Abstract
In this issue of Blood, Fauriat and colleagues find that the expression of KIR2DS1 by human NK cells is associated with a decreased responsiveness to various stimuli in HLA C2/C2 but not in C1/C1 persons.
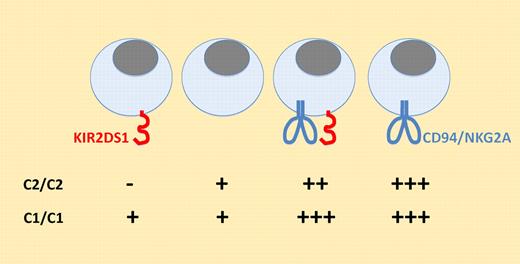
How natural killer (NK)–cell responsiveness is controlled is an unresolved and fascinating question. A major breakthrough in this field of investigations was made when NK cells expressing inhibitory KIR (human) or Ly49 (mouse) capable of interacting with endogenously expressed major histocompatibility complex (MHC) molecules were found to be hyperresponsive to stimulation through various receptors.1-3 This counterintuitive result led to the concept of “licensing” or “education,” a process by which a chronic interaction of inhibitory KIR or Ly49 with their MHC ligands would promote NK-cell responsiveness through unknown mechanisms.4,5 Recent data in mouse models suggested that activating receptors also participate in this educational process but in the opposite direction. Indeed, it was found that overexpression of ligands for an activating Ly496,7 or for NKG2D8,9 led to a decreased responsiveness of NK cells. In this issue of Blood, Fauriat et al investigate the consequences of KIR2DS1 expression on NK-cell responsiveness.10 KIR2DS1 is an activating KIR known to interact with HLA-C molecules of the group 2 but not to those of the group 1. They took advantage of a combination of anti-KIR antibodies to track KIR2DS1 positive NK cells in human peripheral blood mononuclear cells. Next, they set up a 9-color flow cytometry procedure to track every combination of KIR and NKG2A/CD94 molecule expression on NK cells and assess their responsiveness to various stimuli. Of note, this flow cytometry procedure is certainly powerful but potentially limited by steric hindrance of epitopes, considering that 7 antibodies coupled to sometimes very big fluorochromes are used to stain the same cells. Despite this potential technical limitation, data by Fauriat et al clearly indicate that KIR2DS1 influenced NK-cell responsiveness in donors of the C2 group but not in donors of the C1 group. More precisely, they found that KIR2DS1 simple positive NK cells are hyporesponsive to stimulation through various receptors in comparison with KIR-negative NK cells. Moreover, they found that expression of KIR2DS1 by NKG2A-positive NK cells decreases their responsiveness (see figure). These results provide the first evidence that the interaction of an activating KIR with its cognate ligand may modulate NK-cell responsiveness in a physiological setting.
Fine tuning of NK-cell responsiveness by NK-cell receptors. In this model, the interaction of inhibitory receptors with their ligands increases NK-cell responsiveness, whereas the interaction of activating receptors with their ligands has the opposite outcome.
Fine tuning of NK-cell responsiveness by NK-cell receptors. In this model, the interaction of inhibitory receptors with their ligands increases NK-cell responsiveness, whereas the interaction of activating receptors with their ligands has the opposite outcome.
As discussed by the authors, these results fit well with the “rheostat” model of NK-cell responsiveness.11,12 This model proposes that NK-cell responsiveness varies in a wide range, regulated by the level of expression of MHC molecules and the number of inhibitory receptors capable of interacting with these molecules. They propose that activating KIR would be another parameter of this equation, working against responsiveness. However, several issues remain to be addressed. In particular, when, where, and under which conditions does education occur? How do NK cells integrate the various educating stimuli? And perhaps most importantly, how can the same ligand/receptor pair (eg, KIR-L/MHC) increase NK-cell responsiveness in an educating context and inhibit NK-cell activation upon interaction with a target cell? Exploration of signaling complexes downstream activating and inhibitory receptors in educating or triggering conditions may provide clues to understand this complex phenomenon.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■