27 Thus, we aimed to compare the interactions of tumor cells with microvascular endothelial cells (HMVECs) and macrovascular endothelial cells (human umbilical vein endothelial cells [HUVECs]). In contrast to HMVECs, coculture of HUVECs with tumor cells and blood elements resulted in minimal enhancement of E-selectin expression (supplemental Figure 4). Microarray analysis of purified HMVECs and HUVECs from cocultures with LS180 cells and blood elements yielded more than 1000 genes, which were more than 2-fold differently expressed (P < .05). Specific induction of CCL5 and versican was observed in HMVECs, whereas it was absent in HUVECs (Figure 3C and supplemental Figure 3). Versican interactions with CD44 contribute to the retention of inflammatory cells.35 CCL5 and other chemokines are known to bind versican, which thereby modulates the chemotaxis of leukocytes.35 Our findings demonstrate that microvascular endothelial cells are more responsive to tumor cell–induced stimulation than macrovascular endothelial cells.
Microvascular CCL5 expression is stimulated by tumor cells, PMNs, and platelets
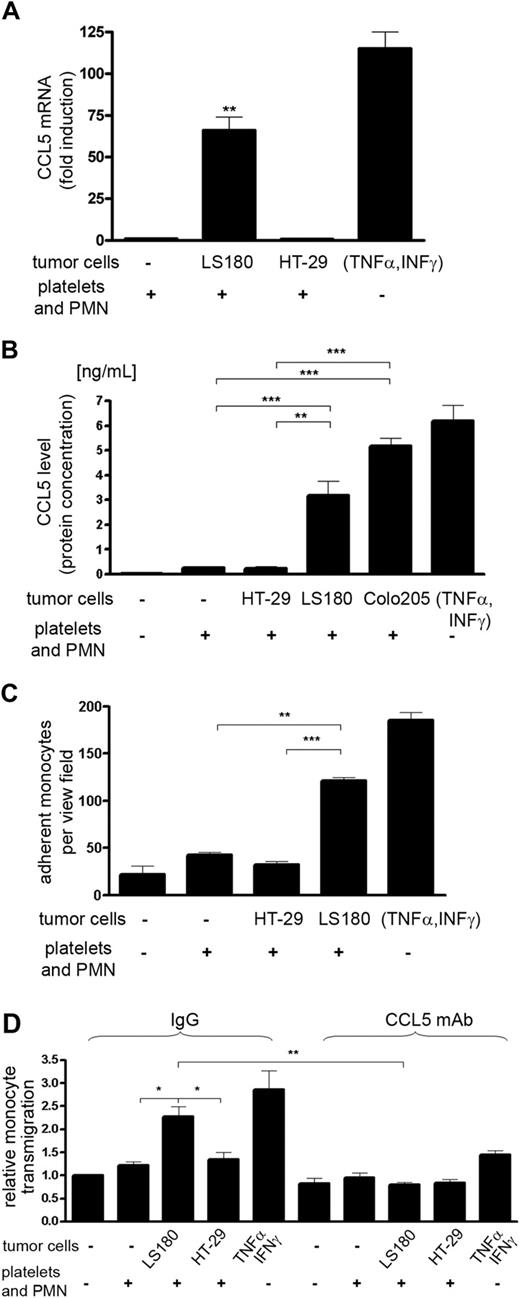
Due to the known association of CCL5 with cancer progression,29,30,36 we further evaluated the functionality of CCL5 expression by HMVECs. CCL5 is chemotactic for different subtypes of leukocytes that have been involved in cancer progression including myeloid cells such as monocytes.37 Thus, we tested the possibility that interactions of tumor cells with PMNs and platelets can stimulate the expression of CCL5 in the microvasculature leading to the recruitment of monocytes. HMVECs were cocultured with platelets, PMNs, and LS180 or Colo205 cells, followed by detection of CCL5 (Figure 4A-B). Significant production of CCL5 was detected both on transcript and protein level. Again, the removal of selectin ligands from tumor cells by O-sialoglycoproteinase treatment significantly reduced secretion of CCL5 by HMVECs (supplemental Figure 6). To ensure that the secreted CCL5 originated from activated HMVECs, adherent cells were extensively washed after coculture and the production of CCL5 was determined in freshly added medium. Thus, we excluded the contribution of platelet-derived CCL5. Tumor cells (LS180 and HT-29) do not express any CCL5.38 Monocyte adherence to and migration toward HMVECs that were previously cocultured with PMNs, platelets, and LS180 cells were increased (Figure 4C-D and supplemental Figure 7), thereby suggesting that the interactions of tumor cells with platelets and PMNs are sufficient for the endothelial activation. Stimulation of HMVECs with TNF-α and interferon γ proved that CCL5 expression can be induced in HMVECs by inflammatory cytokines, and the amounts of CCL5 are relevant for the recruitment of monocytes. Furthermore, the migration of monocytes could be blocked by the addition of an anti-CCL5 antibody (Figure 4D). Similarly, Met-RANTES, a CCR1 and CCR5 receptor antagonist,23,39 led to inhibition of monocyte migration (supplemental Figure 7). Hence, we conclude that selectin-dependent cell-cell interactions between tumor cells, PMNs, and platelets stimulate functional CCL5 secretion by microvascular endothelial cells.
Endothelial CCL5 expression. (A) Detection of C-C chemokine ligand 5 (CCL5) mRNA in HMVECs purified from cocultures as indicated. Mean ± SEM; n = 3. As positive controls, HMVECs were stimulated with TNFα (10 ng/mL) and interferon γ (1 ng/mL). (B) CCL5 protein levels secreted by cocultured HMVECs and measured by cytometric bead array. The de novo CCL5 production was measured in the newly added medium after 12 hours. Mean ± SEM; n = 6-8. (C) Adherence of monocytes to HMVECs activated by different coculture conditions. Monocytes were allowed to adhere to HMVEC layers for 2 hours. Mean ± SEM; n = 4. (D) Migration of monocytes toward cocultured HMVECs. Migration of monocytes was determined in the presence of a CCL5-blocking antibody or of a corresponding isotype control IgG. Mean ± SEM; 3 independent experiments. Statistical significance was determined ANOVA with Bonferonni multiple comparison test; *P < .05, **P < .01, ***P < .001.
Endothelial CCL5 expression. (A) Detection of C-C chemokine ligand 5 (CCL5) mRNA in HMVECs purified from cocultures as indicated. Mean ± SEM; n = 3. As positive controls, HMVECs were stimulated with TNFα (10 ng/mL) and interferon γ (1 ng/mL). (B) CCL5 protein levels secreted by cocultured HMVECs and measured by cytometric bead array. The de novo CCL5 production was measured in the newly added medium after 12 hours. Mean ± SEM; n = 6-8. (C) Adherence of monocytes to HMVECs activated by different coculture conditions. Monocytes were allowed to adhere to HMVEC layers for 2 hours. Mean ± SEM; n = 4. (D) Migration of monocytes toward cocultured HMVECs. Migration of monocytes was determined in the presence of a CCL5-blocking antibody or of a corresponding isotype control IgG. Mean ± SEM; 3 independent experiments. Statistical significance was determined ANOVA with Bonferonni multiple comparison test; *P < .05, **P < .01, ***P < .001.
CCL5 is expressed in the metastatic microenvironment and mediates monocyte recruitment
Results from in vitro experiments provided evidence for the involvement of platelets and leukocytes in tumor cell–induced activation of endothelial cells and CCL5 expression. To determine whether enhanced CCL5 expression contributes to metastasis we analyzed the presence of CCL5 within the microenvironment of metastasizing tumor cells in vivo. We used MC-38GFP colon carcinoma cell line originated from a C57Bl/6J mouse background that was previously shown to metastasize in P- and L-selectin–dependent manner.13,14 C57Bl/6J mice were intravenously injected with MC-38GFP tumor cells and killed at various time points. Lung sections were analyzed for the presence of CCL5 in the vicinity of arrested tumor cells either by immunostaining (Figure 5A-B) or by in situ hybridization (supplemental Figure 8). Although most of the CCL5 signal was associated with CD31+ endothelial cells (Figure 5A), some staining was associated with nonendothelial cells. CCL5 is a secreted, relatively small protein that is not stored within the cell, therefore not all of the CCL5 signal is necessarily associated with the secreting cell.40 Moreover, previous findings showed that CCL5 was detected in pulmonary stromal fibroblasts of human lung adenocarcinoma specimens.36 Thus, activated interstitial fibroblast could be another source of CCL5 in this experimental metastasis model. CCL5 was also reported to be present in platelets and T cells.41,42 In our experiment CCL5 was detected around tumor foci between 12 and 24 hours after tumor cell injection. In the experimental metastasis model, however, platelets are nearly absent after 12 hours in the microenvironment of metastasizing MC-38GFP cells (supplemental Figure 9).14 Furthermore, a reduced CCL5 expression around the tumor foci was observed in mice injected with a P-selectin function-blocking antibody, further supporting the vital role of platelets during the initiation phase of metastasis (supplemental Figure 9). In addition, no association of CD2+ T cells and NK cells was observed at 12 and 24 hours after the injection of MC-38GFP cells (data not shown). Thus, it is unlikely that platelets or T cells significantly contribute to the increased expression of CCL5 during metastatic colonization. Previously we reported an L-selectin–dependent recruitment of monocytes and PMNs to metastasizing tumor cells in the same time period, between 12 and 24 hours after tumor cell injection.14 The temporal and spatial nature of endothelial CCL5 expression led us to consider CCL5 as a factor responsible for the recruitment of monocytes. To test this hypothesis, we determined the presence of monocytes within the tumor cell microenvironment. Indeed, we found the number of F4/80-positive cells to be significantly reduced upon Met-RANTES treatment (Figure 5C-D), thus providing evidence for the function of CCL5 by recruitment of monocytes. At the same time Met-RANTES treatment had no effect on the number of F4/80-positive cells in the lungs, which were not associated with tumor cells (data not shown). The current data suggest that tumor cells, with PMNs and platelets, initiate the activation of endothelium (Figure 4, supplemental Figure 9) and thereby facilitate the creation of a permissive metastatic microenvironment reflected in expression of chemokines (such as CCL5), subsequently mediating the recruitment of monocytes.
CCL5 recruits monocytes to the metastatic microenvironment. (A) Representative immunofluorescence images of CCL5 detection (red) in lung sections from mice injected with MC-38GFP cells (green). Endothelial cells were stained with CD31 antibody (gray) and nuclei by DAPI (blue). CCL5 expression colocalized with endothelial cells (left side of the 12-hour image). Bar represents 20 μm. (B) Quantification of CCL5 signal in a defined area around MC-38GFP tumor cells (15 000 μm3). CCL5 detection was significantly higher at 12 hours compared with 6 hours and 48 hours after tumor cell injection (**P < .01; *P < .05), tested by ANOVA and Bonferroni posthoc test. (C) Representative images of lung sections from mice treated with Met-RANTES, injected with MC-38GFP cells (green), and killed 48 hours later. Monocytes were detected with F4/80 antibody (red) and nuclei by DAPI (blue). Magnification 40×/0.75 NA oil objective. Bar represents 100 μm. Insets show tumor cell microenvironment at higher magnification (63×/1.30 NA oil objective). (D) Quantification of tumor-associated F4/80-positive cells. Statistical analysis of Met-RANTES– and PBS-treated mice (n = 3); ***P < .001.
CCL5 recruits monocytes to the metastatic microenvironment. (A) Representative immunofluorescence images of CCL5 detection (red) in lung sections from mice injected with MC-38GFP cells (green). Endothelial cells were stained with CD31 antibody (gray) and nuclei by DAPI (blue). CCL5 expression colocalized with endothelial cells (left side of the 12-hour image). Bar represents 20 μm. (B) Quantification of CCL5 signal in a defined area around MC-38GFP tumor cells (15 000 μm3). CCL5 detection was significantly higher at 12 hours compared with 6 hours and 48 hours after tumor cell injection (**P < .01; *P < .05), tested by ANOVA and Bonferroni posthoc test. (C) Representative images of lung sections from mice treated with Met-RANTES, injected with MC-38GFP cells (green), and killed 48 hours later. Monocytes were detected with F4/80 antibody (red) and nuclei by DAPI (blue). Magnification 40×/0.75 NA oil objective. Bar represents 100 μm. Insets show tumor cell microenvironment at higher magnification (63×/1.30 NA oil objective). (D) Quantification of tumor-associated F4/80-positive cells. Statistical analysis of Met-RANTES– and PBS-treated mice (n = 3); ***P < .001.
Inhibition of monocyte recruitment confines lung colonization
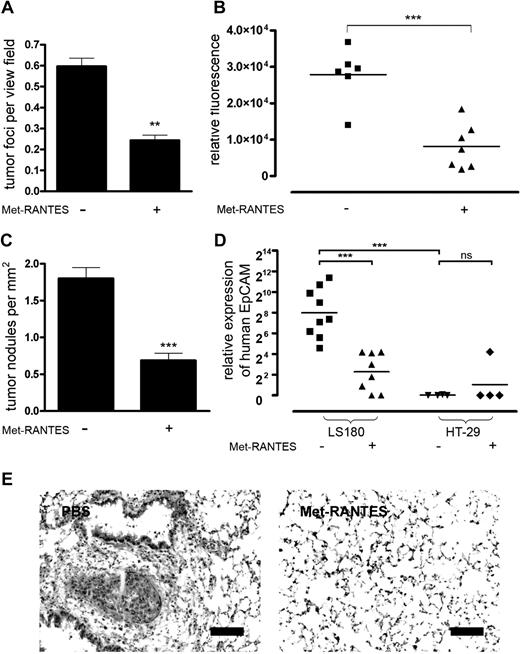
To precisely monitor the effect of Met-RANTES on the initiation steps of metastasis following the entry of tumor cells into the bloodstream we used an experimental metastasis model. When we analyzed mouse lungs 48 hours after tumor cell injection (at the end of Met-RANTES treatment), a significant reduction of surviving tumor cells could be observed (Figure 6A). However, the initial seeding of tumor cells in the lungs was unaffected by the Met-RANTES treatment (data not shown). Previously, we showed that the survival of MC-38GFP cells decreased with the reduction of tumor-associated CD11b+ cells, indicating a positive correlation between leukocyte recruitment and metastasis.14 Treatment of mice with Met-RANTES during the first 48 hours after injection of MC-38GFP cells attenuated metastasis as observed 3 weeks later (Figure 6B). We further analyzed human colon carcinoma cells, LS180 and HT-29 cells, which showed a different capacity to induce CCL5 expression of endothelial cells in vitro (Figure 4). Metastasis was determined by performing quantitative RT-PCR analysis of human EpCAM transcripts and counting the metastatic nodules (Figure 6C-E). Met-RANTES treatment during the first 48 hours after the intravenous injection of tumor cells in immune-deficient mice attenuated metastasis of LS180 cells (Figure 6C-E). In contrast, minimal metastasis of HT-29 cells was observed regardless of Met-RANTES treatment (Figure 6D). These observations indicate that the difference between LS180 cells and HT-29 cells lies in their capability to activate endothelium through selectin-mediated interactions and this defines their metastatic behavior. Moreover, CCR1 and CCR5, the main receptors for CCL5, were expressed on neither MC-38GFP nor LS180 tumor cells, excluding the direct effect of CCL5 signaling on tumor cells via its receptors (supplemental Figure 10). Taken together these data provide evidence that the endothelial CCL5 expression within the microenvironment of metastasizing tumor cells enhances tissue colonization.
CCL5 enhances metastasis of murine and human tumor cells. (A) Survival of MC-38GFP cells in lungs of mice treated with Met-RANTES and killed 48 hours after tumor cell injection (n = 3). (B) Met-RANTES attenuates metastasis of MC-38GFP cells. Mice were treated with Met-RANTES for 48 hours after tumor cell injection and killed 3 weeks later. The extent of metastasis was determined by GFP measurement. (C) Metastasis of LS180 human tumor cells in athymic mice treated with Met-RANTES for 48 hours after tumor cell injection and killed 5 weeks later. Histologic evaluation of mouse lungs from mice injected with LS180 cells for the presence of tumor nodules. (D) Metastasis of LS180 and HT-29 tumor cells in mouse lungs was determined by quantitative RT-PCR analysis of human EpCAM expression and normalized to murine GAPDH levels. **P < .01, ***P < .001 by 2-tailed Student t test; ns indicates not significant. (E) Representative images of hematoxylin-eosin–stained lung sections from mice injected with LS180 cells. Bar represents 100 μm.
CCL5 enhances metastasis of murine and human tumor cells. (A) Survival of MC-38GFP cells in lungs of mice treated with Met-RANTES and killed 48 hours after tumor cell injection (n = 3). (B) Met-RANTES attenuates metastasis of MC-38GFP cells. Mice were treated with Met-RANTES for 48 hours after tumor cell injection and killed 3 weeks later. The extent of metastasis was determined by GFP measurement. (C) Metastasis of LS180 human tumor cells in athymic mice treated with Met-RANTES for 48 hours after tumor cell injection and killed 5 weeks later. Histologic evaluation of mouse lungs from mice injected with LS180 cells for the presence of tumor nodules. (D) Metastasis of LS180 and HT-29 tumor cells in mouse lungs was determined by quantitative RT-PCR analysis of human EpCAM expression and normalized to murine GAPDH levels. **P < .01, ***P < .001 by 2-tailed Student t test; ns indicates not significant. (E) Representative images of hematoxylin-eosin–stained lung sections from mice injected with LS180 cells. Bar represents 100 μm.
Discussion
In this work we provide evidence that cellular interactions of tumor cells with leukocytes and platelets activate microvascular endothelial cells. Endothelial activation was previously described to promote metastasis in different experimental models.4,15,16 Tumor cells, metastasizing to the liver, induced TNFα secretion by Kupffer cells that subsequently activated endothelial cells.15 Inhibition of this activation strongly reduced liver colonization. Reduction of endothelial activation in the pulmonary microvasculature of p38+/− heterozygous mice was associated with decreased lung colonization.16 Here we show that cell-cell interactions leading to pulmonary endothelial activation are, at least in part, P- and L-selectin dependent, although the presence of platelets was essential for this process. This finding is further supported by earlier experiments where the absence of P-selectin and L-selectin–mediated interactions either in selectin-deficient mice or by the removal of mucins by O-sialoglycoproteinase led to an attenuation of metastasis.9,13,14,43 Moreover, the complex interactions among tumor cells, leukocytes, and platelets induced a specific expression profile in microvascular endothelial cells, characterized by the up-regulation of several molecules previously associated with metastasis including CCL5.
The contribution of platelets and leukocytes together with tumor cells to endothelial activation was thoroughly analyzed in vitro (Figures 1,Figure 2–3). Data show that recruitment of platelets together with PMNs is required for endothelial activation and CCL5 expression. Furthermore, enhanced CCL5 expression was biologically active as determined by recruitment of monocytes (Figure 4). In a mouse model, the local expression of CCL5 was detected in the tumor microenvironment (Figure 5A-B). We could show that the recruitment of platelets is essential for the enhanced presence of CCL5 that correlated in a timely manner with the recruitment of monocyte-derived macrophages, F4/80+ cells (Figure 5C-D). Whether the initial recruitment of leukocytes to tumor cells is dependent solely on CCL5 expression induced by selectin-mediated interactions observed in vitro or CCL5 expression is rather a consequence of local activation resulting in monocyte recruitment observed in vivo remains to be determined. Further analysis of initial events during metastasis indicates that the recruitment of monocytes is preceded by the presence of platelets and PMNs, thus further supporting the timely character of events during this process (L.B., manuscript in preparation).
Chemokines and their receptors were shown to contribute to cancer progression either by directly affecting tumor cells or by modulating the tumor stroma.32,44 Expression of CXC chemokine receptor 4 or CCR7 on breast cancer cells was shown to direct metastasis to the lung or lymph nodes.45 Here we provide evidence that the expression of CCL5 within the metastatic microenvironment contributes to tissue colonization by tumor cells. CCL5 was previously associated with the progression of breast cancer in several independent studies, but only a direct effect on tumor cells was examined.29,30,36,46,47 Accordingly, CCL5 derived from mesenchymal stem cells was found to induce a metastatic phenotype through binding to its receptor CCR5 on tumor cells within the primary tumor that increased metastasis to the lungs.29 In our model we observed a CCL5-dependent recruitment of monocytes to metastasizing tumor cells, suggesting a function of CCL5 in shaping the metastatic microenvironment rather than directly affecting tumor cells (supplemental Figure 11). Although platelet-derived CCL5 may additionally promote this process, the absence of platelets at the time of CCL5 detection in the experimental lung metastasis model suggests only a supporting role in this context (supplemental Figure 9). Thus, the local expression of CCL5 likely enhances lung colonization by recruiting innate immune cells to the metastatic microenvironment. This finding was further validated using Met-RANTES in 2 different metastasis models. Met-RANTES treatment during the first 48 hours after tumor cell injection strongly decreased lung colonization by MC-38GFP and LS180 tumor cells. In a previous study, Met-RANTES was successfully tested as an inhibitor of primary tumor growth in a xenograft model and was also linked to the reduction of monocytes in the tumor stroma.37 Innate immune cells are known to promote cancer progression by inducing immune suppression, and promoting angiogenesis and invasion.11,12 Furthermore, the recruitment of immune cells to the metastatic niche before or during initiation of metastasis was found to be important for the successful organ colonization and cancer progression.48-51 In this context, CCL5-mediated activation of monocytes stimulates production of CCL2 and MMP9 that can additionally contribute to the recruitment of inflammatory cells and support the survival of tumor cells.44 We show here a potential mechanism for how CCL5 facilitates spreading of the disease by contributing to the formation of a permissive metastatic microenvironment via the recruitment of innate immune cells, monocytes. Further studies are needed to clarify whether and how CCL5 contributes to local tumor invasion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Eric G. Berger for continuous support, Dr Amanda E. Proudfoot for providing us with Met-RANTES, Andrea Patrignani and Dr Jacob Sabates for help with the microarray experiments, and Bea Berger and Claudia Ruedin for technical assistance.
This work was supported by grants from Swiss National Foundation no. 31003A-116295 (L.B.), and from University of Zürich and Zürich Center for Integrative Human Physiology (H.L.).
Authorship
Contribution: H.L. planned, performed, and analyzed the experiments and wrote the paper; K.-S.S. was responsible for ethical committee approval and critically read the paper; and L.B. designed the project, performed and analyzed the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corresponding author: Lubor Borsig, Institute of Physiology, University of Zürich–Irchel, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; e-mail: lborsig@access.uzh.ch.
References
Supplemental data
Primers for human CCL5 and murine GAPDH were from Qiagen.

![Figure 1. Interactions of tumor cells with platelets and leukocytes activate HMVECs. (A) Human lung microvascular endothelial cells (HMVECs) were cocultured with LS180 or Colo205 cells in the absence or presence of polymorphonuclear leukocytes (PMNs), monocytes, and platelets. Representative flow cytometric data of E-selectin expression on HMVECs are shown. CD105 was used to identify HMVECs. (B) Statistical analysis of multiple coculture experiments (n = 4-6) measuring E-selectin expression on HMVECs. Percentage of E-selectin–positive HMVECs is presented (mean ± SEM). Statistical significance was determined by Bonferroni multiple comparison test. **P < .01; ***P < .001. (C) Representative fluorescence images of p65 localization in HMVECs. Nuclear localization of p65 was determined by colocalization (white) of p65 (red) with DAPI (4,6 diamidino-2-phenylindole [Sigma]; blue. HMVECs were detected by CD31 antibody (green). (D) Relative nuclear localization of p65 in HMVECs determined by Imaris software. Data are presented as mean ± SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/20/10.1182_blood-2008-10-186585/4/m_zh89990944860001.jpeg?Expires=1767726015&Signature=cCnZhfwYA1y0mKmMb4OLXzfwJFD3Fj0XVbhnA88ayifYI6LbecFk9dW6UfFONXRrV~T-6q21ay0LoVdMSzje0kmz-lFxkhxnjA6EKd4fnGdQgIHlOPbk3310mXiCWzwB419DtkayIYyafkc4o1R2F3f-GNBxBEWMcQ4EIIIm6hfcIgPER90J2uxISku5OffkND9eD1EPTjAqTS-Ef6idg~gz4JYj3lHwvJty-NzTj-~BS8~H~Tr38IYhFdYSpnbA6mtyWgrMm0Rcn4mV6biW4Ds81mBol3zdvWloWd6KLwpGdySIz7-2Q5ep3bo8UR3HZDKm84tbkVDtXlKQs1Id2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)