Abstract
There is growing evidence that telomere dysfunction can contribute to human aging. Telomere dysfunction limits lymphopoiesis in aging telomerase knockout (mTerc−/−) mice primarily by the induction of stem cell–extrinsic alterations. The relative contribution of alterations in the stem cell niche and the systemic environment to the impairment of lymphopoiesis in response to telomere dysfunction is currently unknown. This study reveals a minor impact of stem cell–intrinsic defects on the impairment of B and T lymphopoiesis in response to telomere dysfunction. The impairment in B and T lymphopoiesis in aging telomere-dysfunctional mice was mainly due to alterations of the systemic environment. Telomere dysfunction had no significant cell-autonomous effects impairing the function of thymic or bone marrow niches in supporting B and T lymphopoiesis. Moreover, age-related alterations in the cellular composition of the thymic epithelium in telomere-dysfunctional mice were rescued by transplantation of the thymus into a wild-type environment; these rejuvenated thymi supported normal T lymphopoiesis in recipient mice. Together, these data place alterations in the systemic environment on top of the hierarchy of events limiting lymphopoiesis in response to telomere dysfunction.
Introduction
The impairment in B and T lymphopoiesis is a major phenotype of human aging contributing to the reduction in immune function during aging.1,2 The underlying mechanisms that impair B lymphopoiesis in bone marrow and T lymphopoiesis in the involuting thymus in aging humans are still not well understood. Molecular studies on aging indicate that the accumulation of DNA damage and telomere dysfunction can contribute to the evolution of age-related pathology.3,4
Telomeres cap the chromosomal ends, thus preventing chromosomal instability and DNA damage signal induction.5,6 Telomere shortening limits the proliferative capacity of primary human cells to a finite number of cell divisions.7 There is growing evidence that telomere shortening can contribute to the impairment in organ maintenance during human aging,8,9 including the decline in T and B lymphocyte reserves.10 Organ systems with high rates of cell turnover are most sensitive to telomere shortening in mice and humans,11-13 indicating that telomere dysfunction impairs stem cell function, resulting in defects in organ maintenance. Moreover, chronic diseases that accelerate the rate of cell turnover are associated with accelerated telomere shortening and impaired tissue maintenance, including the progressive evolution of lymphopenia and immune dysfunction in chronic HIV infection.14,15
Studies on telomerase knockout mice (mTerc−/−) have shown that telomere dysfunction induces both cell-intrinsic checkpoints16 and environmental alterations,17 limiting the function of hematopoietic stem cells. Of note, the impairment in B lymphopoiesis was strongly associated with telomere dysfunctional environment rather than with telomere dysfunction in hematopoietic stem cells (HSCs).17 It is not known whether the same holds true for the age-related impairment in T lymphopoiesis and the premature involution of the thymus in telomere-dysfunctional mice. In addition, the nature of the cell-extrinsic alterations that impair lymphopoiesis in response to telomere dysfunction remains to be defined.
Parabiosis experiments have shown that systemic acting factors can contribute to the impairment of stem and progenitor cell function in aging wild-type mice, specifically in muscle stem cells and regenerating liver.18 Similarly, a dysregulation of cytokines and growth factors has been observed in aging telomere-dysfunctional mice, contributing to the impairment of B lymphopoiesis and stem cell engraftment in these mice.17 In addition to these effects of the systemic environment, there is experimental evidence that stem cell niches show a decline in capacity to maintain functional stem cells in the germline of aging Drosophila.19,20 It is possible that both a decline in niche function and alterations in the systemic environment could contribute to the impairment of lymphopoiesis in the context of aging and telomere dysfunction. However, an in vivo analysis on the relative contribution of systemic alterations versus stem cell niches is yet to be conducted.
Here we show by syngenic transplantations of HSCs, bone, and thymi that alterations in the systemic environment are the main cause of impaired B and T lymphopoiesis in aging telomere-dysfunctional mice. Alterations in lymphopoietic stem and progenitor cell niches had no measurable influence on lymphopoiesis and were reversible by transplantation of these niches into a wild-type environment. These findings suggest that therapeutic targeting of circulating factors could improve lymphopoiesis in the context of telomere dysfunction and aging.
Methods
Mice
Mice were maintained in a pathogen-free environment and fed with a standard diet. C57BL/6 congenic mice expressing CD45.1 or CD45.2 on leukocytes were used for transplantation experiments. G3mTerc−/− or mTerc+/+ mice (CD45.2) were used as donors, and 2- to 3-month-old wild-type mice (CD45.1) were used as recipients for bone marrow transplantation. Neonatal thymi or bones were isolated from G3mTerc−/− or mTerc+/+ mice and were transplanted under the kidney capsule of 2- to 3-month-old G3mTerc−/− or mTerc+/+ mice side-by-side. All animal experiments were approved by the state government of Baden-Württemberg.
Bone and thymus transplantations
Survival surgery was performed under sterile conditions after intraperitoneal administration of the anesthetic ketamine (100 mg/kg) and xylazine (10 mg/kg) to 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice. A small dorsolateral incision was made to expose the kidney, and a small hole was made in the kidney capsule. Neonatal thymic lobe or 1 hindlimb bone from a G3mTerc−/− or mTerc+/+ donor was placed under the kidney capsule side-by-side in recipient mice. The grafted thymi and bones were analyzed at 9 months after transplantation.
Bone marrow transplantation
Bone marrow cells from 12-month-old G3mTerc−/− or mTerc+/+ (CD45.2) mice were isolated by flushing both tibiae and femurs with sterile phosphate-buffered saline (PBS), and intravenously injected into lethally irradiated (12 Gy) 2- to 3-month-old wild-type mice (CD45.1). At 5 months after bone marrow transplantation, the wild-type recipient mice (reconstituted with either 12-month-old G3mTerc−/− or mTerc+/+ bone marrow cells) were used as recipients for neonatal thymus (G3mTerc−/− or mTerc+/+) transplantation under their kidney capsule. The native thymus and grafted thymus were analyzed at 3 months after the second transplantation.
Flow cytometry
Bone marrow cells were isolated by flushing both tibiae and femurs with sterile PBS, and thymocytes were prepared by smashing the thymus. Thymic epithelial cells were obtained by digestion using collagenase D, DNase1, and dispase1. Cells were filtered and counted before staining with antibodies. Data acquisition was performed on a FACS LSRII (Becton Dickinson), and cell sorting was performed on FACSAria (Becton Dickinson). Data were analyzed on the Diva 6.1 software (Becton Dickinson).
OP9 delta-1 culture
OP9 delta-1 cells were kindly provided by J. C. Zuniga-Pflücker (Department of Immunology, Toronto University) and cultures were carried out as described.21 A total of 50 to 100 purified long-term (LT)–HSCs (lineage−, Sca1+, cKit+, CD34lo/−, Flt3−), multipotent progenitors (MPPs; lineage−, Sca1+, cKit+, CD34+, Flt3+), or common lymphoid progenitors (CLPs; lineage−, Sca1lo, cKitlo, IL7Ra+, Flt3+) were plated on OP9 delta-1 stromal cells in 96-well plates. Flt3 ligand was added at a final concentration of 10 ng/mL and IL-7 was added at a final concentration of 5 ng/mL. Hematopoietic cells were transferred to fresh layers with fresh cytokines at days 8 to 10 of culture. HSC culture was harvested for analysis at day 21. MPP and CLP cultures were analyzed at day 14. Cells were filtered through a nylon mash and stained with the antibody before fluorescence-activated cell sorter (FACS) analysis.
Statistics
We carried out statistical analyses using Microsoft Excel and Graph Pad Prism software. The unpaired Student t test was used to generate P values for all the datasets. Error bars represent SD in all figures.
Results
Alterations in the systemic environment are the main cause of impaired T lymphopoiesis in telomere-dysfunctional mice
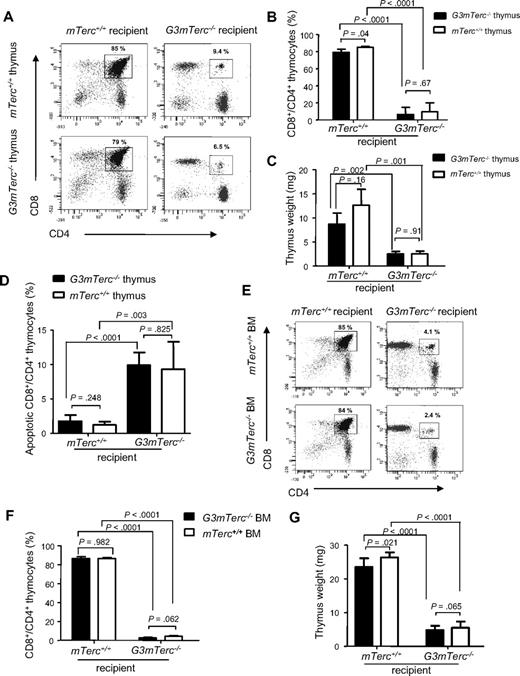
One of the major phenotypes in aging telomere-dysfunctional mice is the premature involution of the thymus resulting in impaired T lymphopoiesis.11,22 In accordance with these data, 10- to 12-month-old G3mTerc−/− mice showed a strongly reduced thymus weight compared with age-matched mTerc+/+ control mice (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To decipher the role of HSCs, the thymic niche, and the systemic macroenvironment in this process, the thymi of newborn G3mTerc−/− and mTerc+/+ mice were transplanted under the kidney capsule of 3-month-old G3mTerc−/− or mTerc+/+ recipients.23 At 9 months after transplantation, the thymus weight, frequency of CD4/CD8 double-positive thymocytes, and total number of thymocytes of the grafted thymi were strongly reduced in G3mTerc−/− recipients compared with mTerc+/+ recipients, independent of the genotype of the thymic graft (Figure 1A-C, supplemental Figure 1B). FACS analysis of annexin V–stained thymocytes revealed an increased rate of apoptosis of CD4/CD8 double-positive thymocytes in the grafted thymi that were transplanted into G3mTerc−/− recipients compared with those transplanted into mTerc+/+ recipients, regardless of the genotype of the thymic graft (Figure 1D).
Telomere dysfunction induces alterations in the systemic environment, impairing thymopoiesis. (A-D) Neonatal thymi from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted thymi were analyzed 9 months after transplantation. (A) Representative FACS profiles and (B) bar graph showing the percentage of CD4 and CD8 double-positive (DP) thymocytes in grafted thymi of recipient mice of the indicated genotypes. (C) Bar graph showing the weight of grafted thymi in recipient mice of the indicated genotypes. (D) Bar graph showing the percentage of annexin V–positive, CD4 and CD8 DP thymocytes in grafted thymi of recipient mice of the indicated genotypes. (E-G) Bone marrow cells (1.5 × 106) from 12-month-old G3mTerc−/− or mTerc+/+ mice were transplanted into lethally irradiated (12 Gy) 2-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 6 mice per group). Thymi were analyzed 8 months after transplantation. (E) Representative FACS profiles and (F) corresponding bar graph showing the percentage of CD4 and CD8 DP cells in the native thymi of mice of the indicated genotypes that received bone marrow transplants. (G) Bar graph showing the weight of the native thymi of mice of the indicated genotypes that received bone marrow transplants. Error bars represent SD in all panels.
Telomere dysfunction induces alterations in the systemic environment, impairing thymopoiesis. (A-D) Neonatal thymi from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted thymi were analyzed 9 months after transplantation. (A) Representative FACS profiles and (B) bar graph showing the percentage of CD4 and CD8 double-positive (DP) thymocytes in grafted thymi of recipient mice of the indicated genotypes. (C) Bar graph showing the weight of grafted thymi in recipient mice of the indicated genotypes. (D) Bar graph showing the percentage of annexin V–positive, CD4 and CD8 DP thymocytes in grafted thymi of recipient mice of the indicated genotypes. (E-G) Bone marrow cells (1.5 × 106) from 12-month-old G3mTerc−/− or mTerc+/+ mice were transplanted into lethally irradiated (12 Gy) 2-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 6 mice per group). Thymi were analyzed 8 months after transplantation. (E) Representative FACS profiles and (F) corresponding bar graph showing the percentage of CD4 and CD8 DP cells in the native thymi of mice of the indicated genotypes that received bone marrow transplants. (G) Bar graph showing the weight of the native thymi of mice of the indicated genotypes that received bone marrow transplants. Error bars represent SD in all panels.
It is known that HSCs/early thymic progenitors from bone marrow constantly repopulate the thymus, thus sustaining adult T thymopoiesis. In agreement with this physiology, more than 98% of the T lymphoid cells in the grafted thymi were recipient-derived (supplemental Figure 1C), whereas the epithelium was graft-derived (data not shown). CD4/CD8 double-positive T-cell progenitors were located within the lobes of the transplanted thymi, showing that the cellular niche for T lymopoiesis was the engrafted thymus rather than kidney capsule itself (supplemental Figure 1D-E). Together, these data indicated that the impairment of thymopoiesis in response to telomere dysfunction was mainly due to alterations in the systemic environment and/or defects in the HSC compartment. To determine the influence of telomere dysfunction in HSCs on thymopoiesis, the native thymus of irradiated G3mTerc−/− and mTerc+/+ mice was analyzed 6 to 8 months after reconstitution with either G3mTerc−/− or mTerc+/+ bone marrow cells. Again, a significant reduction in thymus weight, frequency of CD4/CD8 double-positive thymocytes, and total number of thymocytes was present in G3mTerc−/− recipient mice, independent of the genotype of the reconstituted bone marrow (Figure 1E-G, supplemental Figure 1F). These experiments indicated that the transplantation of hematopoietic stem and progenitor cells with long telomeres did not rescue thymopoiesis in telomere-dysfunctional mice, but environmental defects in G3mTerc−/− mice were dominantly inhibiting thymopoiesis.
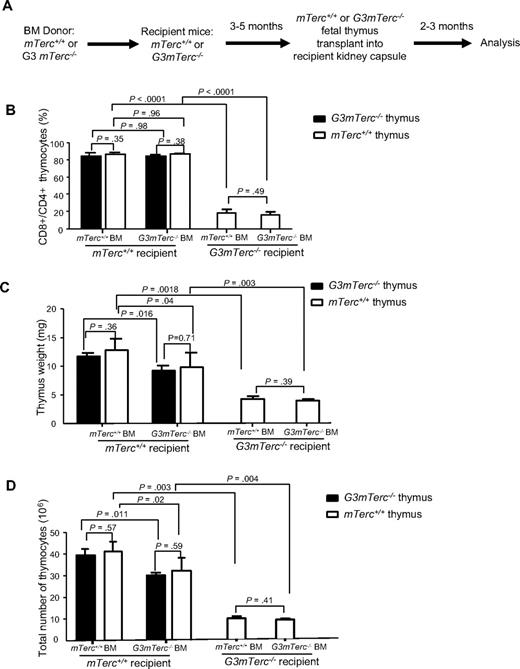
To exclude an effect of irradiation on the thymic niche in mice that underwent bone marrow transplantation, mTerc+/+ and G3mTerc−/− mice were lethally irradiated and transplanted with mTerc+/+ or G3mTerc−/− bone marrow. After bone marrow reconstitution, thymi from neonatal mice were transplanted under the kidney capsules of these recipient mice (Figure 2A-D). An analysis of thymopoiesis in the grafted thymi (2 to 3 months after thymus transplantation) revealed that telomere-dysfunctional HSCs had a minor inhibitory effect on thymopoiesis in mTerc+/+ recipients. Specifically, thymus weight and the total number of thymocytes were slightly reduced in mTerc+/+ mice that received transplants of G3mTerc−/− HSCs compared with mTerc+/+ mice that received transplants of mTerc+/+ HSCs (Figure 2C-D). This impairment in thymopoiesis was independent of the genotype of the grafted thymi. In contrast to these data on mTerc+/+ recipients, the strong reduction in thymopoiesis in G3mTerc−/− mice was not improved by transplantation of mTerc+/+ HSCs (Figure 2B-D, supplemental Figure 2A). In vitro cultures on OP9 delta-1 cells21 confirmed a slight reduction in T lymphopoietic potential of LT-HSCs (lineage−, Sca1+, cKit+, CD34lo/−, Flt3−) of 12-month-old G3mTerc−/− mice compared with age-matched mTerc+/+ mice (supplemental Figure 2B). The T lymphopoietic potential of MPP cells (lineage−, Sca1+, cKit+, CD34+, Flt3+) or CLP cells (lineage−, Sca1lo, cKitlo, IL7Ra+, Flt3+) was not significantly affected by telomere dysfunction (supplemental Figure 2C-D). Together, these data showed that telomere dysfunction in hematopoietic stem and progenitor cells had mild inhibitory effects on thympoiesis in mTerc+/+ mice. In contrast, telomere dysfunction at the organism level induced a strong inhibition of thymopoiesis in G3mTerc−/− mice, which could not be rescued by transplantation of HSCs and thymic niches with long telomere reserves. These defects occurred with a comparable severity in nonirradiated G3mTerc−/− mice (Figure 1A-C, supplemental Figure 1B). Together, these results indicate that the severe impairment in thymopoiesis in telomere-dysfunctional mice was induced by dominant defects in the systemic environment.
Telomere dysfunction in HSCs and the thymic epithelium has minor effects on the impairment in thymopoiesis. (A-D) Two- to 3-month-old mTerc+/+ or G3mTerc−/− recipient mice were lethally irradiated and reconstituted with the bone marrow (1.5 × 106 cells) of 12-month-old G3mTerc−/− or mTerc+/+ donor mice (n = 6 recipients per group). At 3 to 5 months after bone marrow transplantation, neonatal thymi from mTerc+/+ mice or G3mTerc−/− mice were transplanted under the kidney capsules of the recipient mice. Grafted thymi were analyzed at 2 to 3 months after thymus transplantation. (A) Experimental scheme. (B-D) Analysis of the grafted thymi. The bar graphs show (B) the percentage of CD4 and CD8 double-positive thymocytes, (C) the thymus weight, and (D) the total number of thymocytes. Error bars represent SD in all panels.
Telomere dysfunction in HSCs and the thymic epithelium has minor effects on the impairment in thymopoiesis. (A-D) Two- to 3-month-old mTerc+/+ or G3mTerc−/− recipient mice were lethally irradiated and reconstituted with the bone marrow (1.5 × 106 cells) of 12-month-old G3mTerc−/− or mTerc+/+ donor mice (n = 6 recipients per group). At 3 to 5 months after bone marrow transplantation, neonatal thymi from mTerc+/+ mice or G3mTerc−/− mice were transplanted under the kidney capsules of the recipient mice. Grafted thymi were analyzed at 2 to 3 months after thymus transplantation. (A) Experimental scheme. (B-D) Analysis of the grafted thymi. The bar graphs show (B) the percentage of CD4 and CD8 double-positive thymocytes, (C) the thymus weight, and (D) the total number of thymocytes. Error bars represent SD in all panels.
Alterations in the systemic environment are the main cause of impaired B lymphpoiesis in telomere-dysfunctional mice
In addition to thymus involution, a skewing in bone marrow–derived hematolymphopoiesis (decreased B lymphopoiesis, increased myelopoiesis) represents a characteristic phenotype of the aging hematopoietic system in mice and humans.24-26 This skewing in hemato/lymphopoiesis was accelerated in aging telomere-dysfunctional mice.17 To determine the relative contribution of telomere dysfunction–induced changes in the systemic environment compared with telomere dysfunction–induced alterations in the stem cell niche to the skewing of hemato/lymphopoiesis, bones of neonatal G3mTerc−/− and mTerc+/+ mice were transplanted under the kidney capsules of 3-month-old G3mTerc−/− and mTerc+/+ recipients. In agreement with previous studies,27 more than 96% of the hemato/lymphopoietic cells in the grafted bones were host-derived, whereas bone marrow stroma was graft-derived. At 9 months after transplantation, a strong reduction in B lymphopoiesis (less than 5% of total bone marrow cells) and an increase in myelopoiesis was seen in the grafted bones that were transplanted into G3mTerc−/− recipients compared with those transplanted into mTerc+/+ recipients, independent of the genotype of the grafted bones (Figure 3A-C). These data indicated that the skewing of myelolymphopoiesis in response to telomere dysfunction was mainly due to the alterations in the systemic environment or induced by HSC intrinsic defects.
Telomere dysfunction induces alterations in the systemic environment impairing B-cell development. (A-C) Neonatal bones from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted bones were analyzed 9 months after transplantation. (A) Representative FACS profiles and (B-C) bar graphs showing the percentage of (B) B cells and (C) myeloid cells in total bone marrow cells of grafted bones and recipient mice of the indicated genotypes. (D-E) Bone marrow cells (1.5 × 106) from 12-month-old G3mTerc−/− or mTerc+/+ mice were transplanted into lethally irradiated 2-month-old mTerc+/+ recipient mice (n = 6 per group). The bone marrow of recipient mice was analyzed 8 months after transplantation. (D-E) Bar graphs showing percentage of donor-derived (D) B cells and (E) myeloid cells in mTerc+/+ recipient mice that received transplants of G3mTerc−/− or mTerc+/+ bone marrow cells. Error bars represent SD in all panels.
Telomere dysfunction induces alterations in the systemic environment impairing B-cell development. (A-C) Neonatal bones from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted bones were analyzed 9 months after transplantation. (A) Representative FACS profiles and (B-C) bar graphs showing the percentage of (B) B cells and (C) myeloid cells in total bone marrow cells of grafted bones and recipient mice of the indicated genotypes. (D-E) Bone marrow cells (1.5 × 106) from 12-month-old G3mTerc−/− or mTerc+/+ mice were transplanted into lethally irradiated 2-month-old mTerc+/+ recipient mice (n = 6 per group). The bone marrow of recipient mice was analyzed 8 months after transplantation. (D-E) Bar graphs showing percentage of donor-derived (D) B cells and (E) myeloid cells in mTerc+/+ recipient mice that received transplants of G3mTerc−/− or mTerc+/+ bone marrow cells. Error bars represent SD in all panels.
Previous experiments have shown that skewing of myelolymphopoiesis was rescued when the HSCs of 12-month-old G3mTerc−/− mice are transplanted into wild-type recipients followed-up to 3 months after transplantation.17 At this time point, the accumulative age of G3mTerc−/− HSCs was 15 months. Here, we extended the follow-up period of wild-type recipients to 8 months after transplantation with the HSCs of 12-month-old G3mTerc−/− mice (to an accumulative age of 20 months in the G3mTerc−/− HSCs). At this time point, a mild skewing in myelolymphopoiesis was observed in mice reconstituted with G3mTerc−/− bone marrow. Specifically, the percentage of B lymphocytes in total bone marrow was 14.7% plus or minus 1.9% in wild-type mice reconstituted with G3mTerc−/− bone marrow versus 19.9% plus or minus 1.1% in wild-type mice reconstituted with mTerc+/+ bone marrow (Figure 3D; P = .031); the percentage of myeloid cells (CD11b+) was 72.8% plus or minus 2.6% in wild-type mice reconstituted with G3mTerc−/− bone marrow versus 62.1% plus or minus 3.0% in wild-type mice reconstituted with mTerc+/+ bone marrow (Figure 3E; P = .024). These results indicated that telomere dysfunction induced stem cell–intrinsic defects that contributed to the skewing in myelolymphopoiesis in advanced-age G3mTerc−/− HSCs. However, these defects did not resemble the strong suppression in B lymphopoiesis occurring in G3mTerc−/− mice compared with mTerc+/+ mice (Figure 3A-C).
Together, the above data indicate that defects in the systemic environment were the main cause of impaired B and T lymphopoiesis in response to telomere dysfunction, whereas intrinsic alterations in HSCs or the stem cell niches had minor effects and apparently were not the main cause of impaired T and B lymphopoiesis in response to telomere dysfunction. However, it remained possible that alterations in the systemic environment caused secondary changes in local niches that contributed to the phenotype, but were at least in part reversible.
Alterations in the systemic environment induce reversible defects in the thymic epithelial niche
To determine the influence of the systemic environment on the cellular niche, the thymic epithelium was analyzed because this compartment shows major atrophic alterations in response to aging28,29 and telomere dysfunction (supplemental Figures 1A-B, 3A). Flow cytometry revealed increased apoptosis in thymic epithelial cells (TECs; CD45−, G8.8+) from the thymus of 12-month-old G3mTerc−/− mice compared with age-matched mTerc+/+ mice (supplemental Figure 3B). In agreement with these results, a strong reduction in the number of TECs was seen in 12-month-old G3mTerc−/− mice compared with age-matched mTerc+/+ mice (Figure 4A). This atrophy of thymic epithelium was most pronounced in the medullary compartment (Figure 4B-C). Thymus transplantation under the kidney capsules of G3mTerc−/− or mTerc+/+ mice revealed that the atrophy of the thymic epithelium was associated with the systemic environment of the recipients, but not with the genotype of the grafted thymus (Figure 4D-E). Transplantation of severely atrophic thymi of 12-month-old G3mTerc−/− mice under the kidney capsules of 3-month-old mTerc+/+ recipient mice resulted in regrowth of the G3mTerc−/− thymus and reactivation of T lymphopoiesis (Figure 4F-G, supplemental Figure 3C). These data indicate that atrophy of thymic epithelium in telomere-dysfunctional mice can be functionally rescued even at a late stage of thymic involution by re-exposure to the mTerc+/+ wild-type environment.
Telomere dysfunction induces alterations in the systemic environment, impairing thymic epithelial maintenance. (A-C) The thymi of 12-month-old G3mTerc−/− or mTerc+/+ mice were analyzed by FACS (n = 5 mice per group). (A) Bar graph showing the total number of epithelial cells. (B) Representative FACS profiles showing thymus epithelial cells (CD45−, G8.8+), in thymic cortex (CD45−, G8.8+, ly51+) and medulla (CD45−, G8.8+, ly51−). (C) The ratio of cortical to medullary epithelial cells (cTEC/mTEC) in thymi of 12-month-old G3mTerc−/− or mTerc+/+ mice. (D-E) Neonatal thymi from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted thymi were analyzed 9 months after transplantation. (D) Representative FACS profiles and (E) corresponding bar graph showing the ratio of cortical to medullary epithelial cells in the transplanted thymi. (F-G) Thymi from 12-month-old G3mTerc−/− mice were transplanted under the kidney capsules of 2- to 3-month-old mTerc+/+ recipient mice (n = 5 per group). Native nontransplanted thymi and grafted thymi were analyzed 2 to 3 months after transplantation. Bar graphs show (F) thymus weight and (G) percentage of CD4 and CD8 DP cells in total thymocytes of native nontransplanted G3mTerc−/− thymi and grafted thymi 2 to 3 months after transplantation into mTerc+/+ recipients. Error bars represent SD in all panels.
Telomere dysfunction induces alterations in the systemic environment, impairing thymic epithelial maintenance. (A-C) The thymi of 12-month-old G3mTerc−/− or mTerc+/+ mice were analyzed by FACS (n = 5 mice per group). (A) Bar graph showing the total number of epithelial cells. (B) Representative FACS profiles showing thymus epithelial cells (CD45−, G8.8+), in thymic cortex (CD45−, G8.8+, ly51+) and medulla (CD45−, G8.8+, ly51−). (C) The ratio of cortical to medullary epithelial cells (cTEC/mTEC) in thymi of 12-month-old G3mTerc−/− or mTerc+/+ mice. (D-E) Neonatal thymi from G3mTerc−/− or mTerc+/+ mice were transplanted side-by-side under the left and right kidney capsules of 2- to 3-month-old G3mTerc−/− or mTerc+/+ recipient mice (n = 5 per group). The grafted thymi were analyzed 9 months after transplantation. (D) Representative FACS profiles and (E) corresponding bar graph showing the ratio of cortical to medullary epithelial cells in the transplanted thymi. (F-G) Thymi from 12-month-old G3mTerc−/− mice were transplanted under the kidney capsules of 2- to 3-month-old mTerc+/+ recipient mice (n = 5 per group). Native nontransplanted thymi and grafted thymi were analyzed 2 to 3 months after transplantation. Bar graphs show (F) thymus weight and (G) percentage of CD4 and CD8 DP cells in total thymocytes of native nontransplanted G3mTerc−/− thymi and grafted thymi 2 to 3 months after transplantation into mTerc+/+ recipients. Error bars represent SD in all panels.
Discussion
The current study shows that the in vivo impairment of B and T lymphopoiesis in response to telomere shortening is mainly induced by alterations in the systemic environment. Transplantation of HSCs, bones, and thymi showed that the impairment in B and T lymphopoiesis was strongly associated with the telomere status of the recipient mice.
Telomere shortening in hematopoietic stem and progenitor cells had a mild influence on the impairment of lymphopoiesis in mTerc+/+ mice with functional telomeres. In contrast, the telomere status of HSCs did not affect lymphopoiesis in telomere-dysfunctional mice. Of note, transplantation of HSCs with long telomere reserves did not improve thymopoiesis in telomere-dysfunctional mice, indicating that systemic alterations in response to telomere dysfunction have dominant inhibitory effects on thymopoiesis that cannot be overcome by stem cell transplantation.
Previous studies have shown that the transplantation of fetal thymi can result in the engraftment of functionally active thymi in aged mice, indicating that the age-related thymic involution results primarily from changes in the thymic microenvironment.29,30 In contrast to these data on aged wild-type mice, the current study shows that neonatal thymi from mTerc+/+ wild-type mice cannot support functional thymopoiesis in aged telomere-dysfunctional mice. These data suggest that systemic alterations that occur in response to telomere dysfunction are a dominant cause for age-related thymic involution independent of the genotype of the thymic epithelium.
It has been noted that thymi from aged wild-type mice retain some capacity to regenerate mature T-cell progeny.31 Our study shows that telomere dysfunction induces impairments in thymus function that are reversible by transplanting the atrophic thymus from aged G3mTerc−/− mice into young wild-type recipients. In contrast, studies on wild-type mice have revealed experimental evidence for an age-dependent evolution of irreversible defects in thymus function, limiting T lymphopoiesis.32 Our study does not exclude subtle, direct effects of telomere dysfunction on the thymic niche. In fact, the rescue of the thymus weight after transplantation from aged G3mTerc−/− mice into young wild-type recipients was incomplete compared with the weight of young wild-type thymus (Figure 4F, supplemental Figure 1A). However, our study shows that architectural changes in the thymus epithelium and the impairment of thymopoiesis in response to telomere dysfunction are mainly due to environmental alterations, and that these defects are reversible. These results could also be relevant for maintaining the functional reserves of the thymus in aged humans. Along these lines, reinitiation of T lymphopoiesis in humans in response to chemotherapy and autologous bone marrow transplantation was dependent on the re-establishment of thymic structure, and this capacity was reduced with age of the patients.33,34
The impairment in B lymphopoiesis and the increase in myelopoiesis represent additional hallmark features of the aging hematopoietic system in mice and humans; these phenotypes have been associated with an increased number of myeloid-primed HSCs and an accelerated risk of myeloproliferative diseases.27,35,36 The current study shows that the acceleration in skewing of hemato/lymphopoiesis in response to telomere dysfunction17 is mainly due to alterations in the systemic macroenvironment. Bone transplantation experiments showed that telomere dysfunction does not induce cell-intrinsic alterations in the bone marrow niche that lead to a direct impairment in niche function, inducing a skewing in hemato/lymphopoiesis. These results do not exclude the possibility that alterations in the systemic environment influence the function of the bone marrow niche. There is growing evidence that the bone marrow niche has specific molecular functions in maintaining stem cell self-renewal and differentiation.37,38 It is conceivable that alterations in the systemic environment would interfere with some of these specific molecular signaling pathways in the bone marrow microenvironment.
The systemic factors that impair B and T lymphopoiesis in response to telomere dysfunction remain yet to be defined. There are studies suggesting that both processes might be interconnected.39 A variety of systemic acting factors have been shown to affect thymus involution, such as keratinocyte growth factor, IL-7, sex steroids,40-42 serum zinc levels,43 corticosteroids,44 or impaired function of the somatotrophic growth hormone/insulin growth factor axis.45 The latter could be affected by telomere dysfunction, as the accumulation of DNA damage resulted in the suppression of the somatotrophic axis in DNA repair–deficient mice and cells.46,47 Because telomere dysfunction represents a chronic, nonrepairable form of DNA damage, it is possible that similar effects are elucidated by telomere dysfunction.
The degree to which the telomerase knockout mouse model reflects normal human aging remains an open question. Germline deletion of mTerc results in shortened telomeres in all tissues, and the process starts during development in the mice. Therefore, whether the systemic effects described in the present system mirror what would occur during normal aging remains an open question. It has been shown that telomere dysfunction in telomerase knockout mice induced the expression of marker proteins of DNA damage in blood serum.8 Of note, the same marker proteins increase during human aging but not in aging wild-type mice with functional telomeres.8 These findings indicate that mechanisms of telomere dysfunction affecting T and B lymphopoiesis could be relevant for human aging. The current study reveals the first experimental evidence that telomere dysfunction–induced alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in aging telomerase knockout mice. The identification of systemic alterations in response to telomere dysfunction could help to develop therapeutic strategies aiming to preserve lymphopoiesis and immune function in human aging and chronic diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from Deutsche Forschungsgemeinschaft (DFG) to K.L.R. (SFB 518) and by a grant from the Max Planck-Society to Z.J. (Max Plank Partner Group Program on Stem Cell Aging). K.L.R. is supported by DFG (RU745/10-1 and RU745/7-1) and the European Union (Telomarker); Z.J. is supported by the National Natural Science Foundation of China (Grant No. 30771189) and National Science and Technology Major Projects (No. 2009ZX09501-026). G.T., H.-R.R., Z.S., and K.L.R. are funded by SFB 497.
Authorship
Contribution: Z.S. and Z.J. designed and performed research, analyzed data, and wrote the paper; J.W., L.M.G., and G.T. performed research; H.-R.R. designed research; and K.L.R. designed research and wrote the paper.
Conflict-of interest disclosure: The authors declare no competing financial interests.
The current address for Z.S. is Department of Colorectal Surgery, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China.
Correspondence: K. Lenhard Rudolph, Department of Molecular Medicine and Max-Planck Research Group on Stem Cell Aging, University of Ulm, Albert Einstein Allee 11, 89081 Ulm, Germany; e-mail: lenhard.rudolph@uni-ulm.de; or Zhenyu Ju, Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences, Chaoyang Panjiayuan Nanli 5, 100021, Beijing, China; e-mail: zhenyuju@hotmail.com.