Abstract
Human plasmacytoid predendritic cells (pDCs) can be activated during microbial infection through Toll-like receptor engagement. They are also involved in nonmicrobial inflammatory diseases, but their activation pathways in this context remain elusive. To identify Toll-like receptor-independent pDC activators, we performed a systematic analysis of cytokine receptors on primary human pDCs. Six receptors were expressed both at mRNA and protein levels: interleukin-3 receptor (IL-3R), IL-6R, IL-10R, IL-18R, interferon-γ receptor, and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor. Only GM-CSF and IL-3 were able to efficiently promote pDC survival and induce their differentiation into dendritic cells. Allogeneic naive CD4 T cells primed with GM-CSF–activated pDCs produced more interferon-γ and less IL-4 and IL-10 compared with IL-3–activated pDCs, indicating a shift in the Th1/Th2 balance. Our data point at a novel function of GM-CSF, which may serve as a link between a pathologic inflammatory environment, pDC activation, and the modulation of CD4 T-cell responses.
Introduction
Plasmacytoid predendritic cells (pDCs) play a major role in innate immunity by producing large amounts of type 1 interferon1 after Toll-like receptor (TLR) engagement by microbes or microbial products.2 They are involved in antiviral immunity in human and mouse2 and can also respond to CpG-enriched bacterial DNA.3
Besides infection, there is growing evidence that pDCs are also involved in nonmicrobial inflammatory diseases. In some autoimmune settings, such as in psoriasis4 and lupus erythematosus,5 a TLR-dependent pathway of pDC activation has been identified. In other settings, such as allergy,6 rheumatoid arthritis,7 multiple sclerosis,8 Sjögren syndrome,9 and cancer,10 there is no evidence for a TLR-dependent pDC activation, suggesting a prominent role for TLR-independent pathways, through inflammatory mediators and cytokines. However, these pathways remain poorly characterized. Interleukin-3 (IL-3) is the only cytokine known to potently activate pDCs,11 and its physiopathologic significance remains unclear.2
We hypothesized that other cytokines may be able to activate pDCs within a complex inflammatory environment. In this study, we performed a systematic analysis of cytokine receptor expression, which identified granulocyte-macrophage colony-stimulating factor (GM-CSF) as a novel activating factor for human pDCs.
Methods
pDC purification
Buffy coats were obtained from healthy adult donors at the Saint-Antoine Crozatier Blood Bank. pDCs were isolated by flow cytometry as Lin−CD11c−CD4+ as previously described.12 Purity was more than 98%. The study was approved by the Institut Curie Institutional Review Board, and all blood donors gave informed consent for research use of the biologic material in accordance with the Declaration of Helsinki.
Gene expression profiling
Total RNA was extracted from pDCs freshly isolated from the blood of healthy donors, double-amplified, and labeled according to the protocol recommended by Affymetrix for hybridization to Human Genome U133 Plus 2.0 arrays. We extracted the expression levels for cytokine receptor chains from IL-1R to IL-23R, and receptors for other cytokines and growth factors that are known to be important for hematopoietic cells, such as interferon-γ (IFN-γ), tumor necrosis factor (TNF), granulocyte colony stimulating-factor (G-CSF), monocyte colony stimulating factor (M-CSF), stem cell factor (SCF), platelet-derived growth factor (PDGF), and GM-CSF.
Surface cytokine receptor expression on pDCs
Freshly sorted pDCs were stained with specific antibodies: phycoerythrin (PE)–interleukin-3 receptor (IL-3R; BD Biosciences), PE–IL-4R (BD Biosciences), PE–IL-6R (BD Biosciences), fluorescein isothiocyanate (FITC)–IL-7R (eBioscience), allophycocyanin–IL-8R (R&D Systems), PE–IL-10R (BD Biosciences), PE–IL-18R (BD Biosciences), PE–TNFR (BD Biosciences), PE–IFN-γR (R&D Systems), PE–M-CSFR (R&D Systems), and PE–GM-CSFR (BD Biosciences). Isotype-matched antibodies were used as control. The analysis was performed on an LSRII (BD Biosciences).
pDC culture
pDCs were cultured 48 hours in RPMI 10% FCS in the presence of recombinant IL-6 (Miltenyi Biotec), IL-3, IL-10, IL-18, and GM-CSF at 10 ng/mL, IFN-γ at 100 U/mL (all from R&D Systems) and CpG-C (a kind gift from Dr F. Barrat) at 5 μg/mL.
pDC viability
pDC viability was assessed by measuring the percentage of 4,6-diamidino-2-phenylindole-negative pDCs by fluorescence-activated cell sorter after 48 hours of culture with recombinant cytokines.
DC differentiation
pDC maturation was analyzed after 48 hours of stimulation measuring the expression of costimulatory molecules and human leukocyte antigen (HLA)–DR levels by fluorescence-activated cell sorter. We used the following antibodies: FITC-CD80 (BD Biosciences), PECy7-CD86 (eBioscience), PE-ICOS-ligand (eBioscience), FITC-OX40L (Ancell), and Alexa 700-HLA-DR (BioLegend). Isotype-matched antibodies were used as control. pDCs were cytospun in parallel and colored with Giemsa staining. Pictures were taken with a CFW-1308C color digital camera (Scion Corporation) on a Leica DM 4000 B microscope, with a 100×/1.30 oil objective.
pDC cytokine production
pDC supernatants were collected after 48 hours of culture in medium alone, IL-3, GM-CSF, and CpG-C, and frozen at −80°C until assayed. IFN-α was measured by enzyme-linked immunosorbent assay (PBL Biomedical Laboratories). TNF and IL-6 were measured by Cytometric Bead Array Flex Set (BD Biosciences).
CD4 T helper cell differentiation
CD4 naive T cells were sorted as CD4+CD45RA+CD25−CD45RO− by flow cytometry as previously described.13 Purity was higher than 98%. Naive CD4 T cells were cultured for 6 days with allogeneic pDCs at a 5:1 ratio as previously described.14 Supernatants were collected after 24 hours of anti-CD3/CD28 microbeads (Dynal) restimulation. Cytokine measurement was performed by Cytometric Bead Array Flex Set (BD Biosciences). mRNA transcripts were quantified by real-time quantitative polymerase chain reaction on an ABI Prism 7900 sequence detector (Applied Biosystems) with Applied Biosystem predesigned TaqMan Gene Expression Assays and Absolute QPCR ROX mix (Thermo Fisher Scientific).
Statistical analysis
Comparisons between different conditions were performed using the Wilcoxon test. Statistical significance was retained for P values less than .05.
Results and discussion
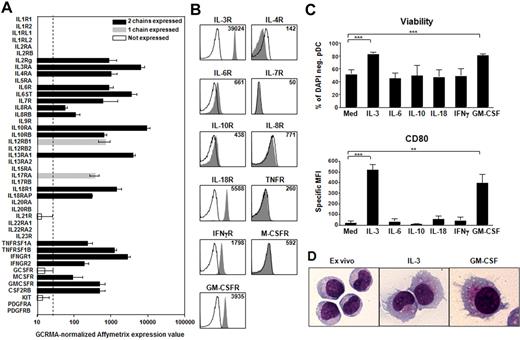
Primary human pDCs were used throughout this study. In a first screening, we used Affymetrix gene expression profiling to assess the expression of 44 cytokine receptor chains corresponding to 26 different cytokines and growth factors (Figure 1A). Eleven cytokine receptors were expressed as heterodimers: IL-3R, IL-4R, IL-6R, IL-7R, IL-8R, IL-10R, IL-18R, TNFR, IFN-γR, M-CSFR, and GM-CSFR (Figure 1A). To confirm surface expression at the protein level, we used flow cytometry. Six receptors were positive on freshly sorted pDCs: IL-3R, IL-6R, IL-10R, IL-18R, IFN-γR, and GM-CSFR; and 5 receptors were negative: IL-4R, IL-7R, IL-8R, TNFR, and M-CSFR (Figure 1B). This discrepancy between mRNA and protein level may be the result of a lower sensitivity of flow cytometry compared with mRNA expression. Thus, in addition to IL-3, our systematic receptor analysis identified 5 cytokines, IL-6, IL-10, IL-18, IFN-γ, and GM-CSF, as potential candidates for activating pDCs.
GM-CSF promotes pDC survival and differentiation into DCs. (A) GCRMA-normalized Affymetrix expression values of cytokine and growth factor receptor chains on pDCs freshly isolated from the blood of healthy donors. The dashed line is the threshold of signal detection. Black bars represent the mRNA levels of the heterodimeric cytokine and growth factor receptors expressed on pDCs; gray bars, the receptors for which we detected only one chain; and white bars, the chains not detectable. Data are the mean of 7 independent experiments each from different donors. Error bars represent SD. (B) Cytokine receptor expression at the surface of freshly isolated pDCs. Filled histograms represent specific staining for receptors; and open histograms, isotype-matched controls. Inset values indicate mean fluorescence intensities over all cells. Data are from one representative of 5 independent pDC experiments each from different donors. (C) pDC viability and maturation after 48 hours of culture in the absence (Med) or presence of recombinant IL-3, IL-6, IL-10, IL-18, IFN-γ, and GM-CSF. Viability is expressed as percentage of 4,6-diamidino-2-phenylindole-negative pDCs. The specific mean fluorescence intensity (MFI) of CD80 is over all the viable pDCs. Data are the mean of 5 or more independent experiments each from different donors. Error bars represent SEM. ***P < .001. **P < .005. (D) Giemsa staining of ex vivo pDCs compared with IL-3– and GM-CSF–treated pDCs (original magnification ×1000).
GM-CSF promotes pDC survival and differentiation into DCs. (A) GCRMA-normalized Affymetrix expression values of cytokine and growth factor receptor chains on pDCs freshly isolated from the blood of healthy donors. The dashed line is the threshold of signal detection. Black bars represent the mRNA levels of the heterodimeric cytokine and growth factor receptors expressed on pDCs; gray bars, the receptors for which we detected only one chain; and white bars, the chains not detectable. Data are the mean of 7 independent experiments each from different donors. Error bars represent SD. (B) Cytokine receptor expression at the surface of freshly isolated pDCs. Filled histograms represent specific staining for receptors; and open histograms, isotype-matched controls. Inset values indicate mean fluorescence intensities over all cells. Data are from one representative of 5 independent pDC experiments each from different donors. (C) pDC viability and maturation after 48 hours of culture in the absence (Med) or presence of recombinant IL-3, IL-6, IL-10, IL-18, IFN-γ, and GM-CSF. Viability is expressed as percentage of 4,6-diamidino-2-phenylindole-negative pDCs. The specific mean fluorescence intensity (MFI) of CD80 is over all the viable pDCs. Data are the mean of 5 or more independent experiments each from different donors. Error bars represent SEM. ***P < .001. **P < .005. (D) Giemsa staining of ex vivo pDCs compared with IL-3– and GM-CSF–treated pDCs (original magnification ×1000).
Next, we studied the effect of these cytokines on 2 major functional outcomes of pDCs: survival and differentiation into DCs. After 48 hours of culture, only IL-3 and GM-CSF were able to maintain pDC viability (Figure 1C) and to induce the up-regulation of costimulatory molecules, such as CD80, CD86, and ICOSL (inducible T-cell co-stimulator ligand) (Figure 1C; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After Giemsa staining, the morphology of GM-CSF–activated pDCs was compatible with a differentiation into DCs, with an increase in cell size, formation of dendrites, vacuolar cytoplasm, and a smaller nucleus (Figure 1D). Overall, our data show that, among a variety of cytokines, only IL-3 and GM-CSF maintain pDC viability and induce their differentiation into DCs. Interestingly, GM-CSF was shown to inhibit pDC development from CD34 hematopoietic precursors.2 Thus, GM-CSF may have a dual role that depends on the site of its production: negative role on pDC development but positive role on peripheral pDC activation. The fact that IL-6, IL-10, IL-18, and IFN-γ were not sufficient to induce pDC activation suggests that their function may be to regulate the response of pDCs to other stimuli. This was already described for IL-10, which was shown to decrease the IL-3-induced survival of pDCs14 and to inhibit type 1 IFN production by TLR-activated pDCs.15
To determine the effect of GM-CSF on pDCs, we performed an extensive phenotypic analysis compared with IL-3 and CpG-C pDCs. We observed an up-regulation of CD86, ICOSL, OX40L, and HLA-DR in GM-CSF pDCs compared with ex vivo pDCs and nonstimulated pDCs (Med). The levels of OX40L and HLA-DR were similar to that induced by IL-3. ICOSL was found to be less expressed in GM-CSF pDCs. The expression of costimulatory molecules was higher in CpG-C pDCs, in accordance with a more mature phenotype induced by TLR activation (Figure 2A-B). We did not detect significant levels of IFN-α, TNF, and IL-6 in supernatants of IL-3- and GM-CSF pDCs compared with CpG-C pDCs (supplemental Figure 2).
GM-CSF-matured pDCs modulate the Th1/Th2 balance during T helper cell differentiation. (A) Surface phenotype of pDCs after 48 hours of culture in medium alone (Med), exogenous cytokines (GM-CSF, IL-3), and CpG-C was assessed by flow cytometry. Filled histograms represent specific staining; open histograms, isotype-matched controls. Inset values indicate mean fluorescence intensities over all viable pDCs. Data are from one representative of 10 independent experiments each from different donors. (B) pDC maturation after 48 hours of culture in medium alone (Med) or with GM-CSF, IL-3, and CpG-C compared with ex vivo pDCs. The specific mean fluorescence intensity (MFI) of CD86, ICSOL, OX40L, and HLA-DR is over all viable cells. Data represent 10 independent experiments each from different donors. Error bars represent median. **P < .005; *P < .05; n.s. indicates not significant. (C) mRNA expression of T cell–derived cytokines after priming with pDCs activated for 48 hours with GM-CSF, IL-3, and CpG-C. GM-CSF pDCs induced higher IFN-γ and less IL-4 and IL-10 mRNA in T cells, compared with IL-3 pDCs. mRNA expression values are normalized on the level of the housekeeping gene β-2-microglobulin (B2M). Data are the mean of 10 independent experiments each from different donors. Error bars represent SEM. **P < .05. *P < .005. (D) Protein expression of T cell–derived cytokines after priming with pDCs activated for 48 hours with GM-CSF, IL-3, and CpG-C. CD4 T cells primed with GM-CSF pDCs produced higher levels of IFN-γ compared with IL-3– and CpG-CpDCs. They produced also more TNF and less IL-4 and IL-10 compared with IL-3 pDCs. Data are the mean of 12 independent experiments, each from a different donor. Error bars represent SEM. **P < .05. *P < .005.
GM-CSF-matured pDCs modulate the Th1/Th2 balance during T helper cell differentiation. (A) Surface phenotype of pDCs after 48 hours of culture in medium alone (Med), exogenous cytokines (GM-CSF, IL-3), and CpG-C was assessed by flow cytometry. Filled histograms represent specific staining; open histograms, isotype-matched controls. Inset values indicate mean fluorescence intensities over all viable pDCs. Data are from one representative of 10 independent experiments each from different donors. (B) pDC maturation after 48 hours of culture in medium alone (Med) or with GM-CSF, IL-3, and CpG-C compared with ex vivo pDCs. The specific mean fluorescence intensity (MFI) of CD86, ICSOL, OX40L, and HLA-DR is over all viable cells. Data represent 10 independent experiments each from different donors. Error bars represent median. **P < .005; *P < .05; n.s. indicates not significant. (C) mRNA expression of T cell–derived cytokines after priming with pDCs activated for 48 hours with GM-CSF, IL-3, and CpG-C. GM-CSF pDCs induced higher IFN-γ and less IL-4 and IL-10 mRNA in T cells, compared with IL-3 pDCs. mRNA expression values are normalized on the level of the housekeeping gene β-2-microglobulin (B2M). Data are the mean of 10 independent experiments each from different donors. Error bars represent SEM. **P < .05. *P < .005. (D) Protein expression of T cell–derived cytokines after priming with pDCs activated for 48 hours with GM-CSF, IL-3, and CpG-C. CD4 T cells primed with GM-CSF pDCs produced higher levels of IFN-γ compared with IL-3– and CpG-CpDCs. They produced also more TNF and less IL-4 and IL-10 compared with IL-3 pDCs. Data are the mean of 12 independent experiments, each from a different donor. Error bars represent SEM. **P < .05. *P < .005.
Because GM-CSF pDC efficiently differentiated into DCs, we tested their ability to prime and polarize allogeneic naive CD4 T cells. GM-CSF pDCs induced higher IFN-γ and TNF and less IL-4 and IL-10, compared with IL-3 pDCs and CpG-C pDCs, both at mRNA and protein level (Figure 2C-D). This represents a previously unrecognized property of GM-CSF in modulating Th differentiation. Interestingly, the reduced IL-10 production by T cells correlated with a reduced expression of ICOSL on GM-CSF pDCs, illustrating an important role of ICOSL in priming T cells for IL-10.13 We did not detect any differences in the levels of CD4 T cell-derived IL-5, transforming growth factor-β (TGF-β; Figure 2D), IL-17, and IL-22 (supplemental Figure 3) between GM-CSF, IL-3, and CpG-C pDCs. Neither did we observe differences in the mRNA levels of the transcription factors T-bet, GATA3, FOXP3, and SOCS3 (supplemental Figure 4).
Our data identified GM-CSF as a novel activator of pDCs. Because GM-CSF is produced in various types of inflammations, including asthma,16 rheumatoid arthritis,17 Sjögren syndrome,18 and psoriasis,19 it is a strong candidate for inducing pDC activation in these settings because the presence of pDCs has been documented.4,7,9,20 GM-CSF is produced by a large variety of cell types, including fibroblasts, endothelial cells, T cells, macrophages, mesothelial cells, epithelial cells, and tumor cells21 and could potentially mediate a cross-talk between pDCs and any of these cell types. Thus, GM-CSF may serve as a link between a pathologic inflammatory environment, pDC activation, and the modulation of CD4 T-cell responses.
Recent studies suggested that GM-CSF can promote tolerance in models of diabetes,22 autoimmune thyroiditis,23 and tumor,24 but the mechanism remains unknown. Although our data show that GM-CSF pDCs prime CD4 T cells to produce increased IFN-γ and decreased IL-10 compared with IL-3 pDCs, we cannot exclude that this IL-10 may play a tolerogenic role in some models. Moreover, pDCs were described to be involved in the generation of regulatory T cells after both TLR-dependent and -independent stimulation13 (C.G., unpublished data, July 2009). Given the well-described environmental plasticity of pDCs,2 further studies will be important to address their ability to promote tolerance or immunity in the context of endogenous or pharmacologic25 GM-CSF activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zofia Maciorowsky and Annick Viguier for the cytofluorimetric cell sorting, David Gentien for the Affymetrix arrays hybridization, and Claire Hivroz and Philippe Benaroch for helpful suggestions and critical reading of the manuscript.
This work was supported by the Leonardo da Vinci Unipharma Graduates Program and Association pour la Recherche contre le Cancer (fellowship; C.G.), European Marie Curie Excellence Grant (R.Z., V.S.), and Fondation pour la Recherche Médicale (fellowship; R.Z.).
Authorship
Contribution: C.G. designed research, performed experiments, and analyzed data; R.Z. performed experiments and analyzed data; and V.S. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vassili Soumelis, Institut Curie, 26 rue d'Ulm, 75005 Paris, France; e-mail: vassili.soumelis@curie.net.