Abstract
The RUNX1/AML1 gene is the most frequently mutated gene in human leukemia. Conditional deletion of Runx1 in adult mice results in an increase of hematopoietic stem cells (HSCs), which serve as target cells for leukemia; however, Runx1−/− mice do not develop spontaneous leukemia. Here we show that maintenance of Runx1−/− HSCs is compromised, progressively resulting in HSC exhaustion. In leukemia development, the stem cell exhaustion was rescued by additional genetic changes. Retroviral insertional mutagenesis revealed Evi5 activation as a cooperating genetic alteration and EVI5 overexpression indeed prevented Runx1−/− HSC exhaustion in mice. Moreover, EVI5 was frequently overexpressed in human RUNX1-related leukemias. These results provide insights into the mechanism for maintenance of pre-leukemic stem cells and may provide a novel direction for therapeutic applications.
Introduction
The RUNX1/AML1 gene encodes the DNA binding α subunit of heterodimeric Runt domain transcription factor, PEBP2/CBF.1 RUNX1 and its partner protein, the non-DNA binding β subunit (PEBP2β/CBFβ), are essential for definitive hematopoiesis and are frequently targeted in human leukemia.2-4 RUNX1 and CBFB are involved in chromosomal translocations, generating fusion proteins that inhibit the activity of wild-type RUNX1 in a dominant-negative manner.5,6 Biallelic point mutations of RUNX1 are frequently found in the acute myeloid leukemia (AML) M0 subtype and familial platelet disorder with predisposition to AML. Monoallelic mutations are found in sporadic myelodysplastic syndrome and AML.7-10 These point mutations make the RUNX1 protein nonfunctional. Hence, loss-of-function of RUNX1 is considered to be the common underlying mechanism for RUNX1-related leukemias.
Despite the prevalence of RUNX1 loss-of-function mutations or dominant-negative fusion proteins, the RUNX1 alteration per se does not cause leukemia. Rather, cells with loss-of-function of RUNX1 remain leukemia-prone and only with acquisition of additional hits do they become fully leukemic.11-14 Conditional deletion of Runx1 in adult mice results in an expansion of immunophenotypically defined hematopoietic stem cell (HSC) compartment and an accumulation of megakaryoblasts and lymphoid progenitors.15-17 The expansion of Runx1-deficient HSC/progenitor compartment is due to higher self-renewal and antiapoptotic properties and results in predisposition to leukemia.18 However, surprisingly, despite the increased number of stem cells, Growney et al16 reported that conditional Runx1 knockout bone marrow (BM) cells are outcompeted by simultaneously transplanted wild-type BM cells in competitive repopulation assay, indicating that Runx1-deficient cells are compromised in reconstituting hematopoiesis in the recipient mice. Also, except for one group describing that Runx1 conditional knockout mice developed lymphoma at later stages of life,17 other groups reported that leukemia/lymphoma did not develop spontaneously. The above studies indicate increased leukemia susceptibility in Runx1-deficient conditions, and at the same time clearly suggest that Runx1-deficient cells require additional genetic changes for leukemic transformation.
Retroviral insertional mutagenesis (RIM) is a powerful tool to identify oncogenes and tumor suppressor genes.19 Injection of replication-competent retrovirus into newborn mice leads to integration of virus into the host genome and activation of oncogenes or disruption of tumor suppressor genes, resulting in leukemia or lymphoma. Retrovirus usually hits multiple genes to induce leukemia or lymphoma.20-23 RIM on conditional Runx1 knockout mice provides an excellent system to identify genes that cooperate with loss-of-function of Runx1 to promote leukemogenesis. Previous RIM studies on heterozygous Runx1 knockout mice have revealed the alterations of the Ras gene family and its upstream factors such as c-Kit and Flt-3 as candidate “second hits” in leukemogenesis. These genes are in fact frequently mutated in human RUNX1-related leukemias.18,22,24
In this study, we show that Runx1 deficiency in HSCs leads to the phenomenon called “stem cell exhaustion” after the initial expansion. Runx1-deficient stem cell maintenance was compromised, probably due to defective niche interaction, resulting in decline of stem/progenitor cell numbers and decreasing contribution of these stem cells to blood cell production. We employed RIM on conditional Runx1 knockout mice and identified overexpression of Evi5 as an additional genetic alteration that prevents the stem cell exhaustion caused by Runx1 deficiency. Together, these 2 genetic alterations maintain an expanded pool of aberrant stem/progenitor cells, which may act as targets for further oncogenic hits.
Methods
Mice
The mice harboring Runx1 allele with exon 4 flanked by loxP sites (Runx1F/+) were generated,25 backcrossed against C57BL/6 mice for 3 generations, and then intercrossed to obtain Runx1F/F mice. They were crossed with interferon-inducible Mx-Cre transgenic mice,26 a gift from Dr K. Rajewsky, to generate Runx1F/F–Tg(Mx1-Cre) mice. For further details, see supplemental Methods (available on the Blood website; click on the Supplemental Materials link at the top of the online article). All mice were maintained in the Biological Resource Center (BRC), Biopolis, Singapore, and all animal experiments followed the strict guidelines set by the National Advisory Committee for Laboratory Animal Research (NACLAR) and were approved by the BRC Institutional Animal Care and Use Committee.
Retroviral insertional mutagenesis
Runx1F/F–Tg(Mx1-Cre) and Runx1F/F mice were mated, and progenies were injected with MoMuLV virus 3 days after birth and with polyinosinic-polycytidylic acid at 1 month of age. Retrovirus-injected Runx1−/− mice and Runx1+/+ littermates were monitored by examining their health condition and by weekly checking of complete blood cell count using an automatic hematology analyzer (Celltac alpha MEK-6358; Nihon Kohden). Necropsy of diseased mice, hematology analysis, and identification of RIS using inverse polymerase chain reaction (PCR) were carried out as previously described.22,23
Flow cytometric analysis
Patient samples
Thirty-five human patients with leukemia belonging to the following categories were screened for expression level of EVI5: AML with t(8;21) (n = 9); inv(16) (n = 7); other AML (n = 10); chronic myeloid leukemia (CML) blast crisis (n = 6); and complete remission from AML (n = 3). Each patient gave informed consent to this study based on the tenets of the revised Helsinki protocol produced by the Institutional Committees for the Protection of Human Subjects and Analysis of the Human Genome. All studies of human samples were approved by the institutional review board of Kumamoto University Hospital.
Additional procedures
For complete information on bone marrow transplantation (BMT) procedures; plasmid construction, retroviral transduction, and in vitro cell culture assays; quantitative real-time PCR (qRT-PCR); luciferase assay; in vivo homing assay; and the BrdU incorporation assay, see the supplemental Methods.
Results
Runx1−/− stem/progenitor cell population declines after the initial expansion
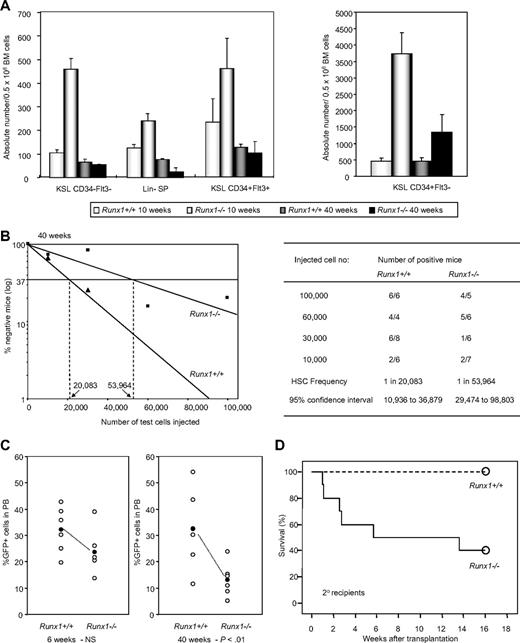
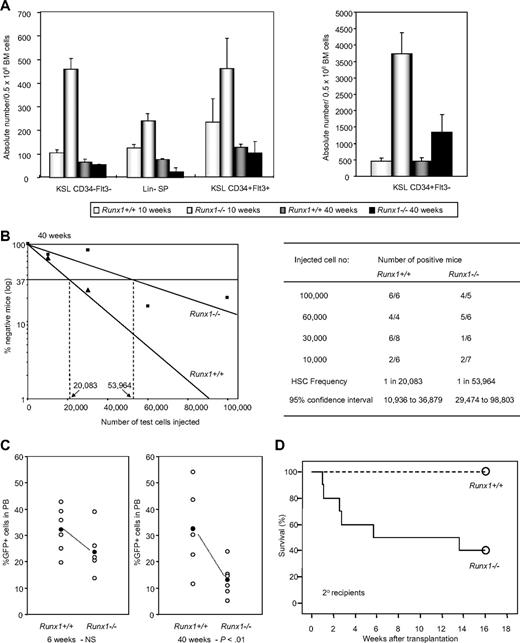
Runx1 knockout (Runx1−/−) BM cells, generated by Cre-recombinase–mediated knockout of Runx1, show an increase in hematopoietic stem/progenitor cell fraction compared with control wild-type (Runx1+/+) mice. However, we found that Runx1−/− HSC expansion is followed by exhaustion, resulting in a progressive decline of stem/progenitor cell numbers. At 10 weeks of age, Runx1−/− mice showed a significant increase in long-term HSCs (c-Kit+Sca1+Lineage− [KSL] CD34−Flt3−), short-term HSCs (KSL CD34+Flt3−), and multipotential progenitors (KSL CD34+Flt3+). However, at 40 weeks of age, Runx1−/− stem/progenitor cell numbers declined significantly and were equivalent to or lesser than corresponding Runx1+/+ cell numbers (Figure 1A, supplemental Figure 1). Side population analysis of lineage-negative cells (lineage-SP) also showed a similar trend of expansion of Runx1−/− HSCs at 10 weeks, followed by decline at 40 weeks (Figure 1A). To analyze the number of functional competitive repopulating units (CRUs) in aged (40 weeks old) Runx1−/− and Runx1+/+ mice, we carried out limiting dilution BMT. The frequency of CRUs in BM of aged Runx1−/− mice was 1 in 53 964, lower than the frequency, 1 in 20 083, in Runx1+/+ littermate controls (Figure 1B). These results suggest that stem cell exhaustion may occur in Runx1−/− mice.
Runx1−/− status leads to stem cell exhaustion. (A) Absolute number of KSL CD34−Flt3− cells, Lin− SP cells, KSL CD34+Flt3+ cells and KSL CD34+Flt3− cells per 0.5 million BM cells from Runx1+/+ and Runx1−/− mice of 2 distinct ages (10 and 40 weeks old). Each group comprises 3 to 4 mice. (B) Limiting dilution analysis using varying numbers of BM cells from 40-week-old CD45.2+Runx1+/+ (▲), or Runx1−/− (■) mice. Mice were considered negative when the percent chimerism was less than 1%. Left panel: estimated frequencies of the repopulating cells are indicated as vertical dashed lines (1 repopulating cell per indicated numbers of BM cells) for each genotype. Right panel: for each indicated number of transplanted cells from CD45.2+Runx1+/+ or Runx1−/− mice, the proportion of mice that are positive for test CD45.2+ cells is given as (number of positive mice)/(number of analyzed mice). Frequencies of HSCs were calculated using Poisson statistics. (C) GFP chimerism in PB of recipients of Runx1+/+ (n = 6) and Runx1−/− (n = 6) cells at 6 and 40 weeks after transplantation. Each open circle represents data from an individual mouse and each closed circle is the average of a cohort. Statistical difference using unpaired Student t test is given at the bottom. NS indicates not significant. (D) Kaplan-Meier survival curves of secondary recipients of mock MIG vector–transfected Runx1+/+ (dashed line; n = 10) and Runx1−/− (solid line; n = 10) BM cells. Circles represent end point of analysis.
Runx1−/− status leads to stem cell exhaustion. (A) Absolute number of KSL CD34−Flt3− cells, Lin− SP cells, KSL CD34+Flt3+ cells and KSL CD34+Flt3− cells per 0.5 million BM cells from Runx1+/+ and Runx1−/− mice of 2 distinct ages (10 and 40 weeks old). Each group comprises 3 to 4 mice. (B) Limiting dilution analysis using varying numbers of BM cells from 40-week-old CD45.2+Runx1+/+ (▲), or Runx1−/− (■) mice. Mice were considered negative when the percent chimerism was less than 1%. Left panel: estimated frequencies of the repopulating cells are indicated as vertical dashed lines (1 repopulating cell per indicated numbers of BM cells) for each genotype. Right panel: for each indicated number of transplanted cells from CD45.2+Runx1+/+ or Runx1−/− mice, the proportion of mice that are positive for test CD45.2+ cells is given as (number of positive mice)/(number of analyzed mice). Frequencies of HSCs were calculated using Poisson statistics. (C) GFP chimerism in PB of recipients of Runx1+/+ (n = 6) and Runx1−/− (n = 6) cells at 6 and 40 weeks after transplantation. Each open circle represents data from an individual mouse and each closed circle is the average of a cohort. Statistical difference using unpaired Student t test is given at the bottom. NS indicates not significant. (D) Kaplan-Meier survival curves of secondary recipients of mock MIG vector–transfected Runx1+/+ (dashed line; n = 10) and Runx1−/− (solid line; n = 10) BM cells. Circles represent end point of analysis.
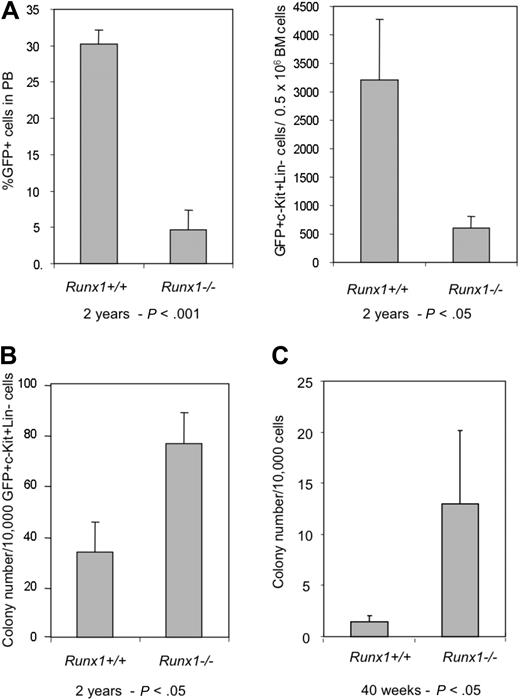
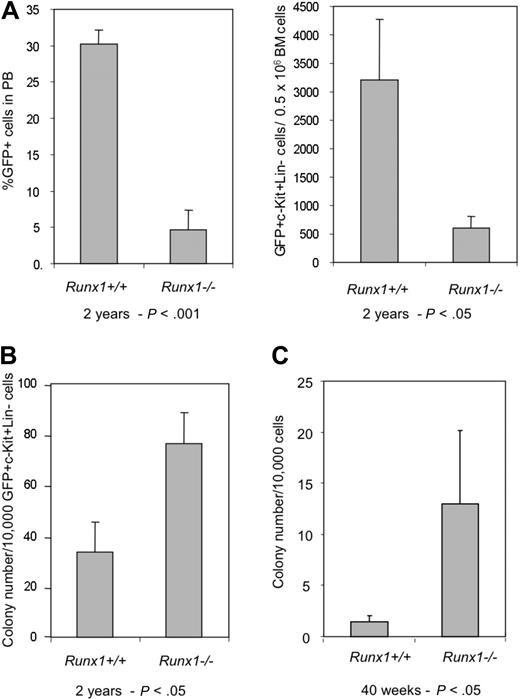
The decline of Runx1−/− HSCs is further observed in another BMT experiment. In the recipient mice that underwent transplantation with BM cells from Runx1−/− and Runx1+/+ mice transfected with MIG (MSCV-IRES-GFP) retroviral vector expressing enhanced green fluorescent protein (EGFP) as a surrogate marker, contribution of donor cells to hematopoiesis was monitored periodically by the percentage of GFP+ cells in the peripheral blood (PB). At 6 weeks after transplantation, the GFP chimerism in PB of the recipients (n = 6) of Runx1+/+ and Runx1−/− cells was comparable, with a mean value of 32.1% and 23.6% respectively. After 40 weeks, the mean GFP chimerism in the recipients of Runx1+/+ remained the same at 32.5%, whereas it was significantly lower (P < .01) at 13.1% in the recipients of Runx1−/− cells (Figure 1C). By 2 years after transplantation, the GFP chimerism in PB of recipients of Runx1−/− cells dropped even further. There was also a concomitant decrease in absolute number of immature Runx1−/− (c-Kit+Lineage−GFP+) cells in the BM of the recipients, again suggesting stem cell exhaustion (Figure 2A).
Aged Runx1−/− stem/progenitor cells maintain proliferative ability. (A) Graphs showing percentage of GFP+ cells in PB and number of GFP+c-Kit+Lin− cells in BM of recipients of Runx1+/+ (n = 3) and Runx1−/− (n = 3) BM cells, 2 years after transplantation. (B) Colony assay of GFP+c-Kit+Lin− cells from recipients of Runx1+/+ (n = 3) and Runx1−/− (n = 3) BM cells, 2 years after transplantation. (C) Colony assay of KSL cells from 40-week-old mice, after 30 days of long-term culture on OP9 stromal cells. Statistical differences using the unpaired Student t test are given at the bottom.
Aged Runx1−/− stem/progenitor cells maintain proliferative ability. (A) Graphs showing percentage of GFP+ cells in PB and number of GFP+c-Kit+Lin− cells in BM of recipients of Runx1+/+ (n = 3) and Runx1−/− (n = 3) BM cells, 2 years after transplantation. (B) Colony assay of GFP+c-Kit+Lin− cells from recipients of Runx1+/+ (n = 3) and Runx1−/− (n = 3) BM cells, 2 years after transplantation. (C) Colony assay of KSL cells from 40-week-old mice, after 30 days of long-term culture on OP9 stromal cells. Statistical differences using the unpaired Student t test are given at the bottom.
To ascertain the phenomenon of Runx1−/− stem cell exhaustion, a secondary transplantation experiment was carried out. Two to 3 primary recipients with similar GFP chimerism were killed at an average of 4 months after transplantation and BM cells were transplanted into 10 lethally irradiated (8 Gy) secondary recipients. Six of 10 recipients of Runx1−/− cells died within 3 months of secondary transplantation due to pancytopenia arising from graft failure, while all the recipients of control Runx1+/+ cells survived beyond that (Figure 1D). Taken together, the above results prove that Runx1−/− status results in progressive stem cell exhaustion.
Surprisingly, colony assay of immature Runx1−/− cells (GFP+c-Kit+Lineage−) from BM of recipient mice showed an increased number of precursors even 2 years after transplantation, similar to the observations made soon after the conditional deletion of the Runx1 gene18 (Figure 2B). Furthermore, long-term culture initiating cell (LTC-IC) assay of KSL cells from BM of aged 40-week-old Runx1−/− mice showed an increased number of progenitor cells after 28 days of culture on OP9 stromal cells (Figure 2C). These results suggest that immature Runx1−/− cells maintain their inherent properties of increased proliferation even after long periods of time. Hence, stem cell exhaustion may not be due to cell intrinsic defects of Runx1−/− stem/progenitor cells.
Runx1−/− status results in increased susceptibility to myeloid leukemia development
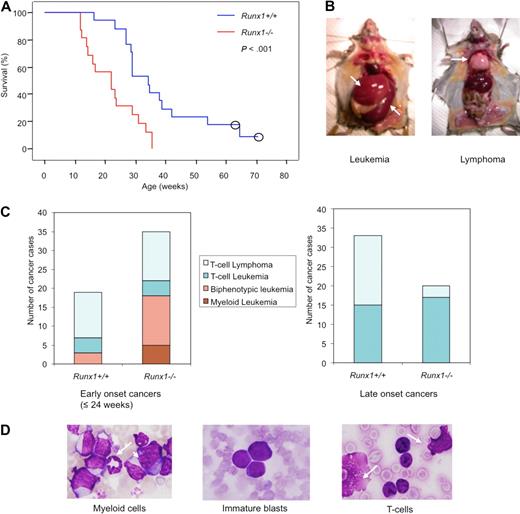
It is conceivable that for Runx1−/− mice to develop leukemia, Runx1−/− stem cells would have to acquire an ability to survive long, possibly through additional genetic hits. Therefore, RIM was employed to induce leukemia in Runx1−/− mice and to identify genes that prevent stem cell exhaustion and aid in leukemia development. Runx1−/− mice showed a significantly shorter latency of leukemia development than wild-type littermates (Figure 3A), thus confirming that the Runx1-deficient status accelerates leukemia development.
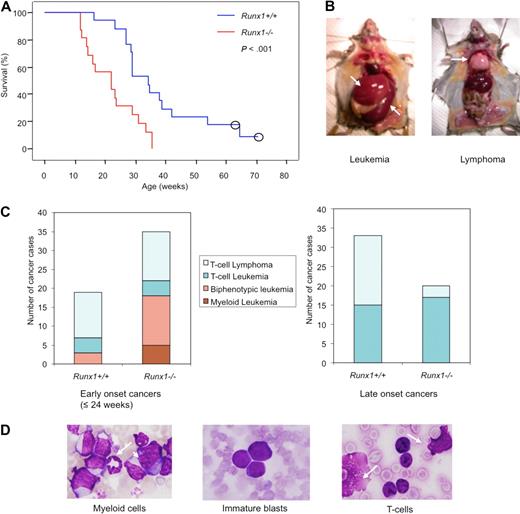
Runx1−/− mice show early onset and high frequency of leukemia with myeloid features in RIM. (A) Kaplan-Meier survival curves of Runx1+/+ (blue line; n = 17) and Runx1−/− (red line; n = 16) mice injected with MoMuLV retrovirus. Kaplan-Meier method showed significant difference between the 2 genotypes (P < .001, Mantel-Cox test). Open circles represent censored cases. (B) Necropsy of diseased mice; leukemic mice usually show enlarged spleen (bottom arrow) and liver (top arrow) while T-cell lymphoma mice show enlarged thymus (arrow). (C) Graphs showing frequency of different types of leukemia/lymphoma, groups 1 to 4, in early-onset (≤ 24 weeks) cancers of Runx1+/+ (n = 19) and Runx1−/− (n = 34) mice; and frequency of leukemia or lymphoma cases in late-onset cancers of Runx1+/+ (n = 33) and Runx1−/− (n = 20) mice. In total, n = 52 for Runx1+/+ mice and n = 54 for Runx1−/− mice. (D) Morphology of cells from PB of representative leukemic case from group 1 showing granulocyte (arrow) and monoblast (arrowhead); group 2 showing immature blasts; and group 3 showing T cells and ghost cells (arrows) which are frequently seen in T-cell malignancy.
Runx1−/− mice show early onset and high frequency of leukemia with myeloid features in RIM. (A) Kaplan-Meier survival curves of Runx1+/+ (blue line; n = 17) and Runx1−/− (red line; n = 16) mice injected with MoMuLV retrovirus. Kaplan-Meier method showed significant difference between the 2 genotypes (P < .001, Mantel-Cox test). Open circles represent censored cases. (B) Necropsy of diseased mice; leukemic mice usually show enlarged spleen (bottom arrow) and liver (top arrow) while T-cell lymphoma mice show enlarged thymus (arrow). (C) Graphs showing frequency of different types of leukemia/lymphoma, groups 1 to 4, in early-onset (≤ 24 weeks) cancers of Runx1+/+ (n = 19) and Runx1−/− (n = 34) mice; and frequency of leukemia or lymphoma cases in late-onset cancers of Runx1+/+ (n = 33) and Runx1−/− (n = 20) mice. In total, n = 52 for Runx1+/+ mice and n = 54 for Runx1−/− mice. (D) Morphology of cells from PB of representative leukemic case from group 1 showing granulocyte (arrow) and monoblast (arrowhead); group 2 showing immature blasts; and group 3 showing T cells and ghost cells (arrows) which are frequently seen in T-cell malignancy.
When the mice became moribund, necropsy was carried out and the disease was divided into leukemia or lymphoma cases. Leukemia cases showed elevated leukocyte counts and hepatosplenomegaly with normal thymus, whereas lymphoma cases showed normal or elevated leukocyte counts and enlarged thymus/lymph node (Figure 3B). Based on combination of leukocyte counts, necropsy, and further immunophenotype and morphologic analyses, tumors were classified into the following 4 groups: group 1, myeloid leukemia (supplemental Figure 2A); group 2, biphenotypic (myeloid and T-cell) leukemia; group 3, T-cell leukemia; and group 4, T-cell lymphoma (supplemental Tables 1-2). Most of the biphenotypic leukemia cases belonging to group 2 were considered to be mixtures of myeloid and lymphoid leukemia originating from different clones, with a certain subset of the leukemic cells expressing both the T-cell and myeloid markers simultaneously (supplemental Figure 2A).
In Runx1−/− mice, 34 of 54 (63.6%) developed early-onset (≤ 24 weeks) leukemia/lymphoma, whereas only 19 of 52 (36.5%) Runx1+/+ mice showed early onset of leukemia/lymphoma. Out of the early-onset cases, 51.4% of Runx1−/− cases and 15.8% of Runx1+/+ cases showed leukemia with myeloid features that fell into groups 1 and 2. The remaining mice developed T-cell leukemia/lymphoma that fell into groups 3 and 4 (Figure 3C). This result indicates that Runx1 knockout status drives myeloid features in leukemias despite the strong T-lymphoid tropism of MoMuLV virus. Some of the group 1 and 2 leukemias recapitulated human RUNX leukemias with accumulation of immature blasts (as seen in AML M0) or accumulation of myeloid cells with differentiation (as seen in AML M2; Figure 3D).
Stemness related genes are preferentially affected in Runx1−/− leukemias
There were 710 retroviral integration sites (RISs) found in 63 Runx1−/− mice and 52 Runx1+/+ mice. These sequences were mapped to the mouse genome to identify the chromosomal location of the sequences and to identify candidate genes at the loci. Twenty loci were affected more than once by retroviral integrations in Runx1−/− or Runx1+/+ mice and these are referred to as common integration sites (CISs; Table 1). The relative locations of these integration sites were compared with the tags from the publicly available Retroviral-Tagged Cancer Gene Database.27 This comparison revealed that 16 CISs correspond to previously known loci where retroviral integration occurred more than once. The other 4 CISs were detected only by our study and have been designated as Slis (Singapore leukemia integration site) and classified as novel CISs (Table 1, supplemental Table 3).
Genes near CISs that are affected with high frequency in Runx1−/− mice, but affected with lower frequency in Runx1+/+ mice, may be specifically involved in leukemogenesis of Runx1−/− mice. Notably, candidate leukemogenic genes near CISs in Runx1-deficient leukemias with myeloid features are more relevant to our study since these leukemias recapitulate human RUNX1-related leukemias. A comprehensive list of genes (near CISs or RISs) that may be involved in tumor progression of each leukemia sample with myeloid features is given in Table 2. Interestingly, 10 of 18 Runx1−/− mice that developed leukemia with myeloid features had integrations near stem cell–related genes such as Gfi1/Evi5, Evi1, and Lmo2. These CISs are rarely affected in T-cell leukemia/lymphoma and preferentially hit in leukemia with myeloid features (supplemental Table 4).
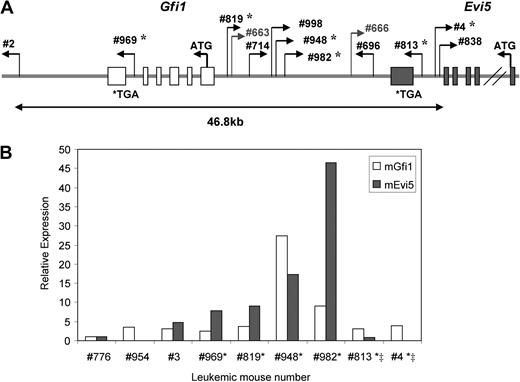
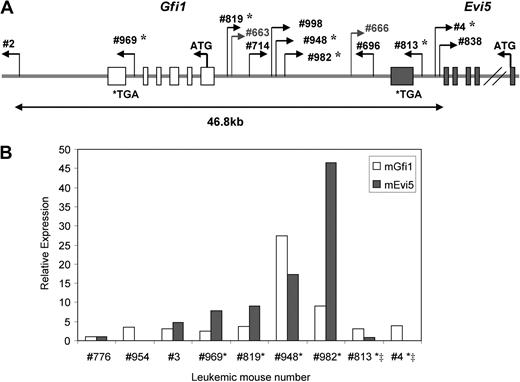
Integrations at the Gfi1/Evi5 locus, the locus where these 2 genes are located in the same direction in no overlapping fashion, were found in 11 of 63 Runx1−/− mice analyzed and only in 2 of 52 Runx1+/+ mice (P < .05, Fisher test). Seven of the 11 Runx1−/− leukemia cases with integrations at the Gfi1/Evi5 locus belonged to groups 1 and 2 (n = 18), which showed early-onset leukemia with myeloid features (Table 2). Gfi1 is a well-known factor involved in stem cell maintenance,28 while Evi5 was recently shown to be a cell-cycle regulator that prevents premature entry of cells into mitosis.29 Expression levels of Gfi1 and Evi5 were examined by qRT-PCR on cDNA from 6 of the available leukemic samples with integrations at this locus and 3 control samples without integration at this locus (Figure 4A). Evi5 overexpression was seen in all affected Runx1−/− cases with integration outside this gene, and became pronounced as the distance between the RIS and the Evi5 gene decreased. This indicates specific, integration site–dependent activation of Evi5 expression. Gfi1 expression was not significantly affected by viral integrations in majority of the cases (Figure 4B). Thus, Evi5 overexpression appears to play a more cooperative role with Runx1 deficiency in leukemogenesis. Out of the integrations that were present only in Runx1−/− mice and not in Runx1+/+ mice, the most frequent were integrations at the Evi1 locus seen in 5 Runx1−/− mice, 3 of which belonged to groups 1 and 2 (Table 2). Evi1 functions in self-renewal, maintenance, and proliferation of stem cells.30,31 Integrations near c-Myc, Cyclin D2, and Cyclin D3 genes were also more frequent in Runx1−/− mice. c-Myc is a well-known protooncogene that causes uncontrolled proliferation of cells when overexpressed. Cyclin D2 and D3 are G1 cyclins and their dysregulation leads to abnormal cycling of cells (Table 2).
Frequent integrations at Gfi1/Evi5 locus in Runx1−/− leukemias lead to overexpression of Evi5. (A) Schematic diagram of retroviral integration sites at Gfi1/Evi5 locus in 2 Runx1+/+ and 11 Runx1−/− leukemias. Numbers are unique to each leukemic mouse. Thin bent arrows represent the retrovirus integration site and its direction of integration. The 2 genes Gfi (light gray) and Evi5 (dark gray) span from their initiation codons (ATG) to stop codons (TGA) with boxes representing exons. *Leukemia cases in which expression of Gfi1 and Evi5 was checked using qRT-PCR. (B) qRT-PCR analysis of Gfi1 and Evi5 expression in leukemic cells harboring integrations at Gfi1/Evi5 locus (*), integrations within the Evi5 gene (‡), and 3 control samples without integrations at this locus. Data are represented as fold change relative to control sample no. 776.
Frequent integrations at Gfi1/Evi5 locus in Runx1−/− leukemias lead to overexpression of Evi5. (A) Schematic diagram of retroviral integration sites at Gfi1/Evi5 locus in 2 Runx1+/+ and 11 Runx1−/− leukemias. Numbers are unique to each leukemic mouse. Thin bent arrows represent the retrovirus integration site and its direction of integration. The 2 genes Gfi (light gray) and Evi5 (dark gray) span from their initiation codons (ATG) to stop codons (TGA) with boxes representing exons. *Leukemia cases in which expression of Gfi1 and Evi5 was checked using qRT-PCR. (B) qRT-PCR analysis of Gfi1 and Evi5 expression in leukemic cells harboring integrations at Gfi1/Evi5 locus (*), integrations within the Evi5 gene (‡), and 3 control samples without integrations at this locus. Data are represented as fold change relative to control sample no. 776.
Overexpression of EVI5 cooperates with Runx1−/− status in long-term maintenance of aberrant stem/progenitor cells in vitro
To examine the details of cooperation with Runx1−/− status, Gfi1, Evi5, and Evi1 were chosen from the RIM screen due to high frequency of viral integrations near these genes in Runx1−/− leukemias compared with wild-type cases. We deduced that they are likely to prevent exhaustion of Runx1−/− stem cells due to their possible function in stem cell maintenance and thus contribute to development of Runx1-related leukemia.
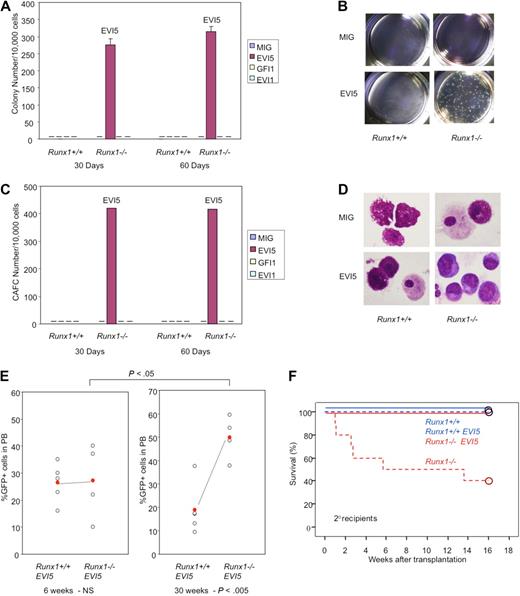
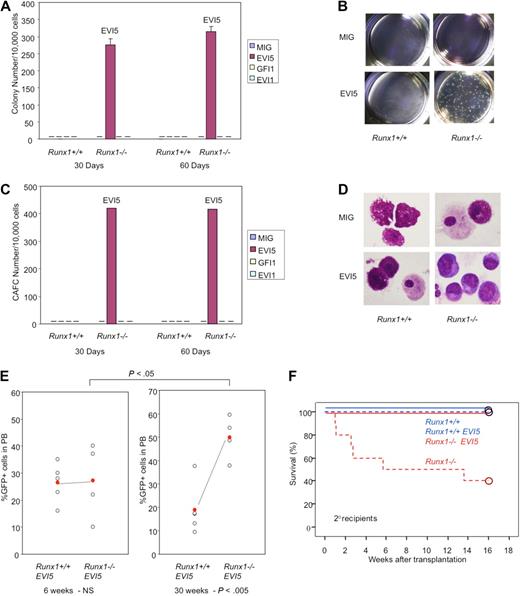
To study the effect of overexpression of these candidate oncogenes in immature hematopoietic cells, the c-Kit+ fraction of BM cells transfected with MIG vector carrying GFI, EVI5, or EVI1 was isolated by FACS and subjected to the experiments. LTC-IC assay was carried out after 30 days' culture of transfected Runx1−/− and Runx1+/+ immature cells (GFP+c-Kit+) on OP9 stromal cells. The plating efficiency (colony number) of Runx1−/− cells with overexpression of EVI5 gene was prominently high while that of mock-transfected Runx1−/− cells or EVI5-transfected Runx1+/+ cells was lost, suggesting that cells overexpressing EVI5 maintain a higher number of stem cells than other combinations (Figure 5A-B). When replated after 30 days, Runx1−/− cells carrying EVI5 also showed a significantly high number of cobblestone area forming cells (CAFCs; Figure 5C). Furthermore, colony assay and CAFC assay after 30 more days of culture of replated cells (total 60 days after initial transfection) still showed a high number of colonies and CAFCs in Runx1−/− cells overexpressing EVI5. GFI1- and EVI1-transfected Runx1+/+ or Runx1−/− cells did not show any colony or CAFCs after 30 days of LTC (Figure 5A,C).
EVI5 overexpression and Runx1−/− status synergize in long-term maintenance of stem cells in vitro and in vivo. Graphic representation of (A) colony assay and (C) CAFC assay of immature cells from Runx1+/+ and Runx1−/− BM cells transfected with mock MIG vector, EVI5, GFI1, or EVI1, after 30 and 60 days of long-term culture. Pictures of (B) colonies and (D) morphology of cells after 30 days of long-term culture. (E) GFP chimerism in recipients of Runx1+/+ (n = 5) and Runx1−/− (n = 4) BM cells transfected with EVI5, 6 and 30 weeks after transplantation. Each open circle represents data from an individual mouse and the closed red circle is the average of a cohort. Statistical difference using unpaired Student t test is given at the bottom and on top. NS indicates not significant. (F) Kaplan-Meier survival curves of secondary recipients of Runx1+/+ (blue; n = 4) and Runx1−/− (red; n = 4) BM cells transfected with mock MIG vector (dashed line) or MIG vector carrying EVI5 (solid line). Circles represent end point of analysis.
EVI5 overexpression and Runx1−/− status synergize in long-term maintenance of stem cells in vitro and in vivo. Graphic representation of (A) colony assay and (C) CAFC assay of immature cells from Runx1+/+ and Runx1−/− BM cells transfected with mock MIG vector, EVI5, GFI1, or EVI1, after 30 and 60 days of long-term culture. Pictures of (B) colonies and (D) morphology of cells after 30 days of long-term culture. (E) GFP chimerism in recipients of Runx1+/+ (n = 5) and Runx1−/− (n = 4) BM cells transfected with EVI5, 6 and 30 weeks after transplantation. Each open circle represents data from an individual mouse and the closed red circle is the average of a cohort. Statistical difference using unpaired Student t test is given at the bottom and on top. NS indicates not significant. (F) Kaplan-Meier survival curves of secondary recipients of Runx1+/+ (blue; n = 4) and Runx1−/− (red; n = 4) BM cells transfected with mock MIG vector (dashed line) or MIG vector carrying EVI5 (solid line). Circles represent end point of analysis.
Morphologic analyses of cells after 30 and 60 days of LTC revealed that Runx1−/− cells overexpressing EVI5 have immature cell morphology characterized by nucleus with fine chromatin and basophilic cytoplasm. Runx1−/− cells transfected with mock vector and Runx1+/+ cells overexpressing EVI5 showed differentiated mast cell and macrophage morphology after 30 days of LTC (Figure 5D).
Taken together, the overexpression of EVI5 strongly cooperates with Runx1−/− status in maintenance and proliferation of stem cells, and overexpression of GFI1 or EVI1 does not show significant cooperation in the OP9 culture. However, colony replating assay showed modest cooperation between Runx1−/− status and EVI1 overexpression (supplemental Figure 3A).
Overexpression of EVI5 and EVI1 prevents exhaustion of Runx1−/− stem cells in vivo
To assess the in vivo effect, EVI5 transfected Runx1−/− or Runx1+/+ cells were transplanted into sublethally irradiated (6 Gy) recipient mice. Recipients of Runx1+/+ cells transfected with EVI5 showed stable GFP chimerism throughout, from 6 weeks to 30 weeks after transplantation, with an average of 20% to 25%. However, the GFP chimerism of mice that underwent transplantation with Runx1−/− cells overexpressing EVI5 increased progressively, with a mean value of 25% at 6 weeks and 50% at 30 weeks (Figure 5E). This is in contrast to the results seen after transplantation of mock vector–transfected Runx1−/− cells described earlier where the contribution of Runx1−/− cells to PB of recipient mice decreased progressively (Figure 1C). Thus, EVI5 cooperates with Runx1−/− status in vivo also by preventing stem cell exhaustion and maintaining an increased number of Runx1-deficient stem cells. A secondary transplantation experiment was repeated to examine whether EVI5 overexpression in Runx1−/− cells could rescue the defects in long-term repopulating abilities of Runx1−/− stem cells. Contrary to the previous results where 60% of the secondary recipients of Runx1−/− cells died within 3 months, all the secondary recipients of Runx1−/− cells overexpressing EVI5 were alive (Figure 5F). We conclude that EVI5 overexpression in Runx1−/− cells can prevent stem cell exhaustion of these cells and render them capable of reconstituting hematopoiesis in the secondary recipients.
As colony replating assay showed mild cooperation between EVI1 overexpression and Runx1−/− status (supplemental Figure 3A), BMT was carried out for EVI1 overexpressing cells as well. Recipients of Runx1+/+ and Runx1−/− cells transfected with EVI1 showed stable GFP chimerism throughout, from 6 weeks to 30 weeks after transplantation, with no significant increase or decrease in GFP chimerism (supplemental Figure 3B). Thus, EVI1 overexpression also seems to rescue Runx1−/− stem cell exhaustion in vivo.
EVI5 is overexpressed in 44% of human patients with AML M2 RUNX leukemia
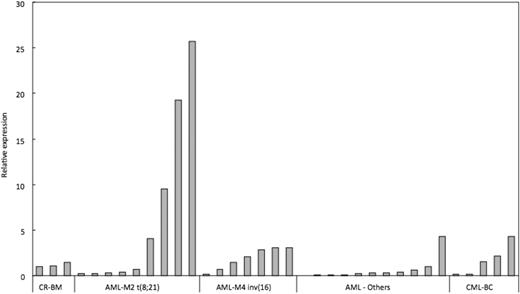
To evaluate whether EVI5 overexpression synergizes with loss-of-function of RUNX1 in human patients with RUNX1-related leukemia, we carried out qRT-PCR on cDNA from patient samples with AML M2 carrying RUNX1-ETO fusion gene or AML M4Eo carrying CBFB-MYH11 fusion gene. These fusion genes are more commonly found RUNX1 alterations and they lead to loss-of-function of RUNX1. Expression of EVI5 in other AML and CML samples without known RUNX1 alterations was also analyzed. cDNA from BM of 3 patients who had undergone complete remission was used as control. Indeed, very significant overexpression of EVI5 was seen in 4 of 9 (44%) AML M2 patients examined. AML M4Eo patients also showed 2- to 3-fold overexpression as compared with control samples and AML samples without RUNX1 alteration (Figure 6). Thus, EVI5 overexpression and concomitant loss-of-function of RUNX1 are often observed in human RUNX1-related leukemia cases, especially in AML M2 carrying RUNX1-ETO fusion gene, suggesting that EVI5 is likely to prevent stem cell exhaustion in human RUNX1-related leukemias.
EVI5 is overexpressed in human RUNX leukemia. qRT-PCR analysis of EVI5 expression in human RUNX1-related leukemia samples: AML M2 with t(8;21) resulting in RUNX1-ETO fusion protein, AML M4 with inv(16) resulting in PEBP2β-SMMHC fusion protein, other AML cases without RUNX1 alteration and CML cases with blast crisis (CML-BC). Data are represented as fold change relative to BM samples undergoing complete remission (CR-BM).
EVI5 is overexpressed in human RUNX leukemia. qRT-PCR analysis of EVI5 expression in human RUNX1-related leukemia samples: AML M2 with t(8;21) resulting in RUNX1-ETO fusion protein, AML M4 with inv(16) resulting in PEBP2β-SMMHC fusion protein, other AML cases without RUNX1 alteration and CML cases with blast crisis (CML-BC). Data are represented as fold change relative to BM samples undergoing complete remission (CR-BM).
Runx1−/− stem cell exhaustion may be due to defective interaction with the niche
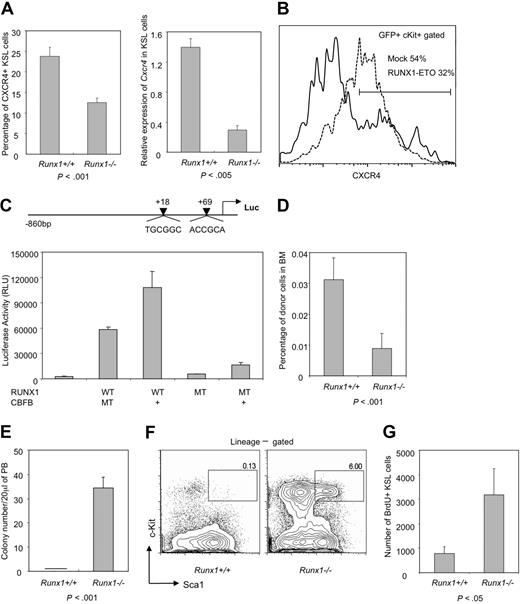
Interaction of stem cells with the stem cell niche is important for maintaining the integrity and self-renewal properties of stem cells.32,33 Analysis of a panel of niche-related factors in immature cell fraction (c-Kit+GFP+) of MIG vector–transfected Runx1+/+ and Runx1−/− BM cells, revealed that one of the most important molecules for interaction with the stem cell niche, Cxcr4, was down-regulated in Runx1−/− cells. However, normal level of Cxcr4 expression was restored after overexpression of EVI5 in Runx1−/− cells (supplemental Figure 4A). The down-regulation of Cxcr4 expression in the Runx1−/− stem/progenitor (KSL) cell fraction was further confirmed by flow cytometry (P < .001) and qRT-PCR (P < .005) (Figure 7A). Cxcr4 expression was also down-regulated in wild-type immature (c-Kit+) BM cells transfected with the dominant-negative chimeric gene RUNX1-ETO, indicating that niche interaction may be altered in human RUNX1-related leukemic cases (Figure 7B). The qRT-PCR result indicates transcriptional regulation of Cxcr4 expression by Runx1. Indeed, 2 RUNX binding sites are present in the CXCR4 promoter region, and luciferase assay using the CXCR4 promoter region showed that RUNX1 transactivates CXCR4 more than 20-fold, in a DNA binding–dependent manner (Figure 7C). Along with Cxcr4, another niche interacting factor, CD49b, which is an α2 integrin, was also down-regulated in immature Runx1−/− cells and its expression restored to normal after overexpression of EVI5 in these cells (supplemental Figure 4A).
Decreased expression of niche factor, Cxcr4, and impaired homing may be responsible for Runx1−/− stem cell exhaustion. (A) Left panel: flow cytometric analysis of Cxcr4 expression on KSL cells from Runx1+/+ (n = 4) and Runx1−/− (n = 3) mice. Right panel: qRT-PCR analysis of expression of Cxcr4 in KSL fraction of Runx1+/+ and Runx1−/− BM cells. Statistical difference using unpaired Student t test is given at the bottom. (B) Expression of Cxcr4 in c-Kit+GFP+ cells from wild-type BM cells transfected with mock MIG vector (dashed line) or RUNX1-ETO (solid line). One representative result of 2 experiments is shown. (C) Structure of the CXCR4 promoter luciferase reporter construct. The 2 arrowheads represent the positions of 2 consensus Runx1 binding sites on the human CXCR4 promoter. Graph represents the result of luciferase assay, showing transcriptional activity of wild-type RUNX1 (WT) or its mutant form R174Q (MT) with (+) or without CBFB, on CXCR4 promoter. (D) Graph showing percentage of CFSE-stained Runx1+/+ or Runx1−/− BM cells found in the recipient BM (n = 4 and 6, respectively), 16 hours after transplantation. Statistical difference using unpaired Student t test is given at the bottom. (E) Graphic representation of colony assay of 20 μL of PB from Runx1+/+ (n = 4) and Runx1−/− (n = 4) mice. (F) FACS analysis of spleen KSL fraction in Runx1+/+ and Runx1−/− mice. One representative flow cytometry profile from 2 experiments is shown. (G) Graph showing absolute number of BrdU+ KSL cells per 1 million BM cells analyzed from Runx1−/− (n = 3) and Runx1+/+ mice (n = 3). Statistical difference using unpaired Student t test is given at the bottom.
Decreased expression of niche factor, Cxcr4, and impaired homing may be responsible for Runx1−/− stem cell exhaustion. (A) Left panel: flow cytometric analysis of Cxcr4 expression on KSL cells from Runx1+/+ (n = 4) and Runx1−/− (n = 3) mice. Right panel: qRT-PCR analysis of expression of Cxcr4 in KSL fraction of Runx1+/+ and Runx1−/− BM cells. Statistical difference using unpaired Student t test is given at the bottom. (B) Expression of Cxcr4 in c-Kit+GFP+ cells from wild-type BM cells transfected with mock MIG vector (dashed line) or RUNX1-ETO (solid line). One representative result of 2 experiments is shown. (C) Structure of the CXCR4 promoter luciferase reporter construct. The 2 arrowheads represent the positions of 2 consensus Runx1 binding sites on the human CXCR4 promoter. Graph represents the result of luciferase assay, showing transcriptional activity of wild-type RUNX1 (WT) or its mutant form R174Q (MT) with (+) or without CBFB, on CXCR4 promoter. (D) Graph showing percentage of CFSE-stained Runx1+/+ or Runx1−/− BM cells found in the recipient BM (n = 4 and 6, respectively), 16 hours after transplantation. Statistical difference using unpaired Student t test is given at the bottom. (E) Graphic representation of colony assay of 20 μL of PB from Runx1+/+ (n = 4) and Runx1−/− (n = 4) mice. (F) FACS analysis of spleen KSL fraction in Runx1+/+ and Runx1−/− mice. One representative flow cytometry profile from 2 experiments is shown. (G) Graph showing absolute number of BrdU+ KSL cells per 1 million BM cells analyzed from Runx1−/− (n = 3) and Runx1+/+ mice (n = 3). Statistical difference using unpaired Student t test is given at the bottom.
We carried out a homing assay to evaluate whether Runx1−/− BM cells are compromised in homing and niche interaction. Five million BM cells from Runx1+/+ and Runx1−/− mice were labeled with 100% efficiency by CFSE, and transplanted into recipient mice. Analysis of recipient BM 16 hours after transplantation revealed that Runx1−/− cells traffic to the BM with significantly reduced efficiency (Figure 7D, supplemental Figure 4B). Thus, altered ability to home and attach to the stem cell niche in the BM may be one of the reasons for Runx1−/− stem cell exhaustion.
To assess whether defects in niche interaction of Runx1-deficient HSCs lead to mobilization of stem/progenitor cells to the PB and spleen, we carried out colony assay of PB and flow cytometry analysis of spleen cells from Runx1−/− and Runx1+/+ mice. PB (20 μL) from each Runx1−/− mouse formed an average of 35 colonies while PB from Runx1+/+ mice did not form any colonies (Figure 7E). Similarly, a significantly higher number of stem/progenitor cells was present in the spleen of Runx1−/− mice (Figure 7F). These results indicate that Runx1 deficiency leads to dramatic egress of HSCs from the BM into the PB and spleen.
We also carried out a BrdU incorporation assay to analyze whether there is increased proliferation of HSC compartment of Runx1−/− mice due to their defective niche interaction and increased cell-cycle entry. As shown in Figure 7G, proliferation of stem/progenitor cells (KSL fraction) was strongly induced in Runx1−/− mice, resulting in approximately 7-fold more BrdU+ stem/progenitor cells in Runx1−/− mice (P < .05). Taken together, all the above results suggest that the interaction between Runx1−/− HSC and its niche may be perturbed, probably due to reduced expression of Cxcr4, resulting in the release of stem cells from the niche, leading to initial expansion and subsequent exhaustion of HSCs (supplemental Figure 5B).
Finally, expression of several genes involved in stem cell function and apoptosis were checked in immature (c-Kit+) cell fraction of mock- or EVI5-transfected (GFP+) BM cells from Runx1+/+ and Runx1−/− mice, by qRT-PCR. Among the candidate genes tested, Bmi-1, important for self-renewal of normal and cancer stem cells,34 and the antiapoptotic gene, Bcl2, which is negatively regulated by Runx family,35,36 were overexpressed in Runx1−/− cells,18 and the expression of these genes was further enhanced by overexpression of EVI5 (data not shown).
Discussion
Loss-of-function of RUNX1 is frequently observed in human leukemia, implying that RUNX1 deficiency may predispose cells to leukemia development. Consistently, our previous study revealed that there is an expansion of the HSC/progenitor compartment in Runx1−/− mice, accompanied by resistance to cell death, senescence, and differentiation, characteristic of a leukemia-susceptible status.18 Three other groups, who generated conditional Runx1 knockout mice independently, also reported the expansion of the HSC/progenitor population.15-17,37 However, there is no spontaneous leukemia development in the Runx1-deficient mice. In this report, we show that the reason for this paradox may be exhaustion of Runx1-deficient HSCs over time. We provided strong evidence for exhaustion of Runx1−/− stem cells using limiting dilution and secondary transplantation experiments. A similar conclusion was reported by others using competitive transplantation experiment.16 Ichikawa et al also suggested that there appears to be a distinct difference in HSC numbers soon after deletion of Runx1 alleles (4 to 9 weeks) and over long periods of time, although they described only the initial phase expansion.37
In order to understand the mechanism of Runx1−/− stem cell exhaustion, we explored cell intrinsic changes and alterations in stem cell niche interaction. Immature Runx1−/− cells expressed higher levels of Bmi1 and Bcl2,18 and they maintained their inherent proliferative ability at 40 weeks of age and even 2 years after transplantation (Figure 2B,C). These results suggest a cell-intrinsic bias toward survival rather than exhaustion of Runx1−/− cells. On the other hand, niche interaction, which is essential for maintaining the functional integrity and quiescence of HSCs, was impaired in immature Runx1−/− cells as evidenced by reduced expression of Cxcr4 in the KSL fraction, defective homing, and mobilization of HSCs from BM into PB and spleen. Conditional Cxcr4 knockout mice show a very similar egress of HSCs from BM to PB and spleen,38 supporting the notion that Cxcr4 could be the downstream factor that affects the niche interaction of Runx1-deficient cells. Quiescent LT-HSCs are found attached to osteoblasts in the endosteal niche of the trabecular bone. Under the steady-state condition, these HSCs are forced to leave their original niche and migrate to another niche due to continuous bone turnover. When there are coexisting wild-type HSCs in the cell milieu, HSCs that lack Runx1 could be outcompeted in establishing adequate interaction with another niche, leading to their slow exhaustion (supplemental Figure 5C). Thus, problems in niche interaction would be a critical issue in leukemia development, particularly at the initial step, and a leukemia initiating clone, or preleukemic stem cells, with Runx1 alteration have to overcome this selective disadvantage for leukemia progression.
RIM to identify cooperating genetic alterations revealed that Runx1−/− mice injected with retrovirus showed shorter latency of leukemia development, thus confirming the leukemic predisposition of Runx1−/− status. There was a high frequency of stem cell–related genes affected in Runx1−/− mice and EVI5 overexpression showed the most significant effect in rescuing stem cell exhaustion, followed by EVI1 overexpression. Our results show that EVI5 may rescue Runx1−/− stem cell exhaustion by restoring expression of Cxcr4 and CD49b (supplemental Figure 4A), which enables HSCs to home back and establish adequate interaction with the niche. It is well known that factors such as CXCR4 and CD44, which mediate homing and interaction with the stem cell niche, are often up-regulated in leukemia and are essential for maintenance of leukemic stem cells.39-41 Thus, the ability of EVI5 to restore Runx1-deficient HSC interaction with its niche, together with other intrinsic factors, may be an important mechanism to rescue stem cell exhaustion, maintain leukemia-initiating stem cells, and promote leukemogenesis. It is not known how EVI5 mediates this effect. The role of EVI5 in the cell cycle may result in the indirect effects seen in HSC/progenitors since cell-cycle regulation is tightly linked to stem cell maintenance. Alternatively, EVI5 contains a conserved GAP domain, which has been shown to be important for actin cytoskeleton reorganization. Hematopoietic stem/progenitor cells from knockout mice of Cdc42GAP, one of the GAP domain family genes, showed impaired cortical F-actin assembly, deficiency in adhesion and migration, and defective homing and engraftment in the stem cell niche, leading to decline in stem cells.42 EVI5 might play a similar role as Cdc42GAP and mediate homing and engraftment of Runx1-deficient HSCs through its GAP domain.
The recipient mice that underwent transplantation with Runx1−/− cells overexpressing EVI5 or EVI1 did not develop leukemia even 1 year after BMT, although the stem cell exhaustion was definitely rescued (Figure 5E-F, supplemental Figure 3B). Further genetic changes, such as strong mitogenic stimuli, are considered to be required for overt leukemia. Indeed, overexpression of oncogenes such as c-Myc, N-Myc, or D-type cyclins that promote cell proliferation was concurrently seen in 5 of 8 Runx1−/− leukemia cases showing Evi5 overexpression in the RIM study (Table 2). In human RUNX1-related leukemia, similar mitogenic events such as mutations in receptor tyrosine kinases including c-KIT and RAS have been previously reported.4,18 In fact, of the 4 human AML M2 cases carrying RUNX1-ETO which showed overexpression of EVI5, 3 cases had concurrent activating mutations in c-KIT or FLT3. Thus, these genetic alterations overlap with each other and act as second and third hits in RUNX1-related leukemia. Interestingly, mitogenic stimuli such as oncogenic Ras are shown to induce apoptosis, senescence, and differentiation, all of which function as negative factors for oncogenesis, and are considered as a vital cellular fail-safe mechanism. However, these detrimental effects due to oncogene stimulation are attenuated by Runx1-deficient status in the development of leukemia.18 Similarly, negative effect of Runx1 deficiency, stem cell exhaustion, is in turn rescued by overexpresssion of Evi5 and Evi1.
Considering the genes that are altered with high frequency in Runx1-related leukemias and the known properties and functions of these genes, we propose the following mechanism of Runx1-related leukemogenesis. Loss-of-function of Runx1 results in increase of stem/progenitor cell fraction and therefore serves as the target cell pool for leukemia. However, maintenance of Runx1−/− stem cells is compromised, probably due to the defect in interaction between HSC and niche, resulting in stem cell exhaustion. Overexpression of stem cell–related gene like Evi5 rescues exhaustion of Runx1-deficient stem cells and maintains a significantly expanded pool of the aberrant cells with enhanced stem cell properties. Mitogenic stimuli such as activation of c-Myc, N-myc, D-type cyclins, Ras, or c-Kit result in overt proliferation of Runx1-deficient cells due to attenuated cellular fail-safe mechanism, thus providing the necessary stimulus for Runx1-deficient cells to develop full-blown leukemia. Such cooperative mechanism may be generally seen in cancer development whereby negative aspects caused by certain oncogenic hits are overcome by the others. Elucidation of these combinatorial mechanisms would provide profound insights into the understanding of oncogenesis and may provide a novel direction for therapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Yanagida, L. Motoda, E. L. Ng, S. S. Nah, L. Q. Chen, and Q. R. Gwee for technical assistance and scientific discussions; N. Copeland and N. Jenkins for the MoMuLV retrovirus; M. Sugai for the MIG retroviral vector; G. P. Nolan for the Phoenix-Eco cell line; K. Morishita for human EVI1 cDNA; K. Rajewsky for Mx-Cre Tg mice; W. Krek for CXCR4 promoter luciferase constructs; and members of the Biological Resource Center, Biopolis, for mouse husbandry.
This work was supported by A*STAR (Agency of Science, Technology and Research), Singapore National Research Foundation, and the Ministry of Education under the Research Center of Excellence Program.
Authorship
Contribution: B.J. and M.O. designed and performed experiments, analyzed data, and wrote the manuscript; N.Y. and C.Q.W. performed experiments; I.T. and D.R.L. provided the conditional Runx1 knockout mice; N.A. provided the patient leukemic samples; and Y.I. analyzed data and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Motomi Osato or Yoshiaki Ito, Institute of Molecular and Cell Biology, 61 Biopolis Dr, Singapore 138673; e-mail: motomi@imcb.a-star.edu.sg or itoy@imcb.a-star.edu.sg.