Abstract
Polycythemia vera (PV) and essential thrombocythemia (ET) are chronic myeloproliferative disorders characterized by an increased incidence of thrombo-hemorrhagic complications. The acquired somatic Janus kinase 2 (JAK2) V617F mutation is present in the majority of PV and ET patients. Because aberrant protein Tyr-phosphorylation has been associated with hematopoietic malignancies, the activity of the tyrosine kinases Src and JAK2 was analyzed in resting and thrombin-stimulated platelets from 13 PV and 42 ET patients. JAK2 was found inactive in healthy and pathological resting cells regardless of the V617F mutation. In addition, Src was inactive in all resting platelets, but in the pathological specimens it was present in a preactivated conformation as a consequence of anomalous dephosphorylation of its inhibitory phospho-Tyr527 residue, likely mediated by Src homology-2 domain-containing protein Tyr-phosphatase-2 (SHP-2), whose constitutive activity correlated with its recruitment to Src. Low thrombin concentration triggered a more rapid Src-signaling activation, higher [Ca2+]c increase, and aggregation in pathological platelets compared with controls. Thrombin-induced Src activation preceded JAK2 activation, which occurred simultaneously in normal and pathological platelets. Our results indicate that a constitutive Src kinase preactivation is implicated in platelet hypersensitivity and likely involved, at least partially, in the functional abnormalities of PV and ET platelets.
Introduction
Philadelphia-negative myeloproliferative diseases (Ph-MPDs) are clonal hematopoietic disorders characterized by overproduction of mature myeloid cells, increased risk of thrombotic and/or hemorrhagic complications, and possible evolution in myelofibrosis (MF) and/or acute leukemia. Whereas essential thrombocythemia (ET) is characterized mainly by an elevated platelet count, polycythemia vera (PV) has a prevalent increase of red cell line often associated with high granulocyte number. The presence of a somatic, acquired mutation (V617F) in the Janus kinase 2 (JAK2) kinase autoinhibitory domain1-5 is considered, at present, as a biologic specific marker for Ph-MPDs, playing a major role in their pathogenesis. In fact it is present in almost all the patients with PV and in approximately half of those with ET and MF.2 Expression of JAK2V617F has been suggested to induce kinase activation in hematopoietic cell lines, and homozygous JAK2 mutation is associated with pronounced trilinear megakaryocytic, erythroid, and granulocytic myeloproliferation.1,3,5 JAK2V617F-positive patients display clinical and laboratory findings different from wild-type patients, including higher white blood cell (WBC) count, hemoglobin concentration, and hematocrit, and lower platelet count.6 ET and PV JAK2V617F-positive patients exhibit a higher risk of cardiovascular events and a more frequent evolution into secondary MF.7,8 However, no clear dose-dependent correlation was found in ET between the JAK2V617F allele burden and clinical symptoms.9 As a rule, both PV and ET mutated subjects have a prognosis worse than wild-type patients.8,10
The relevance of platelets in the occurrence of thrombotic complications in Ph-MPD patients has been widely analyzed and several platelet functional abnormalities have been described.11
Platelet stimulation is accompanied by a dramatic increase in protein tyrosine phosphorylation highlighting an important role for tyrosine kinases in the platelet physiology. Src is a nonreceptor tyrosine kinase particularly abundant in platelets,12 where it plays a pivotal role triggering essential signal transduction pathways including phospholipase C γ2 activation, intracellular Ca2+ movements, and cytoskeleton reorganization.12-14 In thrombin-stimulated platelets Src kinase is required for aggregation.15 The Src activity is modulated by the phosphorylation of 2 tyrosine residues of the kinase: the C-terminal Tyr527, once phosphorylated by Csk, interacts with the Src homology 2 domain of the kinase itself triggering a reorganization of the Src structure that adopts a locked-inactive conformation. Src activation therefore may be achieved by the dephosphorylation of the phospho-Tyr527 by specific tyrosine phosphatase(s), which induce(s) the kinase “opening” and autophosphorylation of the activation loop residue Tyr416, which elicits the enzyme hyperactivation.16 Src kinase is “silent” in resting platelets and its catalytic activity is turned on in response to specific stimuli.
Because it has been shown that aberrant activation of tyrosine kinases plays a crucial role in neoplastic processes and the Src kinase family is involved in hematologic malignancies,17 we analyzed the Tyr phosphorylation signaling mediated by Src and JAK2 tyrosine kinases in resting and thrombin-stimulated platelets of PV and ET patients.
Methods
Materials
[γ32P]adenosine triphosphate (ATP) was purchased from Amersham Pharmacia Biotech. PP2, SU6656, AG490, JAK2-inhibitor-I, calpeptin, and protease inhibitor-cocktail were from Calbiochem and extracellular signal-related kinase1/2 (ERK1/2) was from Cell Signaling. Peptide cdc2(6-20) was kindly provided by Dr Oriano Marin (Padova University). Human alpha-thrombin, phosphatase inhibitor-cocktail, poly(Glu4Tyr), and beta-actin were from Sigma, and human recombinant thrombopoietin was from R&D Systems. Antibodies raised against JAK2, phospho-JAK2, Src-phospho-Tyr416, Src-phospho-Tyr527, Lyn-phospho-Tyr507, signal transducer and activator of transcription 5 (STAT5), and phospho-STAT5 were from Upstate, whereas anti-Src, anti-Fyn, anti-Lyn, anti–Src homology-2 domain-containing Tyr-phosphatase-1 (SHP-1), anti–SHP-2, and anticortactin antibodies were from Santa Cruz Biotechnology. Anti–phospho-tyrosine (PY-20) monoclonal antibody was from ICN Biotechnology.
Healthy donors and patients
This study was approved by the Institutional Ethics Committee of University of Padova and the procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Peripheral blood was withdrawn from 13 PV and 42 ET patients with an increased platelet count more than 450 × 109/L diagnosed in agreement with the World Health Organization criteria,18 from 3 acute myeloid leukemia (AML) and 5 myelodysplastic syndrome (MDS) patients, and from 49 healthy donors with their informed consent. Patient characterization is reported in Tables 1 and 2.
Molecular biology
Granulocytes and platelets were isolated as elsewhere described.19 DNA was extracted from granulocytes with common methods, and RNA, from platelets using TRIZOL (Invitrogen) reagent according to the manufacturer's protocol.
For the detection of JAK2V617F in peripheral blood granulocyte DNA and in platelet RNA the sequence-analysis and allele-specific polymerase chain reactions were used.2 The mutant allele burden was measured in granulocyte DNA by quantitative real-time polymerase chain reaction assay.20 To research JAK2 exon 12 mutations, sequence analysis was used in wild-type JAK2 PV patients.21
The clonality pattern of females was determined by the activation state of the X-chromosome.19
Platelet isolation and treatment
Platelet-rich plasma and washed platelets were prepared from fresh blood.22 Briefly, blood samples were immediately mixed with one-sixth volume of acid citrate-dextrose anticoagulant (85mM sodium citrate, 71mM citric acid, 111mM dextrose, pH 6.5) containing 1 μg/mL prostacyclin, 50 mU/mL hirudin, and 20 μg/mL apyrase. Unless otherwise specified, platelets were resuspended in basal buffer (140mM NaCl, 5mM KCl, 1mM MgCl2, 10mM glucose, and 15mM Tris [tris(hydroxymethyl)aminomethane]/N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4). Aspirin treatment was performed by adding 0.2mM sodium acetylsalicylate (freshly prepared in 0.1M NaHCO3) to platelet-rich plasma and incubating the suspension for 15 minutes at 37°C. Platelets were stimulated at 37°C with alpha-thrombin or thrombopoietin in the presence of 0.5mM CaCl2. Tyrosine kinase inhibitors PP2, SU6656, AG490, and JAK2-inhibitor-I were added to platelet suspensions 10 minutes before stimulation. Tyrosine-phosphatase inhibitors pervanadate (prepared by mixing equimolar solutions of hydrogen peroxide and sodium orthovanadate to final 0.5-mM concentration) and calpeptin were preincubated in platelet suspensions for 5 minutes.
Western blot analysis of cellular lysates
Platelets were lysed by suspension (1 hour at 4°C) in lysis buffer (62mM Tris/HCl, pH 6.8, containing 5% glycerol, 0.5% sodium dodecyl sulfate (SDS), and 0.5% β-mercaptoethanol). Lysates (10 μg protein) were subjected to SDS/polyacrylamide gel electrophoresis (PAGE; 10%), transferred to nitrocellulose membranes, incubated with the indicated antibodies, followed by the appropriate biotinylated second antibodies, and developed by enhanced chemiluminescent detection system (Amersham Pharmacia Biotech).
Immunoprecipitation experiments
Platelets (2 × 108/assay) were lysed by suspension (1 hour at 4°C) in 450 μL of immunoprecipitation (IP) buffer (20mM Tris/HCl, pH 7.5, 10% glycerol, 1% Nonidet P-40, 1mM ethylenediaminetetraacetic acid, 50mM NaCl, 1mM sodium orthovanadate, phosphatase, and protease inhibitor-cocktails). After centrifugation (14 000g for 30 minutes), the supernatants were incubated for 5 hours at 4°C with the appropriate antibody bound to protein A-Sepharose. The immunocomplexes were washed 3 times with 50mM Tris/HCl, pH 7.5, containing protease inhibitor-cocktail and 1mM sodium orthovanadate.
In vitro tyrosine kinase assays
Src and JAK2 were immunoprecipitated as described in “Immunoprecipitation experiments” and in vitro kinase activities of the immunocomplexes were determined in 50 μL of phosphorylation medium containing 50mM Tris/HCl, pH 7.5, 5mM MnCl2, 5mM MgCl2, 30μM [γ33P]ATP (specific activity, 1000 dpm/pmol), 200μM sodium orthovanadate, and either 200μM cdc2(6-20) peptide (Src activity)23 or 1 mg/mL polyGlu4Tyr (JAK2 activity) as substrates. After 10-minute incubation at 30°C, the reactions were stopped and samples were subjected to SDS/PAGE. Substrate 33P phosphorylation was quantified by a Packard Cyclone.
Determination of cytosolic [Ca2+] concentration
[Ca2+]c was determined as previously reported.24 Briefly, platelets were loaded with the fluorescent probe fura 2 acetoxy-methyl-ester, and resuspended at a concentration of 2 × 108 cells/mL in basal buffer supplemented with 0.5mM Ca2+. Fluorescence changes were measured at 37°C in a thermostated, magnetically stirred cuvette using excitation and emission wavelengths of 340 nm and 505 nm, respectively.
Platelet aggregation
Platelet aggregation was monitored with an Elvi Logos aggregometer as previously described.24
Subcellular fractionation
Platelets (4 × 108) were mildly sonicated in 1 mL of isotonic buffer (50mM Tris/HCl, pH 7.5, 250mM saccharose, 1mM orthovanadate, and protease inhibitor-cocktail). Cell debris was pelleted by centrifugation at 1000g for 10 minutes. The supernatant was further centrifuged at 100 000g for 1 hour to separate the cytosol from microsomes. Microsomes were resuspended in 1 mL of the isotonic buffer used for sonication.
[33P]-phospho band 3 preparation
Band 3 was phosphorylated by incubating erythrocyte ghosts (15 μg) at 30°C with p36syk and Lyn tyrosine kinases in the presence of [γ33P]ATP as described.25 After 10-minute incubation the sample was centrifuged at 14 000g and the pellet was washed 3 times with buffer B (25mM imidazole, pH 7, 1mM ethylenediaminetetraacetic acid, 0.02% NaN3, 10% glycerol, 10mM beta-mercaptoethanol, 10 mg/mL leupeptin, 50mM phenylmethylsulphonyl fluoride) and then solved in buffer B.
Tyrosine phosphatase assay
Platelets (106/assay) were lysed in IP-buffer containing 0.1% Nonidet P-40. Tyrosine phosphatase activity was tested by adding 25 μL of lysates to 25 μL of buffer B and 0.3 μg of 33P-phospho band 3. After 1-minute incubation at 30°C, the assays were stopped and subjected to SDS/PAGE. The extent of 33P-phospho band 3 dephosphorylation was evaluated either by analysis on a Packard Cyclone or by autoradiography and counting the excised 33P-band 3 in a scintillation counter.
Statistical analysis
Data are presented as mean (± SD) and compared using 1-way analysis of variance followed by Bonferroni post hoc test. A P value less than .05 was considered statistically significant. All statistics were performed using GraphPad Prism statistical software.
Results
Clinical correlations
In this study 13 PV and 42 ET patients were analyzed. The occurrence of major thrombotic events in these patients did not correlate with the JAK2 mutation status: 58% of mutated PV patients, 25% of mutated ET, and 33% of wild-type ET developed thrombotic complications (Table 1). No correlation was found between major thrombosis and clonality patterns.
Molecular patterns
All but 1 PV patient carried JAK2 V617F mutation (mean allele burden 69% ± 26%); 10 patients had an allele burden more than 50%, 6 of them more than 80%, including 3 more than 90%. In the 26 ET patients carrying JAK2 V617F, the mean allele burden was 37% (± 19%), and in 5 patients the allele burden was between 50% and 80% (Table 2).
JAK2 V617F mutation was also analyzed in the platelet RNA of 9 patients, confirming the results obtained in granulocytes.
Clonality pattern was available in 4 PV (2 monoclonal and 2 skewing) and 18 ET (6 monoclonal, 8 polyclonal, and 4 skewing) females.
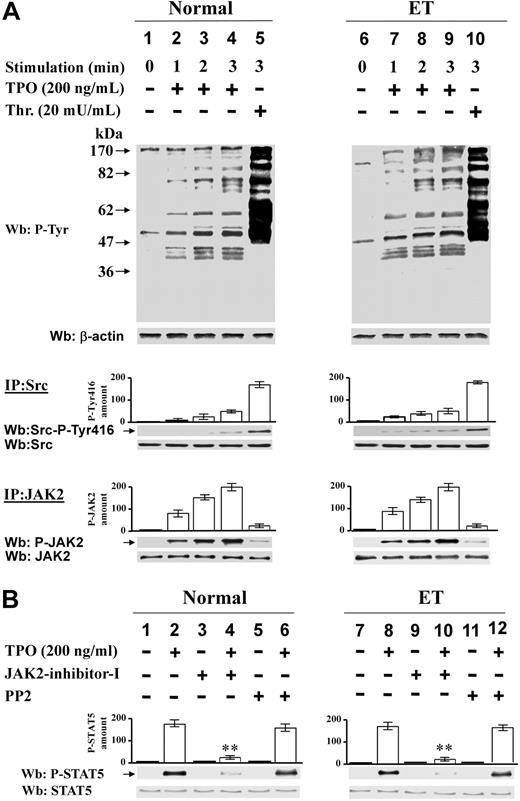
Alpha-thrombin triggers Src and JAK2 activation in platelets of Ph-MPD patients
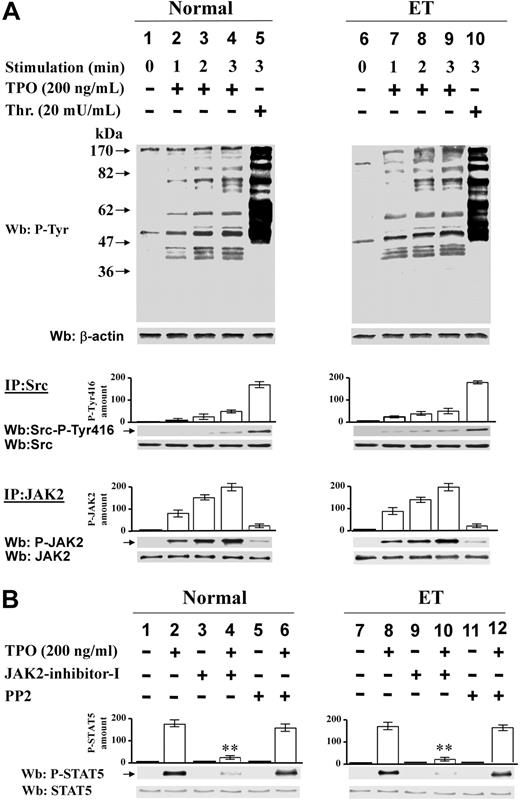
Platelets, freshly prepared from healthy donors or patients with PV or ET, were generally stimulated with thrombin, which was preferred to thrombopoietin because it triggers an intact protein Tyr phosphorylation in PV platelets, whereas the thrombopoietin receptor cMpl was reported to be absent or abnormally expressed in Ph-MPD platelets.26-28 The immunostaining of cellular lysates with anti–P-Tyr antibody shows that the basal protein Tyr phosphorylation is rather weak in both healthy and pathological platelets (Figure 1 lane 1). Cellular stimulation with thrombin (100 mU/mL) induces a rapid increase of Tyr phosphorylation of various proteins in both control and Ph-MPD platelets (Figure 1 lanes 2-3). In the presence of the JAK2 inhibitor AG49029 a general reduction of the thrombin-elicited Tyr phosphorylation is observed in normal, PV, and ET samples, regardless of the presence of wild-type or V617F-mutated JAK2 (Figure 1 lanes 5-6). The Src-specific inhibitor PP230 counteracts the thrombin-induced Tyr phosphorylation more efficiently than AG490 in both normal and pathological platelets (Figure 1 lanes 8-9).
Analysis of protein Tyr phosphorylation in resting and thrombin-challenged platelets isolated from blood of healthy donors or Ph-MPD patients. Platelets were obtained from healthy donors, and PV or ET patients as detailed in “Platelet isolation and treatment.” Cells were stimulated with 100 mU/mL thrombin (Thr) for the indicated times in the presence of vehicle (lanes 1-3), or 10μM AG490 (lanes 4-6), or 10μM PP2 (lanes 7-9). Platelets were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin (loading control) antibodies as described in “Western blot analysis of cellular lysates.” The figure is representative of experiments performed with 20 normal, 11 PV, and 30 ET samples.
Analysis of protein Tyr phosphorylation in resting and thrombin-challenged platelets isolated from blood of healthy donors or Ph-MPD patients. Platelets were obtained from healthy donors, and PV or ET patients as detailed in “Platelet isolation and treatment.” Cells were stimulated with 100 mU/mL thrombin (Thr) for the indicated times in the presence of vehicle (lanes 1-3), or 10μM AG490 (lanes 4-6), or 10μM PP2 (lanes 7-9). Platelets were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin (loading control) antibodies as described in “Western blot analysis of cellular lysates.” The figure is representative of experiments performed with 20 normal, 11 PV, and 30 ET samples.
Resting platelets of Ph-MPD patients contain inactive JAK2 and “potentially active” Src kinase
To throw some light on the Tyr-phosphorylative events triggered by thrombin in ET and PV platelets, the activation state of JAK2 and Src tyrosine kinases was analyzed in resting and stimulated cells.
The Western blot analysis of JAK2 expression in cellular lysates demonstrated that JAK2 protein level is similar in normal and pathological platelets (data not shown). To compare the specific activity displayed by wild-type and mutated JAK2, the kinase was immunoprecipitated from (1) normal platelets containing wild-type JAK2, (2) PV platelets homozygous for JAK2V617F, and (3) ET platelets heterozygous for JAK2V617F. The in vitro kinase activity of JAK2 immunocomplexes (IP) was tested toward the substrate poly(Glu/Tyr). Figure 2A shows that similar amounts of wild-type JAK2, or heterozygous and homozygous JAK2V617F, display similar kinase activity, indicating that the V617F mutation does not affect the in vitro enzyme catalytic activity. The inhibition of the phosphorylative activity observed in the presence of the JAK2 inhibitor AG490 and JAK2-inhibitor-I supports the conclusion that the detected activity is due to JAK2 and not to other coimmunoprecipitated tyrosine kinase(s).
Analysis of JAK2 activation state in resting and thrombin-stimulated platelets. (A) Tyrosine kinase activity of wild-type or V617F-mutated JAK2. Platelets obtained from healthy donors (lanes 1-3), PV patients homozygous for JAK2V617F (lanes 4-6), and ET patients heterozygous for JAK2V617F (lanes 7-9) were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody. The immunocomplexes (IPs) were then analyzed for JAK2 activity tested in vitro toward the substrate polyGlu4Tyr in the presence of [γ33P]ATP and vehicle (lanes 1,4,7), or 10μM AG490 (lanes 2,5,8), or 1μM JAK2-inhibitor-I (lanes 3,6,9) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and transferred to nitrocellulose sheets, which were analyzed for the radioactivity incorporated in polyGlu4Tyr by a Packard Cyclone (columns of the panel) and then immunostained with anti-JAK2 antibody (Wb of the panel). Experiments with AG490 and PP2 were performed with 8 normal, 5 JAK2V617F homozygous PV, and 7 JAK2V617F heterozygous ET samples. Experiments with AG490, JAK-inhibitor-I, and PP2 were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. **P < .001 versus JAK2 activity tested in the absence of inhibitors. (B-C) Platelets, isolated from healthy donors, and PV or ET patients, were treated without (odd lanes) or with 100 mU/mL thrombin (even lanes) for 1 minute in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 1μM JAK2-inhibitor-I (lanes 5-6), or 10μM PP2 (lanes 7-8). (B) Platelet lysates were analyzed by Western blot with an anti–phospho-JAK2 (P-JAK2) antibody that recognizes the activated form of the kinase. Blots were then stripped and reprobed with anti-JAK2 antibody. (C) Platelet lysates were analyzed by Western blot with anti–phospho-STAT5 (P-STAT5) antibody and reprobed with anti-STAT5 antibody. Means of densitometric values are reported above the bands relative to P-JAK2 (B) or P-STAT5 (C). Experiments in panels B and C were performed with 12 normal, 8 PV (JAK2V617F homozygous and heterozygous), and 15 ET samples. Experiments with JAK-inhibitor-I were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. (B) **P < .001 versus P-JAK2 in the absence of JAK2 inhibitors. (C) ** P < .001 and *P < .01 versus P-STAT5 in the absence of inhibitors. #P < .001 PV and ET versus normal platelets.
Analysis of JAK2 activation state in resting and thrombin-stimulated platelets. (A) Tyrosine kinase activity of wild-type or V617F-mutated JAK2. Platelets obtained from healthy donors (lanes 1-3), PV patients homozygous for JAK2V617F (lanes 4-6), and ET patients heterozygous for JAK2V617F (lanes 7-9) were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody. The immunocomplexes (IPs) were then analyzed for JAK2 activity tested in vitro toward the substrate polyGlu4Tyr in the presence of [γ33P]ATP and vehicle (lanes 1,4,7), or 10μM AG490 (lanes 2,5,8), or 1μM JAK2-inhibitor-I (lanes 3,6,9) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and transferred to nitrocellulose sheets, which were analyzed for the radioactivity incorporated in polyGlu4Tyr by a Packard Cyclone (columns of the panel) and then immunostained with anti-JAK2 antibody (Wb of the panel). Experiments with AG490 and PP2 were performed with 8 normal, 5 JAK2V617F homozygous PV, and 7 JAK2V617F heterozygous ET samples. Experiments with AG490, JAK-inhibitor-I, and PP2 were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. **P < .001 versus JAK2 activity tested in the absence of inhibitors. (B-C) Platelets, isolated from healthy donors, and PV or ET patients, were treated without (odd lanes) or with 100 mU/mL thrombin (even lanes) for 1 minute in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 1μM JAK2-inhibitor-I (lanes 5-6), or 10μM PP2 (lanes 7-8). (B) Platelet lysates were analyzed by Western blot with an anti–phospho-JAK2 (P-JAK2) antibody that recognizes the activated form of the kinase. Blots were then stripped and reprobed with anti-JAK2 antibody. (C) Platelet lysates were analyzed by Western blot with anti–phospho-STAT5 (P-STAT5) antibody and reprobed with anti-STAT5 antibody. Means of densitometric values are reported above the bands relative to P-JAK2 (B) or P-STAT5 (C). Experiments in panels B and C were performed with 12 normal, 8 PV (JAK2V617F homozygous and heterozygous), and 15 ET samples. Experiments with JAK-inhibitor-I were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. (B) **P < .001 versus P-JAK2 in the absence of JAK2 inhibitors. (C) ** P < .001 and *P < .01 versus P-STAT5 in the absence of inhibitors. #P < .001 PV and ET versus normal platelets.
The activation state of JAK2 was then analyzed in platelet lysates by Western blot with an anti–phospho-JAK2 antibody, which recognizes the activated enzyme form. Phospho-JAK2 was undetectable in resting platelets of healthy, PV, and ET subjects, irrespective of the presence of JAK2 mutation (Figure 2B lane 1). Thrombin stimulation induces JAK2 phosphorylation in all samples (Figure 2B lane 2). As expected, cell preincubation with AG490 or JAK2-inhibitor-I abrogates JAK2 activation (Figure 2B lanes 2,4,6), whereas PP2 does not significantly affect the kinase phosphorylation (Figure 2B lanes 2,8).
To confirm that both wild-type and mutated JAK2 are not constitutively active in resting Ph-MPD platelets, the presence of phosphorylated STAT5, a major JAK2-signaling target, was analyzed by Western blot with an antibody that recognizes the STAT5-P-Tyr694. STAT5 phosphorylation is undetectable in normal and pathological platelets (JAK2V617F negative or positive) at baseline and becomes evident after stimulation with thrombin (Figure 2C lanes 1-2).
Because an alternative, Src-family–dependent and JAK2-independent pathway of STAT5 phosphorylation has been described,31 we investigated the individual contribution of JAK2 and Src kinase on STAT5 phosphorylation by preincubating the platelets with JAK2 inhibitors (AG490, JAK2-inhibitor-I) or Src inhibitors (PP2, SU6656). Whereas AG490 and JAK2-inhibitor-I greatly reduce (80% and 75%, respectively) the thrombin-induced STAT5 phosphorylation in normal samples, they inhibit this phosphorylation less effectively in Ph-MPD platelets (less than 27%; Figure 2C lanes 2,4,6). At variance, the treatment with PP2 causes an inhibition of only approximately 26% of STAT5 phosphorylation in normal platelets, whereas it strikingly reduces the phosphorylation of this protein in the pathological samples (Figure 2C lanes 2,8). The ineffectiveness of PP2 on JAK2 phosphorylation (Figure 2B lanes 2,8) rules out the hypothesis of a nonspecific PP2 inhibition of JAK2 kinase activity. Similar results were obtained with the Src inhibitor SU6656 (not shown).
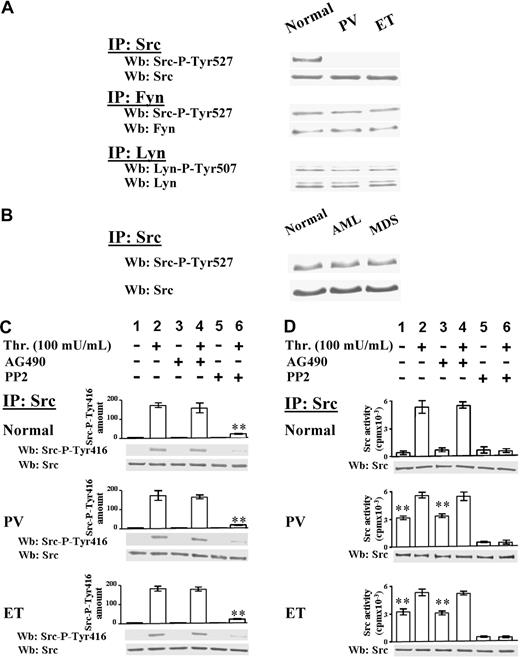
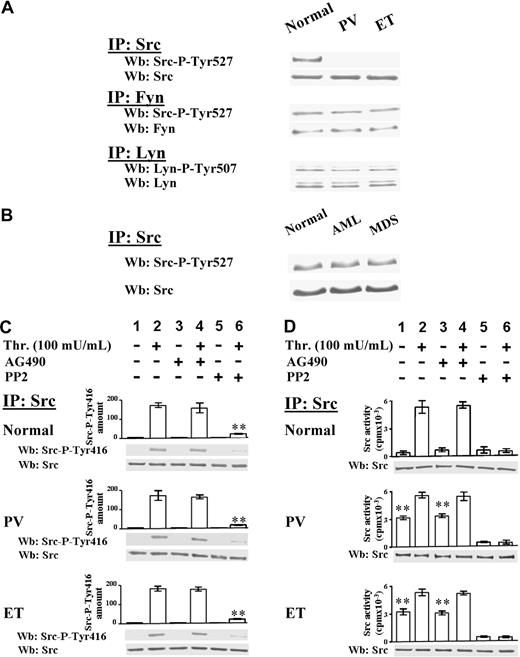
The activation state of 3 kinases of the Src family, namely Src, which is the most expressed member in platelets, Fyn, and Lyn, was then analyzed. The Src family kinases, which were expressed at similar protein level in normal and Ph-MPD platelets (data not shown), were immunoprecipitated from cellular lysates and immunostained with phospho-specific antibodies recognizing their C-terminal phospho-tyrosine (anti–Src-P-Tyr527 for both Src and Fyn, and anti–Lyn-P-Tyr507 for Lyn). In normal platelets all Src kinases cross-react with their phospho-specific antibodies (Figure 3A), consistent with the findings that, in resting cells, Src kinases are present in their “inactive” conformation due to the intramolecular interaction of the C-terminal phospho-tyrosine with their Src homology 2 domain.16 The phospho-specific antibodies interact also with Fyn and Lyn immunoprecipitated from PV and ET platelets, but unexpectedly not with the anti–Src-IP, indicating that Src-Tyr527 is not phosphorylated in the pathological platelets (Figure 3A). Interestingly, in platelets obtained from patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDSs) Src is normally phosphorylated at Tyr527 (Figure 3B).
Analysis of Src activation state in resting and thrombin-stimulated platelets. (A) Platelets, isolated from healthy donors or PV and ET patients, were lysed in IP-buffer and immunoprecipitated with anti-Src, anti-Fyn, or anti-Lyn antibodies. Src-IPs and Fyn-IPs were immunostained with anti–Src-P-Tyr527 antibody, which recognizes the phosphorylated C-terminal sequence of both kinases, and then reprobed with anti-Src or anti-Fyn antibody, respectively. Lyn-IPs were immunostained with anti–Lyn-P-Tyr507 antibody and reprobed with anti-Lyn antibody. The Western blots of Src-IPs are representative of experiments performed with 14 normal samples, 10 PV (JAK2V617F either homozygous or heterozygous), and 18 ET JAK2V617F negative and positive; the Western blots of Fyn-IPs and Lyn-IPs are representative of experiments performed with 4 normal and 4 ET samples. (B) Src kinase was immunoprecipitated from platelet lysates of 5 healthy donors, or 3 AML and 5 MDS patients. Src-IPs were immunostained with anti–Src-P-Tyr527 antibody and then reprobed with anti-Src antibody. (C-D) Platelets, isolated from healthy donors, and PV or ET patients, were incubated with vehicle (lanes 1,3,5) or 100 mU/mL thrombin (lanes 2,4,6) for 1 minute, in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 10μM PP2 (lanes 5-6). Platelets were then lysed in IP-buffer and immunoprecipitated with anti-Src antibody. (C) Src-IPs were analyzed by Western blot with an antibody raised against the Src sequence containing the phospho-Tyr416. Means of densitometric values are reported above the Src-P-Tyr416 bands. Blots were then reprobed with anti-Src antibody. **P < .001 versus Src-P-Tyr416 present in platelets stimulated in the absence of inhibitors. (D) Src-IPs were analyzed for in vitro kinase activity toward the Src-specific peptide substrate cdc2(6-20) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and the gels were analyzed for the radioactivity incorporated in the peptide by a Packard Cyclone (columns of the panel). The gels were then blotted and immunostained with anti-Src antibody. Panels C and D are representative of experiments performed with 12 normal samples, 10 PV (JAK2V617F either homozygous or heterozygous), 18 ET JAK2V617F negative or positive. **P < .001 versus resting normal platelets.
Analysis of Src activation state in resting and thrombin-stimulated platelets. (A) Platelets, isolated from healthy donors or PV and ET patients, were lysed in IP-buffer and immunoprecipitated with anti-Src, anti-Fyn, or anti-Lyn antibodies. Src-IPs and Fyn-IPs were immunostained with anti–Src-P-Tyr527 antibody, which recognizes the phosphorylated C-terminal sequence of both kinases, and then reprobed with anti-Src or anti-Fyn antibody, respectively. Lyn-IPs were immunostained with anti–Lyn-P-Tyr507 antibody and reprobed with anti-Lyn antibody. The Western blots of Src-IPs are representative of experiments performed with 14 normal samples, 10 PV (JAK2V617F either homozygous or heterozygous), and 18 ET JAK2V617F negative and positive; the Western blots of Fyn-IPs and Lyn-IPs are representative of experiments performed with 4 normal and 4 ET samples. (B) Src kinase was immunoprecipitated from platelet lysates of 5 healthy donors, or 3 AML and 5 MDS patients. Src-IPs were immunostained with anti–Src-P-Tyr527 antibody and then reprobed with anti-Src antibody. (C-D) Platelets, isolated from healthy donors, and PV or ET patients, were incubated with vehicle (lanes 1,3,5) or 100 mU/mL thrombin (lanes 2,4,6) for 1 minute, in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 10μM PP2 (lanes 5-6). Platelets were then lysed in IP-buffer and immunoprecipitated with anti-Src antibody. (C) Src-IPs were analyzed by Western blot with an antibody raised against the Src sequence containing the phospho-Tyr416. Means of densitometric values are reported above the Src-P-Tyr416 bands. Blots were then reprobed with anti-Src antibody. **P < .001 versus Src-P-Tyr416 present in platelets stimulated in the absence of inhibitors. (D) Src-IPs were analyzed for in vitro kinase activity toward the Src-specific peptide substrate cdc2(6-20) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and the gels were analyzed for the radioactivity incorporated in the peptide by a Packard Cyclone (columns of the panel). The gels were then blotted and immunostained with anti-Src antibody. Panels C and D are representative of experiments performed with 12 normal samples, 10 PV (JAK2V617F either homozygous or heterozygous), 18 ET JAK2V617F negative or positive. **P < .001 versus resting normal platelets.
The Src autophosphorylation at Tyr416, which leads Src to assume the “hyperactive” conformation,16 was then analyzed. Although anti–Src-IPs do not interact with the anti–Src-P-Tyr416 antibody in resting normal and pathological platelets (Figure 3C lane 1), thrombin stimulation causes the Src-Tyr416 phosphorylation in all samples, a process unaffected by AG490, although almost abolished by PP2 (Figure 3C lanes 2,4,6).
The in vitro kinase activity of Src immunoprecipitated from normal and Ph-MPD platelets was then examined. Consistent with the P-Tyr527–dependent “locked” Src conformation (Figure 3A), the enzyme from resting normal platelets displays low kinase activity, whereas the Src kinase from pathological platelets exhibits a substantial in vitro activity (Figure 3D column 1), consistent with the potentially active Src conformation suggested by the kinase dephosphorylation at both the inhibitory Tyr527 and the activatory Tyr416 (Figure 3A,C). The Src kinase activity strikingly increases (> 12-fold) in thrombin-challenged normal samples, whereas it increases only about 2-fold in stimulated Ph-MPD platelets (Figure 3D columns 1-2). As a consequence, the agonist-induced Src activity reaches almost similar levels in normal and pathological platelets (Figure 3D column 2). The finding that AG490 pretreatment of platelets affects the extent of neither Src autophosphorylation (Figure 3C lanes 2,4) nor Src kinase activity (Figure 3D columns 2,4) demonstrates that the thrombin-triggered Src activation is independent of JAK2 activity.
Ph-MPD platelets are hypersensitive to low thrombin concentration
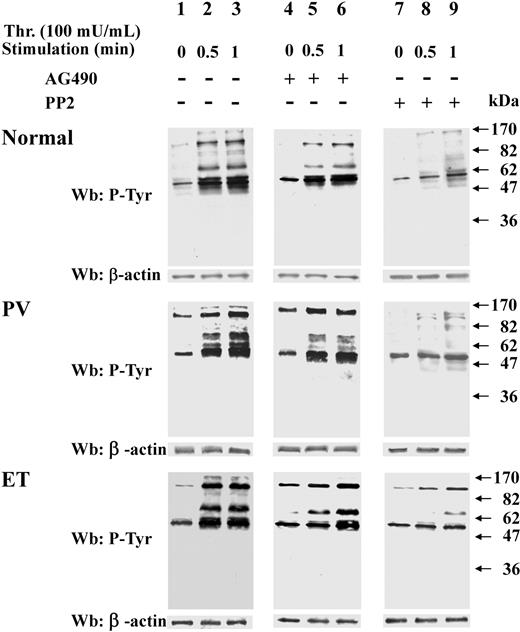
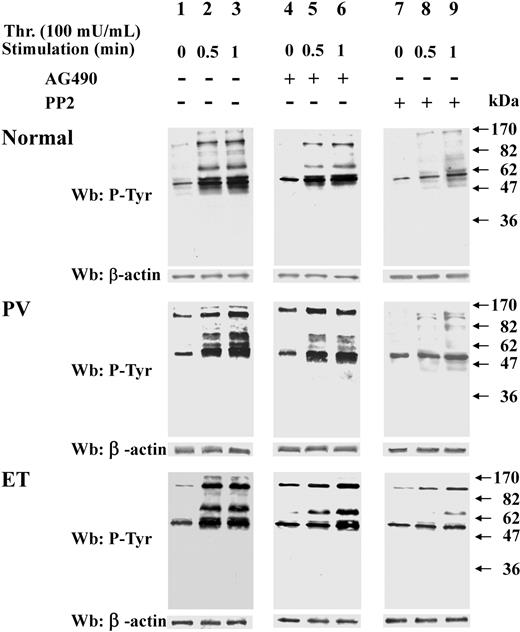
Because resting Ph-MPD platelets contain potentially active Src kinase, their response to relatively weak stimuli was analyzed. To this end, platelets were challenged with low thrombin concentration (20 mU/mL), which elicits a more rapid protein Tyr phosphorylation in Ph-MPD than in normal platelets (Figure 4A). In fact, in control cells the Tyr phosphorylation of some proteins increases gradually during the tested period (2 minutes) after thrombin addition, whereas in both PV and ET samples several Tyr-phosphorylated bands are highly evident already after 30-second stimulation. The different Tyr phosphorylation timing tends to diminish by increasing the stimulus concentration, as shown in Figure 4B, where 100 mU/mL thrombin was used as stimulating agent.
Analysis of protein Tyr phosphorylation, cytosolic [Ca2+] increase, and aggregation triggered by low thrombin concentration in normal and pathological platelets. (A-B) Platelets from healthy donors (lanes 1-4), and PV (lanes 5-8) or ET patients (lanes 9-12), were stimulated with either 20 mU/mL (A) or 100 mU/mL (B) of thrombin for the indicated times. Cells were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin antibodies. (C) [Ca2+]c increase was determined as detailed in “Determination in cytosolic [Ca2+] concentration” in platelets challenged with either 20 mU/mL or 100 mU/mL thrombin. The traces are representative of experiments performed with 10 normal (N), 7 PV, and 10 ET JAK2V617F-negative or -positive samples. (D) Aggregation was determined as detailed in “Platelet aggregation” in platelets pretreated with either vehicle (V) or PP2 and then challenged with 20 mU/mL thrombin. The traces are representative of experiments performed with 4 normal (N) and 4 ET samples.
Analysis of protein Tyr phosphorylation, cytosolic [Ca2+] increase, and aggregation triggered by low thrombin concentration in normal and pathological platelets. (A-B) Platelets from healthy donors (lanes 1-4), and PV (lanes 5-8) or ET patients (lanes 9-12), were stimulated with either 20 mU/mL (A) or 100 mU/mL (B) of thrombin for the indicated times. Cells were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin antibodies. (C) [Ca2+]c increase was determined as detailed in “Determination in cytosolic [Ca2+] concentration” in platelets challenged with either 20 mU/mL or 100 mU/mL thrombin. The traces are representative of experiments performed with 10 normal (N), 7 PV, and 10 ET JAK2V617F-negative or -positive samples. (D) Aggregation was determined as detailed in “Platelet aggregation” in platelets pretreated with either vehicle (V) or PP2 and then challenged with 20 mU/mL thrombin. The traces are representative of experiments performed with 4 normal (N) and 4 ET samples.
The intracellular Ca2+ mobilization represents an upstream event in the platelet activation cascade, therefore the cytosolic [Ca2+]c increase was analyzed in normal and pathological platelets challenged with different thrombin concentration. Interestingly, low thrombin concentration (20 mU/mL) induces a [Ca2+]c rise in PV and ET platelets about 2-fold higher than in normal samples (Figure 4C). At variance, the [Ca2+]c rise elicited by 100 mU/mL thrombin in ET and PV platelets is slightly lower than that of normal platelets (Figure 4C). Consistently, the aggregation, which is a late event of platelet activation, elicited by low-dose thrombin (20 mU/mL) is more rapid (∼ 72% higher initial rate) and extensive in ET compared with normal platelets (Figure 4D), confirming the hypersensitivity of the Ph-MPD samples. The involvement of Src preactivation in the latter process is supported by the finding that the PP2-exerted inhibition of platelet aggregation15 is higher in ET platelets compared with normal samples (∼ 65% and 40%, respectively; Figure 4D).
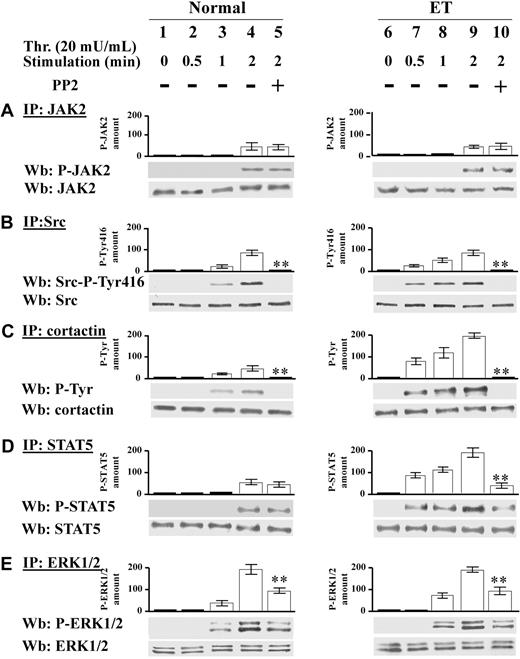
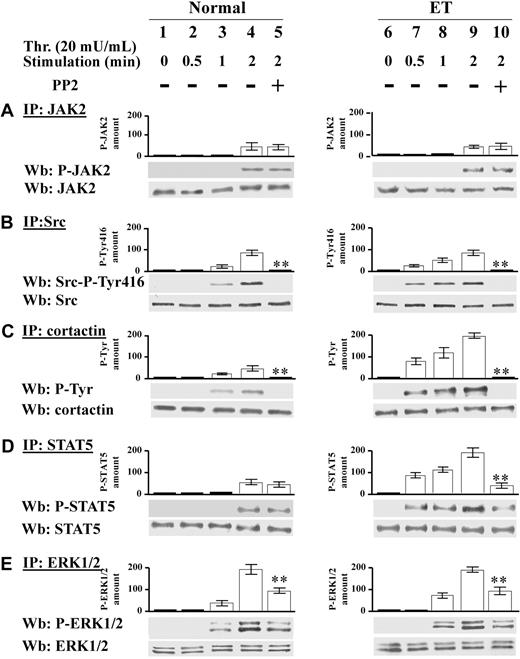
We have demonstrated in Figure 1 that the protein Tyr phosphorylation triggered by relatively high thrombin concentration (100 mU/mL) in Ph-MPD platelets is ascribable more to Src kinase than to JAK2 kinase (Figure 1). To determine the specific contribution of these tyrosine kinases at low agonist concentration, the time course of JAK2 and Src activation was analyzed in normal and ET platelets challenged with 20 mU/mL thrombin. In both normal and ET samples JAK2 activation becomes evident after 2 minutes of thrombin stimulation as judged by the phospho-JAK2 immunostaining (Figure 5A lanes 1-4, 6-9). At variance, the time course of Src activation differs between normal and pathological platelets. In control specimens, Src activation, revealed by Src-Tyr416 autophosphorylation and Tyr phosphorylation of the Src substrate cortactin, appears after 1-minute stimulation (Figure 5B-C lanes 1-4), whereas in pathological cells Src activation is already evident after 30 seconds of thrombin treatment (Figure 5C lanes 6-9). STAT5 phosphorylation is concomitant with JAK2 activation in normal cells, whereas, similarly to Src activation, it occurs earlier in Ph-MPD platelets (Figure 5D lanes 1-4, 6-9). Consistently, the Src inhibitor PP2 abrogates the phosphorylation of Src-Tyr416 and cortactin in both normal and pathological platelets (Figure 5B-C lanes 4-5, 9-10) and drastically decreases STAT5 phosphorylation only in ET cells (Figure 5D lanes 4-5, 9-10).
Time course of JAK2 and Src signaling triggered by 20 mU/mL thrombin. (A-E) Normal (1-5) or ET platelets (6-10) were challenged with 20 mU/mL thrombin for the indicated time in the presence of vehicle (lanes 1-4, 6-9), or 10μM PP2 (lanes 5,10). (A) Platelets were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody and the IPs were subjected to SDS/PAGE, blotted and immunostained with anti–P-JAK2 antibody, stripped, and reprobed with anti-JAK2 antibody. (B) Platelet lysates were immunoprecipitated with anti-Src antibody and the IPs were immunostained with the phospho-specific anti–Src-P-Tyr416 antibody, and reprobed with anti-Src antibody. (C) Platelet lysates were immunoprecipitated with anticortactin antibody and the IPs were immunostained with anti–P-Tyr antibody, and reprobed with anticortactin antibody. (D) IPs obtained by treatment with anti-STAT5 antibody were immunostained with anti–P-STAT5 antibody, and reprobed with anti-STAT5 antibody. (E) IPs obtained with anti-ERK1/2 antibody were immunostained with anti–P-ERK1/2 antibodies, and reprobed with anti-ERK1/2 antibody. Means of densitometric values are reported above the relative phosphorylated bands. Panels A, C, and D are representative of experiments performed with 12 normal and 16 ET samples either JAK2V617F negative or positive, whereas panels B and E are representative of experiments performed with 6 normal and 6 ET samples either JAK2 V617F negative or positive. **P < .001 versus the relative protein Tyr phosphorylation detected after 2 minutes of thrombin stimulation in the absence of PP2.
Time course of JAK2 and Src signaling triggered by 20 mU/mL thrombin. (A-E) Normal (1-5) or ET platelets (6-10) were challenged with 20 mU/mL thrombin for the indicated time in the presence of vehicle (lanes 1-4, 6-9), or 10μM PP2 (lanes 5,10). (A) Platelets were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody and the IPs were subjected to SDS/PAGE, blotted and immunostained with anti–P-JAK2 antibody, stripped, and reprobed with anti-JAK2 antibody. (B) Platelet lysates were immunoprecipitated with anti-Src antibody and the IPs were immunostained with the phospho-specific anti–Src-P-Tyr416 antibody, and reprobed with anti-Src antibody. (C) Platelet lysates were immunoprecipitated with anticortactin antibody and the IPs were immunostained with anti–P-Tyr antibody, and reprobed with anticortactin antibody. (D) IPs obtained by treatment with anti-STAT5 antibody were immunostained with anti–P-STAT5 antibody, and reprobed with anti-STAT5 antibody. (E) IPs obtained with anti-ERK1/2 antibody were immunostained with anti–P-ERK1/2 antibodies, and reprobed with anti-ERK1/2 antibody. Means of densitometric values are reported above the relative phosphorylated bands. Panels A, C, and D are representative of experiments performed with 12 normal and 16 ET samples either JAK2V617F negative or positive, whereas panels B and E are representative of experiments performed with 6 normal and 6 ET samples either JAK2 V617F negative or positive. **P < .001 versus the relative protein Tyr phosphorylation detected after 2 minutes of thrombin stimulation in the absence of PP2.
ERK1/2 phosphorylation state was then analyzed because constitutive ERK activation has been associated with JAK2 mutation in PV erythroid precursor cells.32 In parallel with the lack of basal JAK2 activation, ERK1/2 phosphorylation is undetectable in resting normal and ET platelets (containing either wild-type JAK2 or JAK2V617F; Figure 5E lanes 1,6). Thrombin-elicited ERK1/2 phosphorylation precedes JAK2 activation and is approximately 50% dependent on Src activity in both control and pathological specimens as judged by the PP2 inhibitory effect (Figure 5E lanes 4-5, 9-10).
The tyrosine phosphatase SHP-2 is activated and recruited to Src kinase in resting Ph-MPD platelets
As shown in Figure 3A, in resting pathological platelets the Src kinase displays a potentially active conformation being dephosphorylated at the inhibitory Tyr527. To highlight the mechanism(s) responsible for this “anomalous” kinase conformation occurring in the absence of exogenous stimuli, we analyzed the expression of Csk, the tyrosine kinase that catalyzes the phosphorylation of the Src-Tyr527,16 and found no difference in the Csk protein level between normal and pathological platelets (data not shown). The tyrosine phosphatases SHP-1 and SHP-2 were then analyzed because they are reported to be involved in Src-phospho-Tyr527 dephosphorylation33 and to play a critical role in hematopoietic cell development and function.34,35 SHP-1 and SHP-2 generally display an autoinhibited “closed” conformation in the cytosol of resting cells and their activation requires the opening of their molecules in concomitance with their recruitment to the Tyr-phosphorylated target substrates.36,37 The cellular distribution and activity of these tyrosine phosphatases were analyzed in normal and Ph-MPD resting platelets. Although, as expected, SHP-2 is detectable only in the cytosol of normal cells (Figure 6A lanes 2-3), a phosphatase aliquot is anchored to the membranes of ET platelets (Figure 6A lanes 5-6). A parallel analysis demonstrated that SHP-1 is resident only in the cytosol of both normal and ET cells (Figure 6A lanes 2-3, 5-6). To examine the potential occurrence of an interaction between SHP-2 and Src kinase in ET platelets, coimmunoprecipitation experiments were performed using either anti-Src or anti–SHP-2 antibodies. In normal platelets, cytosolic SHP-2 does not interact with Src kinase (Figure 6B lanes 1-3), which is resident in the membrane fraction (Figure 6A lanes 2-3). At variance, in the pathological cells, SHP-2, which is anomalously present also in the membranes, where Src is normally resident (Figure 6A lane 6), coimmunoprecipitates with the kinase (Figure 6B lane 6). The finding that JAK2 is not detectable in Src-IP and SHP-2-IP indicates that it is not required for Src-SHP-2 association (Figure 6B).
Analysis of SHP-2 basal activity and interaction with Src in normal and ET platelets. (A-B) Platelets from healthy donors (lanes 1-3) or ET patients (lanes 4-6) were disrupted by suspension in 1 mL of lysis buffer (lanes 1,4) or mild sonication in isotonic buffer and the cytosolic (lanes 2,5) and membrane fractions (lanes 3,6) were obtained as described in “Subcellular fractionation.” (A) The different fractions (15 μL) were analyzed by Western blot with anti–SHP-2, whose densitometric values are reported above the relative bands, or anti–SHP-1 or anti-Src antibodies. (B) The different fractions (400 μL) were immunoprecipitated with either anti-Src or anti–SHP-2 antibodies and the IPs were immunostained with anti–SHP-2 or anti-Src or anti-JAK2 antibodies. The panel is representative of data obtained from 12 normal and 12 ET samples (JAK2V617F homozygous and heterozygous), except the immunostaining with JAK2 antibody, which was performed only in 4 normal and 4 ET samples. (C) Normal, PV, and ET platelets were incubated with vehicle (lanes 1-2), or 0.5mM pervanadate (lane 3) or 2 mg/mL calpeptin (lane 4) for 5 minutes. Cells were then lysed and the basal tyrosine phosphatase activity of the lysates was tested in vitro on [33P]phospho band 3 of erythrocytes as detailed in “Tyrosine phosphatase assay.” Samples were then subjected to SDS/PAGE and the 33P-phosphate bound to band 3 was evaluated by autoradiography followed by the counting of the excised 33P-protein bands in a scintillation counter. The phosphatase activity is expressed as 33P-phosphate released from 33P-phospho band 3. Values represent the means of experiments performed in triplicate with 6 normal, 5 PV, and 7 ET samples. **P < .001 versus the phosphatase activity of normal platelets.
Analysis of SHP-2 basal activity and interaction with Src in normal and ET platelets. (A-B) Platelets from healthy donors (lanes 1-3) or ET patients (lanes 4-6) were disrupted by suspension in 1 mL of lysis buffer (lanes 1,4) or mild sonication in isotonic buffer and the cytosolic (lanes 2,5) and membrane fractions (lanes 3,6) were obtained as described in “Subcellular fractionation.” (A) The different fractions (15 μL) were analyzed by Western blot with anti–SHP-2, whose densitometric values are reported above the relative bands, or anti–SHP-1 or anti-Src antibodies. (B) The different fractions (400 μL) were immunoprecipitated with either anti-Src or anti–SHP-2 antibodies and the IPs were immunostained with anti–SHP-2 or anti-Src or anti-JAK2 antibodies. The panel is representative of data obtained from 12 normal and 12 ET samples (JAK2V617F homozygous and heterozygous), except the immunostaining with JAK2 antibody, which was performed only in 4 normal and 4 ET samples. (C) Normal, PV, and ET platelets were incubated with vehicle (lanes 1-2), or 0.5mM pervanadate (lane 3) or 2 mg/mL calpeptin (lane 4) for 5 minutes. Cells were then lysed and the basal tyrosine phosphatase activity of the lysates was tested in vitro on [33P]phospho band 3 of erythrocytes as detailed in “Tyrosine phosphatase assay.” Samples were then subjected to SDS/PAGE and the 33P-phosphate bound to band 3 was evaluated by autoradiography followed by the counting of the excised 33P-protein bands in a scintillation counter. The phosphatase activity is expressed as 33P-phosphate released from 33P-phospho band 3. Values represent the means of experiments performed in triplicate with 6 normal, 5 PV, and 7 ET samples. **P < .001 versus the phosphatase activity of normal platelets.
To assess whether SHP-2 recruitment to the cellular membranes is concomitant with the phosphatase activation, the SHP-2 constitutive activity was analyzed in normal, PV, and ET platelets. Cells were lysed under mild detergent conditions to preserve the SHP-2 native conformation, and the phosphatase activity was tested toward [33P]phospho-Tyr-band 3 of erythrocytes, a SHP-2 substrate.26 PV and ET lysates exhibit about double basal phosphatase activity than control lysates (Figure 6C column 2). Whereas the addition of pervanadate, a wide-spectrum tyrosine phosphatase inhibitor, greatly reduces the P-Tyr-band 3 dephosphorylation in all samples, the SHP-2–selective inhibitor calpeptin38 affects the phosphatase activity only of the pathological samples (Figure 6C columns 3-4), indicating the presence of constitutively active SHP-2 in Ph-MPD platelets.
TPO's effect on JAK2 and Src activation in normal and ET platelets
As mentioned in “Alpha-thrombin triggers Src and JAK2 activation in platelets of Ph-MPD patients,” we did not consider thrombopoietin (TPO) a suitable agonist for this study, however we thought it would be interesting to analyze its effect on Src and JAK2 activation in JAK2V617F-negative and -positive ET platelets responsive to this stimulus.
In agreement with previous results,27 we found that 200 ng/mL TPO is far less effective than 20 mU/mL thrombin in inducing the protein Tyr phosphorylation in both normal and ET platelets (Figure 7A top part). The TPO-triggered Src activation is less than 30% in comparison with that induced by thrombin in control and ET specimens and, consistent with the kinase “preactivated” conformation, Src autophosphorylation appears earlier in the pathological than in normal platelets (Figure 7A middle part). At variance, in all samples, TPO promotes very early and strikingly more efficiently than thrombin the activation of JAK2 (Figure 7A bottom part). Accordingly, TPO-elicited STAT5 phosphorylation is almost abolished by cell treatment with JAK2-inhibitor-I (Figure 7B lanes 2,4,8,10), although it is not significantly affected by Src inhibition in both normal and pathological cells (Figure 7B lanes 2,6,8,12).
Effect of TPO on Src and JAK2 activation in normal and ET platelets. (A) Platelets from healthy donors (lanes 1-5) or ET patients (lanes 6-10) were treated with 200 ng/mL TPO (lanes 2-4, 7-9) or 20 mU/mL thrombin (lanes 5,10) for the indicated times. Cells were lysed and aliquots of the lysates were analyzed by Western blot with anti–P-Tyr antibody (top part of the panel). Platelets were lysed in IP-buffer. Aliquots of the lysates were immunoprecipitated with anti-Src antibody and the IPs were immunostained with anti–Src-P-Tyr416 and reprobed with anti-Src antibodies (middle part of the panel). Aliquots of the lysates were immunoprecipitated with anti-JAK2 antibody and the IP were immunostained with anti-P-JAK2 antibody and reprobed with anti-JAK2 antibody (bottom part of the panel). Means of densitometric values relative to the phosphorylated kinases are reported above the relative bands. (B) Platelets from healthy donors (lanes 1-6) or ET patients (lanes 7-12) were treated without (odd lanes) or with (even lanes) 200 ng/mL TPO for 2 minutes in the absence (lanes 1-2, 7-8), or presence of 1μM JAK-inhibitor-I (lanes 3-4, 9-10), or 10μM PP2 (lanes 5-6, 11-12). Platelet lysates were analyzed by Western blot with anti–P-STAT5 antibody and reprobed with anti-STAT5 antibody. Densitometric values of anti–P-STAT5 are reported above the relative bands and are means of data obtained from 6 normal, 3 JAK2V617-negative, and 3 JAK2V617-positive ET samples. **P < .001 versus STAT5 phosphorylation in the absence of inhibitors.
Effect of TPO on Src and JAK2 activation in normal and ET platelets. (A) Platelets from healthy donors (lanes 1-5) or ET patients (lanes 6-10) were treated with 200 ng/mL TPO (lanes 2-4, 7-9) or 20 mU/mL thrombin (lanes 5,10) for the indicated times. Cells were lysed and aliquots of the lysates were analyzed by Western blot with anti–P-Tyr antibody (top part of the panel). Platelets were lysed in IP-buffer. Aliquots of the lysates were immunoprecipitated with anti-Src antibody and the IPs were immunostained with anti–Src-P-Tyr416 and reprobed with anti-Src antibodies (middle part of the panel). Aliquots of the lysates were immunoprecipitated with anti-JAK2 antibody and the IP were immunostained with anti-P-JAK2 antibody and reprobed with anti-JAK2 antibody (bottom part of the panel). Means of densitometric values relative to the phosphorylated kinases are reported above the relative bands. (B) Platelets from healthy donors (lanes 1-6) or ET patients (lanes 7-12) were treated without (odd lanes) or with (even lanes) 200 ng/mL TPO for 2 minutes in the absence (lanes 1-2, 7-8), or presence of 1μM JAK-inhibitor-I (lanes 3-4, 9-10), or 10μM PP2 (lanes 5-6, 11-12). Platelet lysates were analyzed by Western blot with anti–P-STAT5 antibody and reprobed with anti-STAT5 antibody. Densitometric values of anti–P-STAT5 are reported above the relative bands and are means of data obtained from 6 normal, 3 JAK2V617-negative, and 3 JAK2V617-positive ET samples. **P < .001 versus STAT5 phosphorylation in the absence of inhibitors.
Discussion
In this study we analyzed the Src and JAK2 tyrosine kinase activities in resting and thrombin-stimulated platelets from healthy subjects, and PV and ET patients. Our results demonstrate that, in resting Ph-MPD platelets, Src kinase is present in its anomalously dephosphorylated and potentially active conformation, most likely as a consequence of its interaction with the tyrosine phosphatase SHP-2. The Src signaling activation induced by low thrombin concentration takes place before JAK2 activation and occurs earlier in pathological than in control platelets.
Several findings support the conclusion of an anomalous presence of potentially active Src kinase in resting Ph-MPD platelets:
(1) In healthy resting platelets the phosphorylation of Src-Tyr527 residue correlates with the kinase inactive conformation, whereas in resting PV and ET cells, this residue is dephosphorylated.
(2) SHP-2 tyrosine phosphatase shows anomalous properties in Ph-MPD platelets. Although in healthy subjects SHP-2 is localized exclusively in the cytosol, a phosphatase aliquot is present in the membranes of Ph-MPD platelets, where it interacts with the Src kinase. Because SHP-2 activation is concomitant with the phosphatase recruitment to its cognate substrates,36 our data suggest that this phosphatase might be responsible for the dephosphorylation of the Src phospho-Tyr527 in Ph-MPD platelets. This hypothesis is supported by the constitutive SHP-2–related phosphatase activity measured in Ph-MPD platelets. Interestingly, a constitutive SHP-2 activity has been implicated in other myeloid malignancies.39,40
(3) Consistent with its conformational state (see point 1), Src immunoprecipitates obtained from resting platelets of healthy donors show an almost undetectable in vitro kinase activity, whereas PV and ET Src immunocomplexes display a substantial basal activity. Nevertheless, despite the potentially active Src kinase conformation, pathological resting platelets show a very poor basal protein Tyr phosphorylation, comparable with that of normal cells. These results suggest that in Ph-MPD platelets the Src-mediated Tyr phosphorylation is still under the control of external modulators, which are required for triggering Src-mediated signaling. Upon platelet stimulation by high thrombin concentration, the Src kinase activity in normal and pathological cells reaches similar levels. Thus, although Src is already primed for activation in Ph-MPD platelets, Src signaling occurs only upon cellular stimulation.
The present data are in agreement with other reports showing that the Src activity is involved in hematologic malignancies17 and in the erythropoietin-independent differentiation of erythroid progenitors from PV patients.41
(4) Mild stimulation with low thrombin concentration triggers a faster Src signaling in pathological compared with normal specimens. This is demonstrated by the time courses of Src autophosphorylation at Tyr416 and Tyr phosphorylation of the Src substrates STAT5 and cortactin. It is noteworthy that the Src-mediated phosphorylation of cortactin has been reported to be involved in thrombin-induced platelet cytoskeleton rearrangement and aggregation.42
(5) Analysis of intracellular Ca2+ mobilization and aggregation, an upstream and a downstream event of the platelet activation cascade, promoted by low thrombin concentration, demonstrates that these 2 processes are greater in Ph-MPD than in control cells, supporting the hypothesis of the hypersensitivity of the pathological platelets. The finding that the Src inhibitor PP2 inhibits the aggregation of the pathological platelets more efficiently than that of normal platelets is consistent with the conclusion that Src preactivation is implicated in the anomalous platelet functions.
It has been reported that, despite the evidence of the in vivo occurrence of Ph-MPD platelet activation, the ex vivo platelet functions are impaired.43 The results reported herein obtained with low thrombin concentration, however, demonstrate that washed Ph-MPD platelets are more responsive than normal cells also in ex vivo tests. It is hypothesized that the Src-Tyr527 dephosphorylation might be triggered in vivo by plasma factors characterizing Ph-MPDs, such as high platelet count, high shear stress,43 and high concentration of TPO44 and procoagulant components.45 Interestingly, the shear-mediated platelet morphologic change was reported to be highly dependent on Src activation.46 Pathogenesis of thrombosis in Ph-MPD has been reported to be multifactorial; rheologic abnormalities, anomalous functions of platelets, and enhanced interaction with leukocytes and endothelial cells are possible contributing factors.47 Little correlation between a specific defect and the thrombohemorrhagic symptoms has been found, however the platelet abnormalities seem to contribute somehow to the occurrence of these pathological complications.11,47,48 Even if conclusive evidence is at present lacking, we would hypothesize that Src preactivation and the related platelet hyperactivity to weak stimuli might concur with the onset of the hypercoagulable state, which is present in virtually all PV and ET patients even without clinical manifestations,45 and with Ph-MPD pathogenesis.
The present results also show that the Tyr phosphorylation signaling induced by thrombin in PV and ET platelets is different from that of controls. In particular, whereas in normal platelets STAT5 phosphorylation is dependent mostly on JAK2 activity, in pathological platelets this protein Tyr phosphorylation is greatly ascribable to Src activity. Moreover, low thrombin concentration elicits in the pathological platelets an early Src-dependent STAT5 phosphorylation, which occurs before JAK2 activation. This outcome is consistent with the reported alternative pathway of STAT activation, which is dependent on Src family members and leads to cellular transformation and tumorigenesis.31 The analysis of ERK1/2, members of an alternative signaling pathway downstream of JAK2 V617F,32 demonstrates that their activation is similarly mediated by Src activity.
We also compared for the first time the activity displayed by JAK2 immunoprecipitated from normal or JAK2V617F homozygous platelets and found that native wild-type and mutated JAK2 display similar in vitro kinase activity, which therefore seems not affected by the V617F mutation.
The analysis of JAK2 signaling shows that JAK2 and its downstream effectors STAT5 and ERK1/2 are not phosphorylated in resting platelets from healthy or Ph-MPD subjects (JAK2V617F negative or positive) and that the time courses of JAK2 activation elicited by thrombin as well as by TPO are similar in controls and pathological cells. These results demonstrate that JAK2V617F is not constitutively active in Ph-MPD platelets, in agreement with a report that suggests that V617F mutation is not sufficient to cause constitutive JAK2 activation.49 Consistently, JAK2 phosphorylation was previously reported to be undetectable in resting platelets obtained from PV27 and ET patients harboring normal or mutated kinase.50
Collectively, our results demonstrate that the anomalous activation and recruitment of SHP-2 tyrosine phosphatase to Src kinase correlate with the kinase preactivation, which is implicated in the hypersensitivity of Ph-MPD platelets and likely involved in the functional abnormalities of PV and ET platelets.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Italian Ministry for University and Research (PRIN-2005 [A.D.-D.] and PRIN-2006 [M.L.R.]), AIRC (Italian Association for Cancer Research), and the European Commission (PRO-KINASERESEARCH-503467).
Authorship
Contribution: M.L.R. provided Ph-MPD samples and designed research; A.M.B. designed and performed most experiments; M.S. analyzed JAK2 mutations; M.F. and E.M. performed experiments; R.D. performed the Ca2+ and aggregation experiments and reviewed the paper; F.F. provided intellectual input and reviewed the paper; and A.D.-D. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arianna Donella-Deana, Dipartimento di Chimica Biologica, Viale G Colombo 3, 35131 Padova, Italy; e-mail: arianna.donella@unipd.it.
References
Author notes
M.L.R. and A.M.B. contributed equally to the work.

![Figure 2. Analysis of JAK2 activation state in resting and thrombin-stimulated platelets. (A) Tyrosine kinase activity of wild-type or V617F-mutated JAK2. Platelets obtained from healthy donors (lanes 1-3), PV patients homozygous for JAK2V617F (lanes 4-6), and ET patients heterozygous for JAK2V617F (lanes 7-9) were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody. The immunocomplexes (IPs) were then analyzed for JAK2 activity tested in vitro toward the substrate polyGlu4Tyr in the presence of [γ33P]ATP and vehicle (lanes 1,4,7), or 10μM AG490 (lanes 2,5,8), or 1μM JAK2-inhibitor-I (lanes 3,6,9) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and transferred to nitrocellulose sheets, which were analyzed for the radioactivity incorporated in polyGlu4Tyr by a Packard Cyclone (columns of the panel) and then immunostained with anti-JAK2 antibody (Wb of the panel). Experiments with AG490 and PP2 were performed with 8 normal, 5 JAK2V617F homozygous PV, and 7 JAK2V617F heterozygous ET samples. Experiments with AG490, JAK-inhibitor-I, and PP2 were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. **P < .001 versus JAK2 activity tested in the absence of inhibitors. (B-C) Platelets, isolated from healthy donors, and PV or ET patients, were treated without (odd lanes) or with 100 mU/mL thrombin (even lanes) for 1 minute in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 1μM JAK2-inhibitor-I (lanes 5-6), or 10μM PP2 (lanes 7-8). (B) Platelet lysates were analyzed by Western blot with an anti–phospho-JAK2 (P-JAK2) antibody that recognizes the activated form of the kinase. Blots were then stripped and reprobed with anti-JAK2 antibody. (C) Platelet lysates were analyzed by Western blot with anti–phospho-STAT5 (P-STAT5) antibody and reprobed with anti-STAT5 antibody. Means of densitometric values are reported above the bands relative to P-JAK2 (B) or P-STAT5 (C). Experiments in panels B and C were performed with 12 normal, 8 PV (JAK2V617F homozygous and heterozygous), and 15 ET samples. Experiments with JAK-inhibitor-I were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. (B) **P < .001 versus P-JAK2 in the absence of JAK2 inhibitors. (C) ** P < .001 and *P < .01 versus P-STAT5 in the absence of inhibitors. #P < .001 PV and ET versus normal platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130002.jpeg?Expires=1765065143&Signature=nP~udtWz7pH31Cgs-oLHLAGfmHvZB-lgFaw8Ccf2rx2Grv5qg3G0~9AeOk89zaC9dhS9nSJ6xRx1UxO9jGnKVZ1dUnJABI9VMPCGPPUw-KuXohPFIHpEIdxUUxvFzOedZHuu9NmH0VQL9q0aG8xzwIgHP5OtXsBgr81BNYmMb2zeFd8Ijke8VDtZrolYHDnH1nMmDhOsknS6o6crw8s0AFZnhXwiM5q90Hf9Rta399y1otOg-uPbAZPAtXxivMkZY7f4jDyKXc8Baq8n9vPEzMfQHhYSf0cXtQvyFf-3GTyHktg2NvvyDcFkq3DbZcsxJCcoFX1yKPme62~H6rvfRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Analysis of protein Tyr phosphorylation, cytosolic [Ca2+] increase, and aggregation triggered by low thrombin concentration in normal and pathological platelets. (A-B) Platelets from healthy donors (lanes 1-4), and PV (lanes 5-8) or ET patients (lanes 9-12), were stimulated with either 20 mU/mL (A) or 100 mU/mL (B) of thrombin for the indicated times. Cells were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin antibodies. (C) [Ca2+]c increase was determined as detailed in “Determination in cytosolic [Ca2+] concentration” in platelets challenged with either 20 mU/mL or 100 mU/mL thrombin. The traces are representative of experiments performed with 10 normal (N), 7 PV, and 10 ET JAK2V617F-negative or -positive samples. (D) Aggregation was determined as detailed in “Platelet aggregation” in platelets pretreated with either vehicle (V) or PP2 and then challenged with 20 mU/mL thrombin. The traces are representative of experiments performed with 4 normal (N) and 4 ET samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130004.jpeg?Expires=1765065143&Signature=zffKEEHFT9ctfxmeRpzhQCzxi5FK9AMnwq~-6YUTuM723d2SvE-txtlB8ZcczV66LjTJ0yNYoYLqKy5yqlCiKxm8qYdsKIWRbkYhFn6mHVHbFY-r2Z753uGaorFytTa~YxYK~F7KjVsOkfyBYlFgzqdA~009HKeoHGcPT3QASwxf6YhgO7nyW6UsMUtMmcFB046e6peoHkwnrRJU29~sz2SzV4EV1TV9JEAwkRce-T0Xjz4soF4Zei12GEKH~V21sXX4VzUVKVZBBRbFERL4OD-tZQHm-CD4PYRk-ZAbVDlez~vfOk9Bj1VXnSKhbNCZcr1DoKzMlfk7EAiojhP0qg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Analysis of SHP-2 basal activity and interaction with Src in normal and ET platelets. (A-B) Platelets from healthy donors (lanes 1-3) or ET patients (lanes 4-6) were disrupted by suspension in 1 mL of lysis buffer (lanes 1,4) or mild sonication in isotonic buffer and the cytosolic (lanes 2,5) and membrane fractions (lanes 3,6) were obtained as described in “Subcellular fractionation.” (A) The different fractions (15 μL) were analyzed by Western blot with anti–SHP-2, whose densitometric values are reported above the relative bands, or anti–SHP-1 or anti-Src antibodies. (B) The different fractions (400 μL) were immunoprecipitated with either anti-Src or anti–SHP-2 antibodies and the IPs were immunostained with anti–SHP-2 or anti-Src or anti-JAK2 antibodies. The panel is representative of data obtained from 12 normal and 12 ET samples (JAK2V617F homozygous and heterozygous), except the immunostaining with JAK2 antibody, which was performed only in 4 normal and 4 ET samples. (C) Normal, PV, and ET platelets were incubated with vehicle (lanes 1-2), or 0.5mM pervanadate (lane 3) or 2 mg/mL calpeptin (lane 4) for 5 minutes. Cells were then lysed and the basal tyrosine phosphatase activity of the lysates was tested in vitro on [33P]phospho band 3 of erythrocytes as detailed in “Tyrosine phosphatase assay.” Samples were then subjected to SDS/PAGE and the 33P-phosphate bound to band 3 was evaluated by autoradiography followed by the counting of the excised 33P-protein bands in a scintillation counter. The phosphatase activity is expressed as 33P-phosphate released from 33P-phospho band 3. Values represent the means of experiments performed in triplicate with 6 normal, 5 PV, and 7 ET samples. **P < .001 versus the phosphatase activity of normal platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130006.jpeg?Expires=1765065143&Signature=V3gk5q8NlbDrDlfDlYhWDF9C5LMZyV9JZSkL8Th7WMymfExKvlRDk6~71Hr2SiItPN223Bwb0YPW0xzwb8YQZjLvAuztYI7fjStTu1DHZVD3J-cquniXWfZXFpWpSFOX6zHzyPKRsFXgtqTP~1MtfCHLpZQh4w-B-Kt-yRJt0aKdVnG2WJ9uHpjQvdatFTJQDlHfSrJwAENMP29B8ILL~ozSgeX6MeeVo4z0b0aaP3M2CDXDBYmnvCD-J9Kc9NsM7FLifESFdpreZUoRaKk3s-a37kx1jKDzhABPYKB9my-gu7~JIvjAwlXTbUGJplZ-1yyDkO5IYc3vohCvbyNPzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Analysis of JAK2 activation state in resting and thrombin-stimulated platelets. (A) Tyrosine kinase activity of wild-type or V617F-mutated JAK2. Platelets obtained from healthy donors (lanes 1-3), PV patients homozygous for JAK2V617F (lanes 4-6), and ET patients heterozygous for JAK2V617F (lanes 7-9) were lysed in IP-buffer and immunoprecipitated with anti-JAK2 antibody. The immunocomplexes (IPs) were then analyzed for JAK2 activity tested in vitro toward the substrate polyGlu4Tyr in the presence of [γ33P]ATP and vehicle (lanes 1,4,7), or 10μM AG490 (lanes 2,5,8), or 1μM JAK2-inhibitor-I (lanes 3,6,9) as described in “In vitro tyrosine kinase assays.” Samples were subjected to SDS/PAGE and transferred to nitrocellulose sheets, which were analyzed for the radioactivity incorporated in polyGlu4Tyr by a Packard Cyclone (columns of the panel) and then immunostained with anti-JAK2 antibody (Wb of the panel). Experiments with AG490 and PP2 were performed with 8 normal, 5 JAK2V617F homozygous PV, and 7 JAK2V617F heterozygous ET samples. Experiments with AG490, JAK-inhibitor-I, and PP2 were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. **P < .001 versus JAK2 activity tested in the absence of inhibitors. (B-C) Platelets, isolated from healthy donors, and PV or ET patients, were treated without (odd lanes) or with 100 mU/mL thrombin (even lanes) for 1 minute in the absence (lanes 1-2) or presence of 10μM AG490 (lanes 3-4), or 1μM JAK2-inhibitor-I (lanes 5-6), or 10μM PP2 (lanes 7-8). (B) Platelet lysates were analyzed by Western blot with an anti–phospho-JAK2 (P-JAK2) antibody that recognizes the activated form of the kinase. Blots were then stripped and reprobed with anti-JAK2 antibody. (C) Platelet lysates were analyzed by Western blot with anti–phospho-STAT5 (P-STAT5) antibody and reprobed with anti-STAT5 antibody. Means of densitometric values are reported above the bands relative to P-JAK2 (B) or P-STAT5 (C). Experiments in panels B and C were performed with 12 normal, 8 PV (JAK2V617F homozygous and heterozygous), and 15 ET samples. Experiments with JAK-inhibitor-I were performed with 4 normal, 3 JAK2V617F homozygous PV, and 4 JAK2V617F heterozygous ET samples. (B) **P < .001 versus P-JAK2 in the absence of JAK2 inhibitors. (C) ** P < .001 and *P < .01 versus P-STAT5 in the absence of inhibitors. #P < .001 PV and ET versus normal platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130002.jpeg?Expires=1765200567&Signature=mp7fW8oes1MJfwA99pPH87xGTCHDDOM8undF-2eXJssFsJ1HKPUgT~SK8eyILEqHddKcmF9fv2ukIxqipumifxCL3z47-NTaYAhkmwR1YI2YZ1DfffZEubtRoq0f9rUAg~8Xk5RcrxXXbS7LiW6QbS0v9Cde8DBndpNRlThflLcAdRU1anaqdlSTUu0nRoYzqbn4HlXenzAafp~WsxkxQhz9il0ET45lS78CsXxgVudxcTpcH129rrhS~j-fgs21DnAUtI6Q~4wksEuT-v6BJQMwF23SpEgK8-Kxdw1FR0~Z-EhKdX-tuIV-4g0T2AZkzD3S90oBrC9e4HchFwA3jQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Analysis of protein Tyr phosphorylation, cytosolic [Ca2+] increase, and aggregation triggered by low thrombin concentration in normal and pathological platelets. (A-B) Platelets from healthy donors (lanes 1-4), and PV (lanes 5-8) or ET patients (lanes 9-12), were stimulated with either 20 mU/mL (A) or 100 mU/mL (B) of thrombin for the indicated times. Cells were then lysed and analyzed by Western blot with anti–P-Tyr and β-actin antibodies. (C) [Ca2+]c increase was determined as detailed in “Determination in cytosolic [Ca2+] concentration” in platelets challenged with either 20 mU/mL or 100 mU/mL thrombin. The traces are representative of experiments performed with 10 normal (N), 7 PV, and 10 ET JAK2V617F-negative or -positive samples. (D) Aggregation was determined as detailed in “Platelet aggregation” in platelets pretreated with either vehicle (V) or PP2 and then challenged with 20 mU/mL thrombin. The traces are representative of experiments performed with 4 normal (N) and 4 ET samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130004.jpeg?Expires=1765200567&Signature=eGnsB8pC1c5U510blud~HbwE0visMsPD71hGBjYywL8p~tRbkXSqmBDLTYqA4eFtCvvhVqEcV54AbfFcYvnRA~3laffWJivCZPLGXL9Ui2ECmMNY3lnWf-mKPg0gRQRqk4hUiUq4Rxurs-Es00deBWs-qjM6kCOeB2YtYNMX~d19tft2ldcVZqqfu3fwGifnlXpJAyvs1Czj7MlL-B5KGJsZQQ~g-Vq4Zk1weMoeV7qZsoWXXCbAy9PJDzKRray3iedSAoCwtGGtoJWHkG~TQyC9sW~S15JNco5sPJzPOWXQ9yo6n5Axtt1xEetwz5~8DYW~sQvafcaJ6TRuh1g5kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Analysis of SHP-2 basal activity and interaction with Src in normal and ET platelets. (A-B) Platelets from healthy donors (lanes 1-3) or ET patients (lanes 4-6) were disrupted by suspension in 1 mL of lysis buffer (lanes 1,4) or mild sonication in isotonic buffer and the cytosolic (lanes 2,5) and membrane fractions (lanes 3,6) were obtained as described in “Subcellular fractionation.” (A) The different fractions (15 μL) were analyzed by Western blot with anti–SHP-2, whose densitometric values are reported above the relative bands, or anti–SHP-1 or anti-Src antibodies. (B) The different fractions (400 μL) were immunoprecipitated with either anti-Src or anti–SHP-2 antibodies and the IPs were immunostained with anti–SHP-2 or anti-Src or anti-JAK2 antibodies. The panel is representative of data obtained from 12 normal and 12 ET samples (JAK2V617F homozygous and heterozygous), except the immunostaining with JAK2 antibody, which was performed only in 4 normal and 4 ET samples. (C) Normal, PV, and ET platelets were incubated with vehicle (lanes 1-2), or 0.5mM pervanadate (lane 3) or 2 mg/mL calpeptin (lane 4) for 5 minutes. Cells were then lysed and the basal tyrosine phosphatase activity of the lysates was tested in vitro on [33P]phospho band 3 of erythrocytes as detailed in “Tyrosine phosphatase assay.” Samples were then subjected to SDS/PAGE and the 33P-phosphate bound to band 3 was evaluated by autoradiography followed by the counting of the excised 33P-protein bands in a scintillation counter. The phosphatase activity is expressed as 33P-phosphate released from 33P-phospho band 3. Values represent the means of experiments performed in triplicate with 6 normal, 5 PV, and 7 ET samples. **P < .001 versus the phosphatase activity of normal platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/3/10.1182_blood-2008-12-196535/4/m_zh89990947130006.jpeg?Expires=1765200567&Signature=Av2Fn64b6XcPbXB4ZjhaWdQDye8Y4sDwrPSWSUT0fP8x44ack8HL3vKHzzsSNaqbp1MGoI9tXcD-CsVzG6r0y5642AdTltE96IXSZahbHI257lkUiH22fVUSL6~Ft4Vt9N5uGk0eRN-CteCd7U-eua1WNkq66wjANOwGabZYSt6UejMx0IdnYEurwNPn4A4c0HJV5yUhnoyHWiksTtCpZWQSBzuRGfRO1r9r48bJRqX4AlwiKu-X3fSAHsLPS6WvIeVQigVkVeA8pt24o~XFSGQ5KtXN-EKavl9TwtNrSY80BsA2vuhg0-852a5ye9bPV1xDJnL42EyRgAtLLIoQ1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)