Abstract
Chronic lymphocytic leukemia (CLL) is the most prevalent human leukemia and is characterized by the progressive accumulation of long-lived malignant B cells. Here we show that human B-CLL cells selectively express high levels of Toll-like receptor 9 (TLR9) mRNA and proteins. Treating B-CLL cells with TLR9 agonists, type B CpG oligodeoxynucleotides (CpG-B ODNs), induces significant morphologic and phenotypic activation, altered cytokine production, reversal of signal transducer, and activator of transcription 1 (STAT1) phosphorylation state, followed by profound apoptosis of B-CLL cells that is CpG-B ODN treatment time- and dose-dependent. TLR9-CpG ODN ligation-induced apoptosis of B-CLL cells is confirmed by viable cell counts, annexin V/propidium iodide and tetramethyl-rhodamine ethylester staining, Western blots of the activation, and cleaved caspases and poly (ADP-ribose) polymerase. Triggering TLR9 by CpG-B ODN leads to nuclear factor-κB-dependent production of autocrine interleukin-10, which activates JAK/STAT pathway-dependent tyrosine phosphorylation of STAT1 proteins and thereby provokes an apoptosis pathway in B-CLL cells. Treating B-CLL cells in vitro or in vivo with CpG-B ODN reduces the number of leukemia cells that engraft in NOD-scid mice. These findings provide new understanding of CpG ODN-mediated antitumor effects and support for the development of TLR9-targeted therapy for human CLL.
Introduction
Chronic lymphocytic leukemia (CLL) accounts for 25% to 30% of all leukemias and is the most prevalent type of leukemia in the Western world. Despite major advances in our understanding of the genetics, biology, diagnosis, clinical staging, and management of CLL,1-5 the overall median survival of CLL patients remains approximately 6 years and is shorter for patients in the high-risk group. The disease is characterized by the progressive accumulation of long-lived malignant B cells with a characteristic CD19+CD5+CD23+BCRlow phenotype. These B-CLL cells accumulate in the G0 phase of the cell cycle with a low proliferative index and have acquired resistance to apoptosis via aberrant up-regulation of prosurvival mechanisms.6-10 Because B-CLL cells do not divide at a high rate, they are less susceptible to radiation therapy and chemotherapy. Alternative therapeutic approaches are needed to achieve longer survival and ultimately a cure in CLL patients. Immunotherapy is a promising approach for the treatment of CLL.11-15 B-cell surface differentiation antigens and receptors can serve as tissue-specific targets for immunotherapy. Antibodies (Abs), such as the anti-CD20 (rituximab),16-18 the anti-CD52 (alemtuzumab, Campath-1H),19-21 and the anti-CD40 antagonist Ab (HCD122),22 are being used clinically to achieve a complete and prolonged remission in CLL patients. However, most CLL patients who undergo antibody-based treatments eventually relapse. New immunotherapeutic strategies are needed to effectively prevent and treat leukemia recurrences.
Toll-like receptors (TLRs) are pattern recognition receptors that trigger innate and adaptive immune responses. The human TLR family includes 10 different TLRs that compose an elegant pathogen recognition system for host defense in innate immunity.23 Triggering TLRs activates a set of common proinflammatory genes and leads to the expression of antimicrobial effector molecules as well as inflammatory cytokine production. Agonists for human TLR1 to TLR9 have been identified and are being developed as new drugs and vaccine adjuvants to treat cancer, allergies, and infectious diseases.24,25 We are interested in exploring TLR-targeted immunotherapy of CLL with TLR agonists and in elucidating the molecular mechanism(s) underlying TLR agonist-mediated antitumor effects. Although the TLR expression profile in human B-CLL cells is unknown, studies have shown that TLR9 agonists, CpG oligodeoxynucleotides (CpG ODN), can activate B-CLL cells to up-regulate cell surface expression of costimulatory molecules and induce cytokine production.26,27 In this study, we show that human B-CLL cells express high levels of TLR9. Triggering TLR9 with CpG-B ODN leads to nuclear factor-κB (NF-κB)–dependent production of autocrine interleukin-10 (IL-10), which activates JAK/STAT pathway-dependent tyrosine phosphorylation of signal transducer and activator of transcription 1 (STAT1) and thereby provokes an apoptotic death pathway in B-CLL cells.
Methods
Cell preparations
Blood samples from 23 untreated CLL patients were drawn after obtaining written informed consent approved by the University of Minnesota Institutional Review Board in accordance with the Declaration of Helsinki. The sex, age, Rai stage, percentage of blood CD5+CD19+CD23+ B-CLL cells, and cytogenetic and mutational status of these CLL patients are summarized in Table 1. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density gradient centrifugation. B-CLL cells in PBMCs were purified using untouched B-cell isolation kit (Miltenyi Biotec) to more than 95% of CD5+CD19+ cells. Normal B and T cells were purified from PBMCs of healthy blood donors using B-cell or T-cell isolation kit (Miltenyi Biotec) to more than 98% of CD19+ cells or CD3+ cells, respectively.
Characteristics of B-CLL patients and CpG ODN-induced apoptosis
| CLL patient . | Sex . | Age, y . | Rai stage . | B-CLL cells, percentage* . | Cytogenetic or mutational status† . | B-CLL cell counts after treatment, percentage‡ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No CpG . | CpG 2216 . | CpG PB4 . | CpG 2006 . | CpG 685 . | ||||||
| 1 | Female | 39 | IV | 92.3 | del (13q), p53 mutations | 78.5 | 68.2 | 76.9 | 10.2 | 7.7 |
| 2 | Male | 62 | IV | 79.8 | del (11q), del (13q), complex karyotype | 79.8 | 62.9 | 63.3 | 31.3 | 15.2 |
| 3 | Female | 43 | 0 | 76.9 | NA | 64.4 | 57.8 | 59.4 | 17.1 | 12.9 |
| 4 | Male | 58 | IV | 85.4 | del (13q), complex karyotype | 76.4 | 72.8 | 82.2 | 23.2 | 17.5 |
| 5 | Male | 49 | IV | 71.4 | del (11q), del (13q), del (Y) | 82.4 | 84.3 | 72.2 | 15.2 | 13.3 |
| 6 | Male | 48 | IV | 97.8 | del (11q) | 76.4 | 72.9 | 91.3 | 78.8 | 78.8 |
| 7 | Female | 50 | 0 | 43.0 | del (13q) | 75.9 | 67.8 | 79.5 | 40.0 | 9.3 |
| 8 | Female | 56 | 0 | 46.7 | NA | 85.2 | 67.5 | 83.7 | 16.5 | 9.9 |
| 9 | Female | 50 | 0 | 77.9 | NA | 90.2 | 68.4 | 79.6 | 46.0 | 12.8 |
| 10 | Male | 76 | IV | 63.0 | del (13q), p53 mutations | 89.0 | 78.5 | 87.0 | 68.9 | 66.0 |
| 11 | Female | 59 | IV | 88.2 | del (11q), del (Y) | 72.4 | 62.5 | 64.0 | 39.0 | 7.7 |
| 12 | Female | 75 | III | 92.9 | del (13q) | 79.2 | 70.4 | 76.5 | 20.0 | 8.6 |
| 13 | Male | 62 | II | 80.2 | del (13q), del (17p), p53 mutations | 82.0 | 53.6 | 80.6 | 11.6 | 10.5 |
| 14 | Male | 60 | I | 73.8 | del (13q) | 86.3 | 92.1 | 76.0 | 11.3 | 10.0 |
| 15 | Male | 78 | I | 68.0 | No abnormalities | 84.6 | 90.3 | 91.8 | 64.2 | 63.0 |
| 16 | Male | 51 | I | 51.7 | No abnormalities | 72.8 | 86.4 | 86.5 | 19.5 | 16.7 |
| 17 | Male | 57 | IV | 92.3 | del (11q) | 67.9 | 52.7 | 58.2 | 63.8 | 51.8 |
| 18 | Male | 53 | III | 70.3 | del (13q) | 74.9 | 73.3 | 73.4 | 13.2 | 10.7 |
| 19 | Male | 70 | IV | 91.7 | del (11q), del (13q) | 77.7 | 74.4 | 81.0 | 32.7 | 26.7 |
| 20 | Male | 76 | IV | 80.0 | del (13q) | 80.2 | 79.5 | 81.9 | 50.7 | 42.1 |
| 21 | Female | 82 | II | 64.9 | NA | 80.4 | 78.6 | 86.3 | 25.2 | 21.9 |
| 22 | Male | 55 | III | 88.1 | del (17p) | 77.6 | 75.4 | 73.8 | 15.6 | 16.3 |
| 23 | Male | 68 | 0 | 57.1 | del (13q) | 70.7 | 70.4 | 67.3 | 17.7 | 10.4 |
| CLL patient . | Sex . | Age, y . | Rai stage . | B-CLL cells, percentage* . | Cytogenetic or mutational status† . | B-CLL cell counts after treatment, percentage‡ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No CpG . | CpG 2216 . | CpG PB4 . | CpG 2006 . | CpG 685 . | ||||||
| 1 | Female | 39 | IV | 92.3 | del (13q), p53 mutations | 78.5 | 68.2 | 76.9 | 10.2 | 7.7 |
| 2 | Male | 62 | IV | 79.8 | del (11q), del (13q), complex karyotype | 79.8 | 62.9 | 63.3 | 31.3 | 15.2 |
| 3 | Female | 43 | 0 | 76.9 | NA | 64.4 | 57.8 | 59.4 | 17.1 | 12.9 |
| 4 | Male | 58 | IV | 85.4 | del (13q), complex karyotype | 76.4 | 72.8 | 82.2 | 23.2 | 17.5 |
| 5 | Male | 49 | IV | 71.4 | del (11q), del (13q), del (Y) | 82.4 | 84.3 | 72.2 | 15.2 | 13.3 |
| 6 | Male | 48 | IV | 97.8 | del (11q) | 76.4 | 72.9 | 91.3 | 78.8 | 78.8 |
| 7 | Female | 50 | 0 | 43.0 | del (13q) | 75.9 | 67.8 | 79.5 | 40.0 | 9.3 |
| 8 | Female | 56 | 0 | 46.7 | NA | 85.2 | 67.5 | 83.7 | 16.5 | 9.9 |
| 9 | Female | 50 | 0 | 77.9 | NA | 90.2 | 68.4 | 79.6 | 46.0 | 12.8 |
| 10 | Male | 76 | IV | 63.0 | del (13q), p53 mutations | 89.0 | 78.5 | 87.0 | 68.9 | 66.0 |
| 11 | Female | 59 | IV | 88.2 | del (11q), del (Y) | 72.4 | 62.5 | 64.0 | 39.0 | 7.7 |
| 12 | Female | 75 | III | 92.9 | del (13q) | 79.2 | 70.4 | 76.5 | 20.0 | 8.6 |
| 13 | Male | 62 | II | 80.2 | del (13q), del (17p), p53 mutations | 82.0 | 53.6 | 80.6 | 11.6 | 10.5 |
| 14 | Male | 60 | I | 73.8 | del (13q) | 86.3 | 92.1 | 76.0 | 11.3 | 10.0 |
| 15 | Male | 78 | I | 68.0 | No abnormalities | 84.6 | 90.3 | 91.8 | 64.2 | 63.0 |
| 16 | Male | 51 | I | 51.7 | No abnormalities | 72.8 | 86.4 | 86.5 | 19.5 | 16.7 |
| 17 | Male | 57 | IV | 92.3 | del (11q) | 67.9 | 52.7 | 58.2 | 63.8 | 51.8 |
| 18 | Male | 53 | III | 70.3 | del (13q) | 74.9 | 73.3 | 73.4 | 13.2 | 10.7 |
| 19 | Male | 70 | IV | 91.7 | del (11q), del (13q) | 77.7 | 74.4 | 81.0 | 32.7 | 26.7 |
| 20 | Male | 76 | IV | 80.0 | del (13q) | 80.2 | 79.5 | 81.9 | 50.7 | 42.1 |
| 21 | Female | 82 | II | 64.9 | NA | 80.4 | 78.6 | 86.3 | 25.2 | 21.9 |
| 22 | Male | 55 | III | 88.1 | del (17p) | 77.6 | 75.4 | 73.8 | 15.6 | 16.3 |
| 23 | Male | 68 | 0 | 57.1 | del (13q) | 70.7 | 70.4 | 67.3 | 17.7 | 10.4 |
B-CLL indicates B-cell chronic lymphocytic leukemia; ODN, oligodeoxynucleotide; and NA, patients have asymptomatic, indolent course of CLL; cytogenetic data not available.
Data are percentage of CD19+CD5+CD23+ B-CLL cells presented in peripheral blood samples collected from individual CLL patients. Immunophenotyping of the CD19+CD5+CD23+ expressed on B-CLL cells was determined by flow cytometric analysis.
Cytogenetic abnormalities and p53 mutations/deletions of individual CLL patients. Interphase fluorescence in situ hybridization analysis was performed using Vysis LSI ATM (11q22.3), LSI D13S319 (13q14.3), and LSI p53 (17p13.1) probes.
Purified B-CLL cells from individual patients were cultured in media with or without the indicated CpG-A or CpG-B ODN for 5 days. B-CLL cells of all treatment groups were counted, stained with TMRE, and determined by flow cytometry. Data are percentage of viable B-CLL cells remaining at day 5 cultures.
CpG oligodeoxynucleotides
Phosphorothioated unmethylated type A CpG ODNs (2216, 5′-ggGGGACGATCGTCgggggG-3′; PB4, 5′-tcgGACGATCGTCgggggG-3′; 1002, 5′-ggGGTCGTTCGTCGTTgggggG-3′) and type B CpG ODNs (2006, 5′-tcgtcgttttgtcgttttgtcgtt-3′; 684, 5′-tcgacgttcgtcgttcgtcgttc-3′; 685, 5′-tcgtcgacgtcgttcgttctc-3′) were purchased from Intergrated DNA Technologies. Lowercase letters represent phosphorothioate linkage, and upper letters represent phosphodiester linkage. CpG ODNs were resuspended in Tris-ethylenediaminetetraacetic acid buffer, diluted in phosphate-buffered saline (PBS), and tested at a final concentration of 5 μg/mL or as indicated.
RT-PCR for TLRs
RNA was extracted from 2 × 106 purified B-CLL, normal B, or T cells, reversely transcribed to cDNA, and analyzed for TLR1-10 expression and real-time reverse-transcribed polymerase chain reaction (RT-PCR) for TLR9 expression. All PCR parameters and primer sequences for TLR1 to TLR-10 and β-actin were as published.28 All RT-PCR reagents were from Invitrogen.
B-CLL cell cultures
To determine the immune-stimulatory effects of CpG ODNs, purified B-CLL cells (1 × 106/well) were cultured in 10% human AB serum RPMI 1640 media with or without CpG ODN in 48-well plates. After 3 days, cells were harvested and assessed for morphologic changes by Giemsa staining29 and for phenotypic changes by flow cytometry. To determine CpG ODN treatment time or doses on apoptosis induction, B-CLL cells (2 × 105/well) were cultured in media with or without CpG ODNs at 5 μg/mL for up to 9 days or at different doses (0.1-10 μg/mL) for 5 days in 96-well plates. Several wells of B-CLL cells from each culture condition were harvested at the indicated time points, counted, and stained with tetramethyl-rhodamine ethylester (TMRE, Invitrogen) or annexin V-FITC Apoptosis Detection Kit I (BD Biosciences). To determine the minimum treatment dose and exposure time required for CpG ODNs to achieve a 50% reduction of leukemia cells, B-CLL cells (4 × 105/well) were cultured in 96-well plates in media with different concentrations of CpG ODNs for the indicated exposure time lengths. At the end of each designated treatment time point, cells were washed twice in the wells, resuspended in fresh media, and continued in culture for a total of 7 days. B-CLL cells of all treatment groups were then harvested, counted, stained with TMRE, and determined by flow cytometry. Viable B-CLL cell number was calculated by multiplying total cell counts with the percentage of TMRE-positive cells of each culture condition.
Flow cytometry
Cell surface markers were analyzed by staining with fluorescent Abs against CD3, CD5, CD19, CD23, CD40, CD45, CD54, CD80, CD86, HLA-ABC, HLA-DR, or isotype control Ab (BD Biosciences). Apoptotic B-CLL cells were assessed by TMRE or annexin V/propidium iodide (PI) staining. Mean fluorescence intensity (MFI) and positive cell percentages were determined.
T-cell proliferation assay
B-CLL cells cultured with or without CpG ODNs for 3 days were irradiated at 30 Gy and used as stimulators to purified allogeneic T cells (1 × 105/well) in mixed leukocyte reaction (MLR) assays. Plates were incubated at 37°C for 5 days and pulsed with 1 μCi/well of 3H-thymidine for the last 18 hours before harvesting. All determinations were conducted in triplicate and 3H-thymidine incorporation (cpm) was determined.
NOD-scid mouse xenograft models
Eight-week-old female NOD-scid IL2Rγnull mice, a NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mouse strain that lacks of T, B, and natural killer (NK) cells (The Jackson Laboratory), were used. To determine whether CpG 2006 pretreated B-CLL cells were programmed to undergo apoptosis in vivo, groups of mice were injected intraperitoneally with 4 × 107 purified B-CLL cells with or without 48 hours of preincubation with CpG 2006. To determine whether administration of CpG 2006 can inhibit the engraftment and survival of B-CLL cells in vivo, mice were intraperitoneally injected with 4 × 107 B-CLL cells followed by 5 daily intraperitoneal injection with CpG 2006 (5 mg/kg body weight) or PBS. At day 5, cells from peritoneal cavity and spleen of each mouse were harvested, stained with anti-hCD45/CD19 Abs, and analyzed by flow cytometry. The number of B-CLL cells present in peritoneal cavity and spleen were calculated by multiplying total cell counts with the percentage of hCD45+/CD19+ cells.
Western blots
Protein lysates were prepared from 5 × 106 purified B-CLL, normal B, and T cells. Western blot was performed using primary Abs specific for TLR9, total, tyrosine- or serine-phophorylated STAT1 (Santa Cruz Biotechnology), cleaved caspase-3, caspase-9, or poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology), respectively, and secondary anti–rabbit IgG-horseradish peroxidase (Santa Cruz Biotechnology), and visualized by enhanced chemiluminescence (Pierce Chemical). Blots were reprobed with an anti–β-actin Ab (Santa Cruz Biotechnology) as a loading control. Signals intensity was quantified using a Fluorochem 8000 imaging system (Alpha Innotech).
Assays for blocking CpG ODN-induced apoptosis of B-CLL cells
To block apoptosis with caspase inhibitors, B-CLL cells (2 × 105/well) were pretreated with 20μM pan-caspase, caspase-3, or caspase-9 inhibitor (R&D Systems) for 2 hours in 96-well plates and cultured in media with CpG 2006. To block apoptosis with inhibitors specific for NF-κB and JAK/STAT pathways, B-CLL cells were pretreated with 5 μg/mL caffeic acid phenethyl ester (CAPE) and/or 50μM AG490 (Calbiochem) for 1 hour and cultured in media with or without CpG 2006. Kinetic expressions of tyrosine- or serine-phosphorylated STAT1 were determined by Western blot. Culture supernatants at 72 hours were assessed for cytokine production. AG490 pretreatment on CpG 2006-induced cleavage of caspase-9, caspase-3, and PARP in B-CLL cells were determined at day 5 by Western blots. To block apoptosis with STAT1 antisense ODN, B-CLL cells were pretreated with 12.5μM STAT1 phosphorothioated antisense ODN (5′-CCACTGAGACATCCTGCCACC-3′)30 or sense ODN (5′-GGTGGCAGGATGTCTCAGTGG-3′) from Operon Biotechnologies for 24 hours and cultured in media with or without CpG 2006. Total, tyrosine- or serine-phosphorylated STAT1 expression was assessed by Western blot at 0, 24, and 48 hours. B-CLL cells in cultures were assessed for phenotypic changes at day 3 and apoptosis at day 5.
Cytokine assays
Serum-free culture supernatants from B-CLL cells incubated with or without CpG 2006 were collected at 72 hours or the indicated time points and assessed for cytokines. IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) were assayed with the Fluorokine MAP immunoarray (R&D Systems) using the Luminex 100 analyzer. IFN-α, IFN-β, and transforming growth factor-β1 (TGF-β1) were determined by enzyme-linked immunosorbent assay (R&D Systems). The lower limits of each cytokine detection were as follows (pg/mL): IL-1β, 0.15; IL-2, 0.2; IL-4, 0.7; IL-6, 0.5; IL-8, 0.2; IL-10, 0.3; IFN-γ, 4.0; TNF-α, 0.4; G-CSF, 1.0; GM-CSF, 2.0; VEGF, 0.4; IFN-α, 3.0; IFN-β, 50; TGF-β1, 4.6. The background level of TGF-β1 in serum-free medium alone is at 200 pg/mL.
Neutralizing IL-10 in B-CLL cell cultures
Anti–IL-10 (10 μg/mL) or anti–TNF-α (10 μg/mL) Ab (R&D Systems) was added to B-CLL cell cultures with or without CpG 2006. The expression of tyrosine- or serine-phosphorylated STAT1 in B-CLL cells was assessed by Western blot at the indicated time points. In some experiments, graded doses (0.02-50.0 ng/mL) of exogenous rh-IL-10 (R&D Systems) were added to B-CLL cell cultures with or without the presence of anti–IL-10 or anti–TNF-α Ab. B-CLL cells in cultures with or without CpG 2006 and/or rh-IL-10 (5 ng/mL) were assessed for phenotypic changes at day 3 and apoptosis at day 5. Tyrosine- or serine-phosphorylated STAT1 expression in B-CLL cells cultured with or without IL-10 (5 ng/mL) in the presence or absence of anti–IL-10 Ab (10 μg/mL) were determined by Western blot at 24 hours.
Data analysis
Data from experiments are expressed as the mean plus or minus SD. Statistical analysis of the results between groups was performed by Student t test. Values of P less than .05 were considered significant.
Results
Human B-CLL cells express high levels of TLR9 and can be potently activated by CpG-B ODNs
B-CLL cells from blood samples of CLL patients express a characteristic CD19+CD5+CD23+ malignant B-cell phenotype. Like normal B cells, B-CLL cells expressed TLR1, TLR6, TLR7, TLR9, and TLR10 (Figure 1A; and data not shown). Real-time RT-PCR results showed that TLR9 gene expression was significantly higher (3.2-fold) in B-CLL cells than in normal B cells (Figure 1B). Western blot analysis confirmed that TLR9 protein expression was 3.5-fold higher in B-CLL cells than in normal B cells (Figure 1C). To study the immune-stimulatory effects of TLR9 agonists on B-CLL cells, 2 distinctive classes of CpG-A and CpG-B ODNs31 were used. Freshly isolated B-CLL cells display a typical leukemic B-cell morphology (Figure 1D). B-CLL cells cultured in media alone or with CpG-A ODN (CpG 2216) displayed a typical CLL morphology with cytologic features similar to that of fresh B-CLL cells. In contrast, CpG-B ODN (CpG 2006)–stimulated B-CLL cells formed aggregated cell clusters and underwent dramatic cytologic changes with marked cellular enlargement and abundant basophilic cytoplasm within 3 days in culture (Figure 1D). Fresh B-CLL cells are positive for HLA-ABC and HLA-DR but express negligible levels of CD40, CD54, CD80, and CD86. B-CLL cells cultured in media or with CpG 2216 for 3 days only modestly increased B-CLL cell surface expression of CD54, CD86, and HLA-DR (2.9-, 2.5-, and 2.2-fold, respectively). In contrast, more substantial and significant increases of surface expression of CD40, CD54, CD86, HLA-ABC, HLA-DR (11.5-, 12.5-, 5.2-, 4.5-, and 4.6-fold, respectively) on B-CLL cells were detected within 3 days after CpG 2006 stimulation (Figure 1E-F). We have comparatively tested additional CpG-A and CpG-B ODNs and consistently found that CpG-B ODNs strongly activate, whereas CpG-A ODNs weakly activate, B-CLL cells to up-regulate cell surface CD40 expression (Figure 1G). Fresh B-CLL cells are poor stimulators to allogeneic T cells in MLR.32,33 Whereas untreated and CpG 2216-treated B-CLL cells induced weak proliferative responses from allogeneic T cells, CpG 2006-treated B-CLL cells induced a strong proliferation of allogeneic T cells in MLR (Figure 1H). These results demonstrate that triggering TLR9 with CpG-B ODNs potently activates B-CLL cells and induces phenotypic changes, consistent with the enhanced immunogenicity.
Human B-CLL cells express high levels of TLR9 and can be potently activated by CpG-B ODNs. Purified B-CLL cells from CLL patients 1 to 5 in Table 1 were used and tested. (A) RT-PCR results of TLR1-10 expression profile in B-CLL cells from 5 CLL patients. (B) RT-PCR results of TLR9 mRNA expression and (C) Western blot results of TLR9 protein expression in B-CLL cells from 5 patients were compared with that of normal B and T cells from 5 healthy blood donors, and normalized to the expression of β-actin. Data are presented as the mean ± SD in the adjacent bar diagram. *P < .01, B-CLL cells and B cells versus T cells. **P < .01, B-CLL cells versus B cells. (D) Morphologies of fresh and day 3 cultured B-CLL cells with or without CpG-A or CpG-B ODN were examined. Images of B-CLL cells in cultures were acquired on an Olympus CKX41 inverted microscope with an Olympus DP12 digital microscope camera at 200× magnification. Images of Giemsa staining of B-CLL cells were acquired on a Zeiss Axioplan 2 Upright Microscope (Carl Zeiss Inc) with a Spot RT color digital camera (Diagnostic Instruments), captured with a Zeiss Plan-Apochromat 63×/1.4 oil objective lens at 630× magnification, and processed with Spot advanced 4.6 software. (E) Expression of surface markers (shaded histogram) on fresh or day 3 cultured B-CLL cells with or without CpG-A or CpG-B ODN was analyzed by flow cytometry, indicated with MFI number, and overlaid with isotype control (unshaded histogram). Data are representative results from 1 of 5 CLL patients. (F) MFI fold increases of the indicated surface molecules on day 3 cultured versus uncultured B-CLL cells. Data are the mean ± SD from 5 independent experiments. *P < .01. (G) MFI fold increases of CD40 expression on day 3 cultured B-CLL cells with CpG-A or CpG-B ODNs versus B-CLL cells cultured in media only. Data are the mean ± SD from 5 independent experiments. *P < .01. (H) Graded doses of irradiated day 3 cultured B-CLL cells with or without CpG ODNs were used as stimulators to allogeneic T cells in MLR assays. Data are representative results with B-CLL cells from 4 CLL patients. The error bars represent the SD of triplicates. The independent experiments were performed with B-CLL cells derived from different CLL patients
Human B-CLL cells express high levels of TLR9 and can be potently activated by CpG-B ODNs. Purified B-CLL cells from CLL patients 1 to 5 in Table 1 were used and tested. (A) RT-PCR results of TLR1-10 expression profile in B-CLL cells from 5 CLL patients. (B) RT-PCR results of TLR9 mRNA expression and (C) Western blot results of TLR9 protein expression in B-CLL cells from 5 patients were compared with that of normal B and T cells from 5 healthy blood donors, and normalized to the expression of β-actin. Data are presented as the mean ± SD in the adjacent bar diagram. *P < .01, B-CLL cells and B cells versus T cells. **P < .01, B-CLL cells versus B cells. (D) Morphologies of fresh and day 3 cultured B-CLL cells with or without CpG-A or CpG-B ODN were examined. Images of B-CLL cells in cultures were acquired on an Olympus CKX41 inverted microscope with an Olympus DP12 digital microscope camera at 200× magnification. Images of Giemsa staining of B-CLL cells were acquired on a Zeiss Axioplan 2 Upright Microscope (Carl Zeiss Inc) with a Spot RT color digital camera (Diagnostic Instruments), captured with a Zeiss Plan-Apochromat 63×/1.4 oil objective lens at 630× magnification, and processed with Spot advanced 4.6 software. (E) Expression of surface markers (shaded histogram) on fresh or day 3 cultured B-CLL cells with or without CpG-A or CpG-B ODN was analyzed by flow cytometry, indicated with MFI number, and overlaid with isotype control (unshaded histogram). Data are representative results from 1 of 5 CLL patients. (F) MFI fold increases of the indicated surface molecules on day 3 cultured versus uncultured B-CLL cells. Data are the mean ± SD from 5 independent experiments. *P < .01. (G) MFI fold increases of CD40 expression on day 3 cultured B-CLL cells with CpG-A or CpG-B ODNs versus B-CLL cells cultured in media only. Data are the mean ± SD from 5 independent experiments. *P < .01. (H) Graded doses of irradiated day 3 cultured B-CLL cells with or without CpG ODNs were used as stimulators to allogeneic T cells in MLR assays. Data are representative results with B-CLL cells from 4 CLL patients. The error bars represent the SD of triplicates. The independent experiments were performed with B-CLL cells derived from different CLL patients
Triggering TLR9 with CpG-B ODN activates B-CLL cells to undergo apoptosis
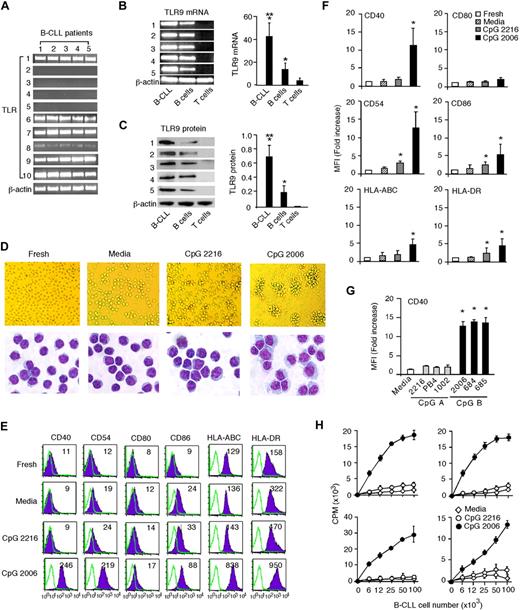
B-CLL cells can be sustained in culture for weeks despite undergoing spontaneous apoptosis. Kinetic analysis of B-CLL cell apoptosis showed that 10% to 25% of cells became apoptotic during a 9-day culture in media alone and 70% to 80% of the starting number of B-CLL cells could be recovered from days 5 to 9. The viability and cell counts of the CpG 2216-treated B-CLL group on days 1 to 9 were similar to that of B-CLL cells cultured in media only. Interestingly, CpG 2006-stimulated B-CLL cells maintained good viability during the first 2 days and then underwent profound apoptosis and greatly decreased the annexin V/PI-negative, TMRE-positive viable B-CLL cell number from days 3 to 9 (Figure 2A). Kinetic analysis showed significant decreases of annexin V/PI-negative, TMRE-positive viable B-CLL cells in cultures with CpG 2006 or CpG 685 at days 3 to 9 (Figure 2B). Similarly, the addition of CpG-B ODNs into patients' PBMCs containing different percentages (96%, 79%, or 47%) of B-CLL cells invariably induced the apoptosis and decreased the number of B-CLL cells in culture, in contrast to the results of B-CLL cells cultured in media only or with CpG-A ODNs (Figure 2C). The results of CpG-B ODN-induced apoptosis of B-CLL cells are consistent in 18 of 23 CLL patients (Table 1; Figure 2D). We also found that apoptotic B-CLL cells (R1 gate) lost their characteristic expression of CD23 and decreased surface expression of CD5 and CD19, whereas viable B-CLL cells (R2 gate) remained positive for CD23 (data not shown). B-CLL cells from 5 patients (patients 6, 10, 15, 17, and 20) were insensitive to CpG-B ODN treatment. Further studies are needed to determine whether B-CLL cells insensitive to CpG-B ODN treatment are from specific cytogenetic CLL subgroups and potentially correlate with the presence of certain cytogenetic aberrations. Nevertheless, these findings demonstrate that CpG-B ODN triggering of TLR9 activates B-CLL cells to undergo apoptosis.
TLR9 signaling by CpG-B ODN induces apoptotic death of B-CLL cells. (A) B-CLL cells were cultured in media with or without CpG 2216 or CpG 2006 for 9 days. Annexin V/PI-positive, TMRE-negative apoptotic (R1 gate) and annexin V/PI-negative, TMRE-positive viable (R2 gate) B-CLL cells in cultures were determined at the indicated time points. Flow cytometry data shown are representative for day 7 culture with indicated number (%) of viable B-CLL cells. Kinetic changes of viable B-CLL cell number in cultures are summarized in the adjacent bar graph. Data are the mean ± SD from 5 independent experiments with B-CLL cells from different CLL patients. *P < .01, viable B-CLL cells in media with versus without CpG ODN at each time point. (B[b]) B-CLL cells were cultured in media with or without the indicated CpG ODNs. Kinetic changes of annexin V/PI-negative, TMRE-positive B-CLL cells in cultures were determined. Data are representative results from 1 of 5 independent experiments with B-CLL cells from different CLL patients. The number (%) of viable B-CLL cells is indicated. (C) Representative results showing the addition of CpG-B ODNs to unpurified patient PBMCs containing different percentages (96%, 79%, or 45%) of B-CLL cells. (D) Data are results of day 5 cultures from independent experiments with purified B-CLL cells from the 23 CLL patients listed in Table 1. Each dot represents an individual patient, and the horizontal bar represents the median level. *P < .01, B-CLL cell numbers in cultures with versus without CpG ODNs. Annexin V/PI staining and TMRE staining were concurrently performed to determine the number of viable B-CLL cells in cultures with B-CLL cells from 10 CLL patients, and the results were comparable (data not shown).
TLR9 signaling by CpG-B ODN induces apoptotic death of B-CLL cells. (A) B-CLL cells were cultured in media with or without CpG 2216 or CpG 2006 for 9 days. Annexin V/PI-positive, TMRE-negative apoptotic (R1 gate) and annexin V/PI-negative, TMRE-positive viable (R2 gate) B-CLL cells in cultures were determined at the indicated time points. Flow cytometry data shown are representative for day 7 culture with indicated number (%) of viable B-CLL cells. Kinetic changes of viable B-CLL cell number in cultures are summarized in the adjacent bar graph. Data are the mean ± SD from 5 independent experiments with B-CLL cells from different CLL patients. *P < .01, viable B-CLL cells in media with versus without CpG ODN at each time point. (B[b]) B-CLL cells were cultured in media with or without the indicated CpG ODNs. Kinetic changes of annexin V/PI-negative, TMRE-positive B-CLL cells in cultures were determined. Data are representative results from 1 of 5 independent experiments with B-CLL cells from different CLL patients. The number (%) of viable B-CLL cells is indicated. (C) Representative results showing the addition of CpG-B ODNs to unpurified patient PBMCs containing different percentages (96%, 79%, or 45%) of B-CLL cells. (D) Data are results of day 5 cultures from independent experiments with purified B-CLL cells from the 23 CLL patients listed in Table 1. Each dot represents an individual patient, and the horizontal bar represents the median level. *P < .01, B-CLL cell numbers in cultures with versus without CpG ODNs. Annexin V/PI staining and TMRE staining were concurrently performed to determine the number of viable B-CLL cells in cultures with B-CLL cells from 10 CLL patients, and the results were comparable (data not shown).
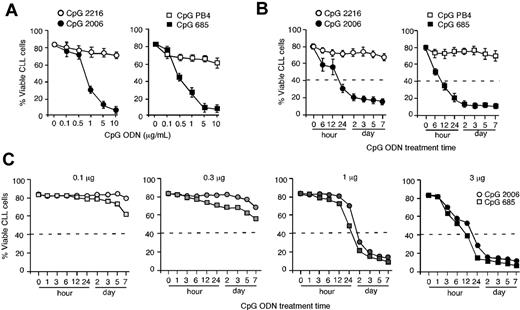
CpG-B ODN-induced apoptosis of B-CLL cells is treatment time- and dose-dependent
Purified B-CLL cells were incubated with graded doses of CpG ODNs in a 5-day culture. CpG-B ODNs (2006 or 685) but not CpG-A ODNs (2216 or PB4) potently induced apoptosis and decreased B-CLL cell number in a CpG ODN dose-dependent manner (Figure 3A). A 50% reduction of B-CLL cells was achieved with just 12- to 24-hour exposure to CpG 2006 or CpG-B 685 at 3 μg/mL but was unachievable with the same dose of CpG 2216 or CpG PB4 at all treatment time points up to 7 days (Figure 3B). A 50% reduction of B-CLL cells was also achieved with 48-hour exposure to CpG 2006 or CpG 685 at 1 μg/mL but was unachievable when the dose of CpG 2006 or CpG 685 dropped to 0.3 μg/mL or lower in the 7-day culture (Figure 3C). These findings demonstrate that CpG-B ODN-induced apoptosis of B-CLL cells is treatment time- and dose-dependent.
CpG-B ODN-induced apoptosis in B-CLL cells is treatment time- and dose-dependent. (A) B-CLL cells were cultured with various doses of the CpG ODNs for 5 days. Data are the mean ± SD from 3 independent experiments with B-CLL cells from different CLL patients. (B) B-CLL cells were exposed to the indicated CpG ODNs (3 μg/mL) for different time lengths, washed, and cultured in media for a total of 7 days. Data are the mean ± SD from 3 independent experiments with B-CLL cells from different CLL patients. (C) B-CLL cells were exposed to different doses of CpG-B ODNs for different time lengths, washed, and cultured in media for a total of 7 days. Data are representative results from 1 of 2 experiments. The dashed lines indicate the required treatment time and/or dose of CpG ODN to reach the 50% decrease of B-CLL cells.
CpG-B ODN-induced apoptosis in B-CLL cells is treatment time- and dose-dependent. (A) B-CLL cells were cultured with various doses of the CpG ODNs for 5 days. Data are the mean ± SD from 3 independent experiments with B-CLL cells from different CLL patients. (B) B-CLL cells were exposed to the indicated CpG ODNs (3 μg/mL) for different time lengths, washed, and cultured in media for a total of 7 days. Data are the mean ± SD from 3 independent experiments with B-CLL cells from different CLL patients. (C) B-CLL cells were exposed to different doses of CpG-B ODNs for different time lengths, washed, and cultured in media for a total of 7 days. Data are representative results from 1 of 2 experiments. The dashed lines indicate the required treatment time and/or dose of CpG ODN to reach the 50% decrease of B-CLL cells.
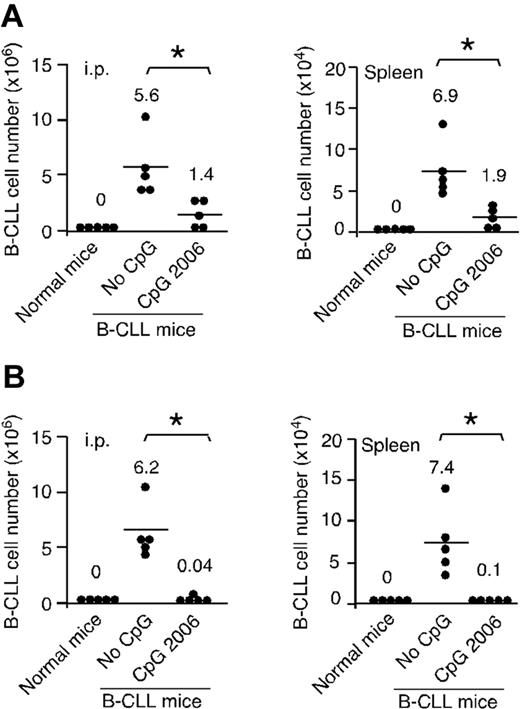
CpG-B ODN treatment reduces the number of B-CLL cells engrafted in NOD-scid mice
To determine whether in vitro pretreatment of B-CLL cells with CpG 2006 can influence the engraftment and survival of leukemia cells in vivo, B-CLL cells with or without preincubation with CpG 2006 were injected intraperitoneally into NOD-scid mice. No human CD45+/CD19+ cells were detected in normal NOD-scid control mice. In contrast, hCD45+/CD19+ B-CLL cells were detected in the peritoneal cavity and spleen of the NOD-scid mice of No-CpG group. Mice injected with CpG 2006 pretreated B-CLL cells had significantly decreased B-CLL cells, at 25% and 27.5% of the B-CLL cell numbers recovered from the mice of No-CpG group, respectively, confirming in vivo the in vitro finding that CpG 2006-treated B-CLL cells are programmed to undergo apoptosis (Figure 4A). In addition, in vivo administration of CpG 2006 significantly decreased the number of B-CLL cells engrafted in the peritoneal cavity and spleen of NOD-scid mice, at 0.64% and 1.4% of the B-CLL cell numbers recovered from the No-CpG group mice, respectively (Figure 4B). These findings demonstrate that treatment of B-CLL cells with CpG 2006 in vitro and in vivo significantly inhibits the engraftment and survival of B-CLL cells in NOD-scid mice.
CpG ODN treatment reduces B-CLL cell engrafted in NOD-scid mouse xenograft models. (A) B-CLL cells with or without CpG 2006 pretreatment for 2 days were intraperitoneally injected into NOD-scid mice. After 5 days, B-CLL cells engrafted within the peritoneal cavity and spleen of NOD-scid mice were harvested and determined. (B) NOD-scid mice were intraperitoneally injected with fresh B-CLL cells followed by 5 daily intraperitoneal injections of CpG 2006 or PBS. After 5 days, the number of B-CLL cells within host peritoneal cavity and spleen were harvested and determined. (A-B) Results from 5 mice per group with B-CLL cells derived from different patients. Each dot represents an individual mouse, and the horizontal bar represents the median level. *P < .01, the number of B-CLL cells in mice with versus without CpG 2006 treatments. Naive NOD-scid mice were used as control.
CpG ODN treatment reduces B-CLL cell engrafted in NOD-scid mouse xenograft models. (A) B-CLL cells with or without CpG 2006 pretreatment for 2 days were intraperitoneally injected into NOD-scid mice. After 5 days, B-CLL cells engrafted within the peritoneal cavity and spleen of NOD-scid mice were harvested and determined. (B) NOD-scid mice were intraperitoneally injected with fresh B-CLL cells followed by 5 daily intraperitoneal injections of CpG 2006 or PBS. After 5 days, the number of B-CLL cells within host peritoneal cavity and spleen were harvested and determined. (A-B) Results from 5 mice per group with B-CLL cells derived from different patients. Each dot represents an individual mouse, and the horizontal bar represents the median level. *P < .01, the number of B-CLL cells in mice with versus without CpG 2006 treatments. Naive NOD-scid mice were used as control.
CpG ODN-induced apoptosis of B-CLL cells depends on NF-κB and JAK/STAT pathways
The molecular mechanisms involved in CpG-B ODN-mediated apoptosis of B-CLL cells are unknown. Treatment of B-CLL cells with CpG 2006 led to cleavage of caspase-9, caspase-3, and PARP in B-CLL cells at days 3 to 7 cultures (Figure 5A). Pretreatment of B-CLL cells with pan-caspase, caspase-3, or caspase-9 inhibitor significantly reduced CpG 2006-induced apoptosis of B-CLL cells (Figure 5B). These findings demonstrate that TLR9 signaling by CpG ODN activates the apoptotic pathway elements in B-CLL cells committing them to death, and this effect can be inhibited by specific caspase inhibitors.
CpG ODN-induced apoptosis of B-CLL cells depends on NF-κB and JAK/STAT signaling pathways and tyrosine phosphorylation of STAT1. (A) Cleavage of caspase-9, caspase-3, and PARP in B-CLL cells cultured with CpG 2006 were determined by Western blots at the indicated time points. Data are representative results from 2 of 3 independent experiments with B-CLL cells from different CLL patients. (B) B-CLL cells were cultured with or without CpG 2006 for 5 days in the presence or absence of pan-caspase, caspase-3, or caspase-9 inhibitor. Data are the mean ± SD from 3 independent experiments. *P < .01, B-CLL cell numbers versus CpG 2006 only group. (C) B-CLL cells with or without CAPE and/or AG490 pretreatment were cultured in media with or without CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in CAPE and/or AG490 pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 5 independent experiments. Western blots of AG490 pretreatment on CpG 2006-induced activation and cleavage of caspase-9, caspase-3, and PARP in B-CLL cells at day 5 cultures. (D[b]) Western blot time course of CpG 2006-induced tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells from 5 patients. Data are densitometrically assessed and presented as the mean ± SD in the adjacent bar diagram on the right. *P < .01, STAT1 expression in B-CLL cells before versus at each time point after CpG 2006 stimulation. (E) Western blots of tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells with or without CAPE or AG490 pretreatment and cultured in media with or without CpG 2006 stimulation were determined at the indicated time points. Data are representative results of 3 independent experiments. (F) Western blots of STAT1 antisense ODN on total, tyrosine-, or serine-phosphorylated forms of STAT1 expression in B-CLL cells cultured with or without CpG 2006 for 2 days. Data are representative results of 3 independent experiments. (G) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured in the presence or absence of CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in STAT1 antisense ODN or STAT1 sense ODN pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 3 independent experiments. (H) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured with CpG 2006 for 3 days. Expression of surface markers (shaded histogram) on fresh or cultured B-CLL cells was analyzed by flow cytometry, indicated with MFI number, and overlaid with isotype control (unshaded histogram). Data are representative results from 1 of 5 independent experiments. The adjacent diagrams on the right are MFI fold increases of the indicated surface molecules on day 3 cultured versus uncultured B-CLL cells from 5 CLL patients. *P < .01. The horizontal bar represents the median level with the indicated fold increase number.
CpG ODN-induced apoptosis of B-CLL cells depends on NF-κB and JAK/STAT signaling pathways and tyrosine phosphorylation of STAT1. (A) Cleavage of caspase-9, caspase-3, and PARP in B-CLL cells cultured with CpG 2006 were determined by Western blots at the indicated time points. Data are representative results from 2 of 3 independent experiments with B-CLL cells from different CLL patients. (B) B-CLL cells were cultured with or without CpG 2006 for 5 days in the presence or absence of pan-caspase, caspase-3, or caspase-9 inhibitor. Data are the mean ± SD from 3 independent experiments. *P < .01, B-CLL cell numbers versus CpG 2006 only group. (C) B-CLL cells with or without CAPE and/or AG490 pretreatment were cultured in media with or without CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in CAPE and/or AG490 pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 5 independent experiments. Western blots of AG490 pretreatment on CpG 2006-induced activation and cleavage of caspase-9, caspase-3, and PARP in B-CLL cells at day 5 cultures. (D[b]) Western blot time course of CpG 2006-induced tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells from 5 patients. Data are densitometrically assessed and presented as the mean ± SD in the adjacent bar diagram on the right. *P < .01, STAT1 expression in B-CLL cells before versus at each time point after CpG 2006 stimulation. (E) Western blots of tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells with or without CAPE or AG490 pretreatment and cultured in media with or without CpG 2006 stimulation were determined at the indicated time points. Data are representative results of 3 independent experiments. (F) Western blots of STAT1 antisense ODN on total, tyrosine-, or serine-phosphorylated forms of STAT1 expression in B-CLL cells cultured with or without CpG 2006 for 2 days. Data are representative results of 3 independent experiments. (G) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured in the presence or absence of CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in STAT1 antisense ODN or STAT1 sense ODN pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 3 independent experiments. (H) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured with CpG 2006 for 3 days. Expression of surface markers (shaded histogram) on fresh or cultured B-CLL cells was analyzed by flow cytometry, indicated with MFI number, and overlaid with isotype control (unshaded histogram). Data are representative results from 1 of 5 independent experiments. The adjacent diagrams on the right are MFI fold increases of the indicated surface molecules on day 3 cultured versus uncultured B-CLL cells from 5 CLL patients. *P < .01. The horizontal bar represents the median level with the indicated fold increase number.
TLR9 uses a myeloid differentiation factor 88-dependent pathway to induce expression of NF-κB-dependent target genes that promote inflammatory responses and cytokine production. Thus, a potential mechanism for CpG-B ODN to induce apoptosis of B-CLL cells is via autocrine cytokine stimulation by CpG ODN-triggered B-CLL cells. Testing this hypothesis, we examined whether the NF-κB signaling pathway inhibitor CAPE or the JAK/STAT pathway inhibitor AG490 could block CpG 2006-induced apoptosis of B-CLL cells. Pretreatment of B-CLL cells with CAPE and/or AG490 at the indicated treatment time and dose did not directly affect the viability of B-CLL cells but significantly reduced CpG 2006-induced apoptosis of B-CLL cells (Figure 5C). Moreover, pretreatment of B-CLL cells with AG490 effectively blocked CpG 2006-induced cleavage of caspase-9, caspase-3, and PARP in B-CLL cells (Figure 5C). These findings suggest that apoptosis induced by TLR9-CpG ODN ligation is dependent on the NF-κB and JAK/STAT signaling pathways. Preventing apoptosis by AG490 suggests that CpG ODN-induced apoptosis probably occurs via an indirect mechanism involving autocrine cytokines that provoke apoptosis of B-CLL cells.
CpG ODN alters STAT1 phosphorylation, thereby provoking apoptosis in B-CLL cells
Cytokines implicated in the pathogenesis of B-CLL have used the JAKs and STATs to transmit growth and survival signals. Constitutive activation of STAT1 can play a key role in leukemogenesis of acute and chronic leukemias.10 STAT1 is constitutively phosphorylated on a carboxy terminal serine residue (Ser727) in B-CLL cells but not in normal B cells.7 In contrast, tyrosine phosphorylation was not detected on any STATs in B-CLL cells.7 Our results confirmed that freshly isolated B-CLL cells expressed STAT1 constitutively phosphorylated on serine (pSer727-STAT1) but not tyrosine (pTyr701-STAT1; Figure 5D[b]). TLR9-CpG ODN ligation induced a significant increase of pTyr701-STAT1 expression in CpG 2006-treated B-CLL cells, which was detected at 24 hours, and increased steadily over the 72-hour culture (Figure 5D). The expression of pSer727-STAT1 in B-CLL cells was detected before and after CpG 2006 stimulation, transiently increased at 24 to 48 hours, and was maintained at high levels over the 72-hour culture. Pretreatment of B-CLL cells with CAPE or AG490 alone did not activate or alter STAT1 phosphorylation; however, CAPE and AG490 each effectively abolished CpG 2006-induced pTyr701-STAT1 expression without affecting pSer727-STAT1 expression in B-CLL cells (Figure 5E). These data provide additional evidence that CpG-B ODN-induced apoptosis of B-CLL cells occurs via the JAK/STAT pathway. Together with the finding that CAPE and AG490 each block CpG 2006-induced apoptosis in B-CLL cells, CpG ODN-induced tyrosine phosphorylation of STAT1 correlated with the induction of apoptosis.
To definitively determine whether STAT1 tyrosine phosphorylation impacts CpG ODN-induced apoptosis of B-CLL cells, antisense ODN against STAT1 was introduced to B-CLL cells before CpG 2006 stimulation. Pretreatment of B-CLL cells with STAT1 antisense ODN completely abolished pTyr701-STAT1, pSer727-STAT1, and total STAT1 expression in B-CLL cells (Figure 5F). STAT1 antisense ODN did not affect the survival of untreated B-CLL cells but significantly prevented CpG 2006-mediated apoptosis of B-CLL cells. The control STAT1 sense ODN did not affect the survival of untreated B-CLL cells and the apoptosis of CpG 2006-treated B-CLL cells (Figure 5G). Interestingly, pretreatment of B-CLL cells with these non-CpG STAT1 antisense or sense ODN did not interfere with CpG 2006-induced activation of B-CLL cells (Figure 5H). These results demonstrate that TLR9 signaling by CpG 2006 leads to tyrosine phosphorylation of STAT1, thereby provoking apoptosis of B-CLL cells.
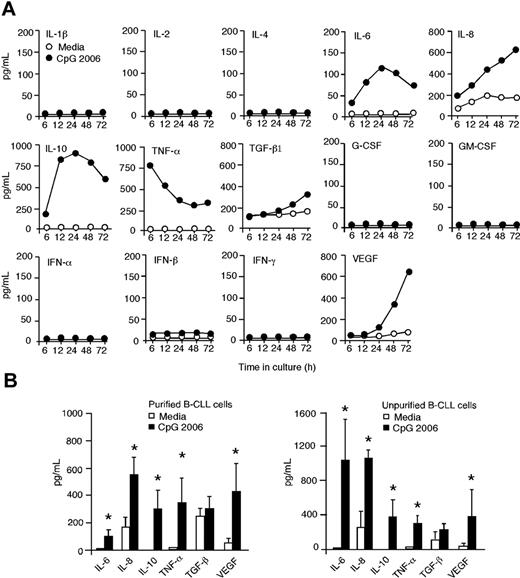
CpG ODN induces IL-10 to provoke STAT1 phosphorylation and apoptosis in B-CLL cells
One important biologic feature of CLL is the capacity of B-CLL cells to produce autocrine cytokines that impact their survival and apoptosis. To determine whether cytokine(s) are responsible for STAT1 tyrosine phosphorylation and apoptosis in CpG-activated B-CLL cells, supernatants from B-CLL cell cultures with or without CpG 2006 were assessed at time points up to 72 hours. Triggering TLR9 with CpG 2006 led to the production of IL-6, IL-8, IL-10, TNF-α, and VEGF by B-CLL cells (Figure 6A). A profound increase of IL-10 production was detected at 6 hours, peaked at 24 hours, and remained at high levels over the 72-hour culture. TNF-α production was significantly increased, peaked within 6 hours, decreased gradually but maintained at elevated levels over the 72-hour culture. No detectable levels of IL-1β, IL-2, IL-4, G-CSF, GM-CSF, IFN-α, IFN-β, and IFN-γ were produced by B-CLL cells in cultures with or without CpG 2006 (Figure 6A). The amounts (pg/mL) of IL-6 (98.7 vs 1.7), IL-8 (560.1 vs 178.8), IL-10 (287.6 vs undetectable), TNF-α (331.8 vs 2.9), and VEGF (417.5 vs 52.5) produced by B-CLL cells with or without CpG 2006 were significantly different (Figure 6B). TGF-β1 was slightly increased, but the difference was insignificant (313.3 ± 77.9 vs 246.3 ± 58.0). Similar cytokine profiles were detected in supernatants from unpurified PBMCs containing high percentages (> 70%) of B-CLL cells in cultures with or without CpG 2006 (Figure 6B).
TLR9 signaling by CpG ODN induces autocrine cytokine production by B-CLL cells. (A) Kinetics of cytokine production by purified B-CLL cells in cultures with or without CpG 2006 at the indicated time points (hours). The data shown are representative results from 1 of 3 experiments with CLL cells from different patients. (B) Aggregated results (n = 5, mean ± SD) of cytokine production by purified B-CLL cells or unpurified PBMCs containing more than 70% of B-CLL cells from patients 1 to 5 in cultures with or without CpG 2006 for 72 hours. *P < .01, each cytokine production by B-CLL cells in cultures with versus without CpG 2006.
TLR9 signaling by CpG ODN induces autocrine cytokine production by B-CLL cells. (A) Kinetics of cytokine production by purified B-CLL cells in cultures with or without CpG 2006 at the indicated time points (hours). The data shown are representative results from 1 of 3 experiments with CLL cells from different patients. (B) Aggregated results (n = 5, mean ± SD) of cytokine production by purified B-CLL cells or unpurified PBMCs containing more than 70% of B-CLL cells from patients 1 to 5 in cultures with or without CpG 2006 for 72 hours. *P < .01, each cytokine production by B-CLL cells in cultures with versus without CpG 2006.
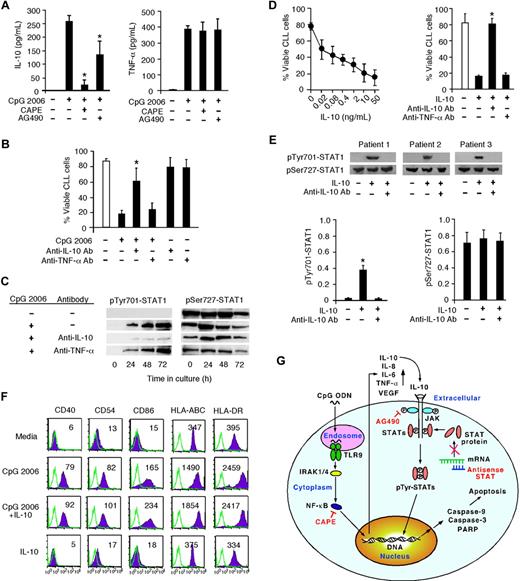
The profound increases of IL-10 and TNF-α production by CpG 2006-activated B-CLL cells suggest that these cytokines may have a role in the apoptotic induction of B-CLL cells. Intriguingly, treating B-CLL cells with CAPE before CpG 2006 stimulation significantly blocked IL-10 production, reduced the elevated IL-6, IL-8, and VEGF production, but did not affect TNF-α production by CpG 2006-activated B-CLL cells (Figure 7A; data not shown). Treating B-CLL cells with AG490 slightly lowered IL-10 production but did not influence TNF-α production by CpG 2006-activated B-CLL cells. These results suggest that IL-10 might be the cytokine responsible for STAT1 modulation and apoptotic induction in CpG 2006-activated B-CLL cells. To test this hypothesis, anti–IL-10 or anti–TNF-α Abs were added into CpG 2006-stimulated B-CLL cell cultures. The addition of anti–IL-10 Abs significantly protected the survival of B-CLL cells, whereas anti–TNF-α Abs did not influence the survival of B-CLL cells in CpG 2006-stimulated cultures (Figure 7B). Moreover, anti–IL-10 Abs, but not anti–TNF-α Abs, significantly inhibited tyrosine phosphorylation of STAT1 (Figure 7C). Furthermore, adding exogenous rh-IL-10 into B-CLL cell cultures induced apoptotic B-CLL cells in an IL-10 dose-dependent manner, which could be specifically blocked by anti–IL-10 Abs (Figure 7D). These results confirmed that IL-10 can induce apoptosis of B-CLL cells, and demonstrated that TLR9 signaling by CpG-B ODN actually uses autocrine IL-10 to mediate the induction of apoptosis of B-CLL cells. In addition, treatment of B-CLL cells with IL-10 leads to tyrosine-phosphorylated STAT1 expression in B-CLL cells, which can be effectively blocked by anti–IL-10 Abs (Figure 7E). Notably, the addition of IL-10 into B-CLL cell cultures did not directly induce the activation of B-CLL cells or affect CpG 2006-induced activation of B-CLL cells (Figure 7F). Together, these findings demonstrate the molecular mechanism of TLR9-CpG ODN ligation induces apoptosis of human B-CLL cells is via the altered production of autocrine IL-10, which activates tyrosine-phosphorylation of STAT1 and thereby provokes apoptosis of B-CLL cells (Figure 7G).
CpG ODN uses autocrine IL-10 to provoke STAT1 tyrosine phosphorylation and apoptosis in B-CLL cells. (A) IL-10 and TNF-α production at 72 hours by CpG 2006-stimulated B-CLL cells with or without CAPE or AG490 pretreatment. Data are the mean ± SD from 5 independent experiments. *P < .01, IL-10 production by CpG 2006-stimulated B-CLL cells with versus without CAPE or AG 490 pretreatments. (B) Viable B-CLL cell number in day 5 cultures with or without CpG 2006 and in the presence or absence of anti–IL-10 or anti–TNF-α Abs. Data are the mean ± SD from 5 independent experiments. *P < .01, the viable B-CLL cell number of anti–IL-10 Ab group versus the CpG 2006 only group. (C) Western blot time course of tyrosine-phosphorylated or serine-phosphorylated STAT1 expression in CpG 2006-stimulated B-CLL cells in the presence or absence of anti–IL-10 or anti–TNF-α Abs. The data shown are representative results from 1 of 2 experiments. (D) Dose effect of IL-10 on B-CLL cell apoptosis in culture at day 5. Data are the mean ± SD from 7 independent experiments with B-CLL cells from 7 individual CLL patients (left panel). The number (%) of viable B-CLL cells in IL-10 (5 ng/mL) cultures with or without anti–IL-10 or anti–TNF-α Abs was determined at day 5. Data are the mean ± SD from 5 independent experiments with B-CLL cells from different CLL patients (right panel). *P < .01, B-CLL cell numbers in IL-10 cultures with versus anti–IL-10 or anti–TNF-α Abs. (E) Western blot of tyrosine-phosphorylated or serine-phosphorylated STAT1 expression in B-CLL cells cultured with or without IL-10 in the presence or absence of anti–IL-10 Abs was determined at 24 hours of culture. The data shown are representative results from 3 of 5 independent experiments. Data from the 5 CLL patients are densitometrically assessed and presented as the mean ± SD in the adjacent bar diagrams. *P < .01, STAT1 expression in B-CLL cells in IL-10 cultures with versus without anti–IL-10 Abs to the media only group. (F) Expression of the indicated surface markers on B-CLL cells cultured with or without CpG 2006 and/or IL-10 was analyzed at day 3, indicated with MFI number, and overlaid with isotype control. Data are representative results from 1 of 3 independent experiments with B-CLL cells derived from different CLL patients. (G) Schematic representation of TLR9-CpG ODN ligation induced apoptotic pathway in B-CLL cells. CpG ODN is recognized by TLR9 and ligated with it in the endosome engaging an intracellular pathway and leading to NF-κB activation and translocation. Binding of activated NF-κB fragments to DNA induces the production of cytokines (eg, IL-10, TNF-α). Extracellular binding of IL-10 with its receptor activates JAK/STAT pathway-dependent tyrosine phosphorylation of STAT proteins, leading to the activation and cleavage of caspase-9, caspase-3, and PARP, and consequent apoptosis of B-CLL cells. CAPE specifically inhibits NF-κB activation. AG490 blocks JAK phosphorylation, thus blocking the JAK/STAT signaling pathway. STAT1 antisense ODN blocks STAT monomer production from mRNA.
CpG ODN uses autocrine IL-10 to provoke STAT1 tyrosine phosphorylation and apoptosis in B-CLL cells. (A) IL-10 and TNF-α production at 72 hours by CpG 2006-stimulated B-CLL cells with or without CAPE or AG490 pretreatment. Data are the mean ± SD from 5 independent experiments. *P < .01, IL-10 production by CpG 2006-stimulated B-CLL cells with versus without CAPE or AG 490 pretreatments. (B) Viable B-CLL cell number in day 5 cultures with or without CpG 2006 and in the presence or absence of anti–IL-10 or anti–TNF-α Abs. Data are the mean ± SD from 5 independent experiments. *P < .01, the viable B-CLL cell number of anti–IL-10 Ab group versus the CpG 2006 only group. (C) Western blot time course of tyrosine-phosphorylated or serine-phosphorylated STAT1 expression in CpG 2006-stimulated B-CLL cells in the presence or absence of anti–IL-10 or anti–TNF-α Abs. The data shown are representative results from 1 of 2 experiments. (D) Dose effect of IL-10 on B-CLL cell apoptosis in culture at day 5. Data are the mean ± SD from 7 independent experiments with B-CLL cells from 7 individual CLL patients (left panel). The number (%) of viable B-CLL cells in IL-10 (5 ng/mL) cultures with or without anti–IL-10 or anti–TNF-α Abs was determined at day 5. Data are the mean ± SD from 5 independent experiments with B-CLL cells from different CLL patients (right panel). *P < .01, B-CLL cell numbers in IL-10 cultures with versus anti–IL-10 or anti–TNF-α Abs. (E) Western blot of tyrosine-phosphorylated or serine-phosphorylated STAT1 expression in B-CLL cells cultured with or without IL-10 in the presence or absence of anti–IL-10 Abs was determined at 24 hours of culture. The data shown are representative results from 3 of 5 independent experiments. Data from the 5 CLL patients are densitometrically assessed and presented as the mean ± SD in the adjacent bar diagrams. *P < .01, STAT1 expression in B-CLL cells in IL-10 cultures with versus without anti–IL-10 Abs to the media only group. (F) Expression of the indicated surface markers on B-CLL cells cultured with or without CpG 2006 and/or IL-10 was analyzed at day 3, indicated with MFI number, and overlaid with isotype control. Data are representative results from 1 of 3 independent experiments with B-CLL cells derived from different CLL patients. (G) Schematic representation of TLR9-CpG ODN ligation induced apoptotic pathway in B-CLL cells. CpG ODN is recognized by TLR9 and ligated with it in the endosome engaging an intracellular pathway and leading to NF-κB activation and translocation. Binding of activated NF-κB fragments to DNA induces the production of cytokines (eg, IL-10, TNF-α). Extracellular binding of IL-10 with its receptor activates JAK/STAT pathway-dependent tyrosine phosphorylation of STAT proteins, leading to the activation and cleavage of caspase-9, caspase-3, and PARP, and consequent apoptosis of B-CLL cells. CAPE specifically inhibits NF-κB activation. AG490 blocks JAK phosphorylation, thus blocking the JAK/STAT signaling pathway. STAT1 antisense ODN blocks STAT monomer production from mRNA.
Discussion
This study provides the first demonstration that TLR9 signaling by CpG-B ODN leads to an NF-κB-dependent production of autocrine IL-10, which activates JAK/STAT pathway-dependent tyrosine phosphorylation of STAT1 proteins and thereby provokes an apoptotic death pathway in B-CLL cells. These findings provide new understanding of CpG ODN-mediated direct antitumor effects and new insights into the biology and therapy of human B-cell leukemias. Although the molecular mechanism(s) responsible for the survival and accumulation of B-CLL cells remains unknown, studies have suggested that B-CLL cells probably acquire resistance to apoptosis via aberrant up-regulated activities of kinase proteins in signaling pathways.6-10 Constitutive activation of STATs can play an important role in leukemogenesis of B-CLL.10 STAT1 has a proapoptotic role by transmitting apoptotic signals and inducing proapoptotic regulatory genes, such as caspases, fas ligand, and TNF-related apoptosis-inducing ligand.34-36 Constitutive serine phosphorylation without tyrosine phosphorylation of STATs may enhance prosurvival signals in B-CLL cells.7,36 Our results confirm that B-CLL cells expressed STAT1 constitutively phosphorylated on serine, but not tyrosine, and demonstrate that TLR9 signaling by CpG-B ODN induces tyrosine phosphorylation of STAT1 in B-CLL cells. We think that this TLR9-CpG ODN ligation triggered NF-κB, IL-10, JAK/STAT pathway represents the main pathways for CpG-B ODN-mediated apoptosis in B-CLL cells. In a CD154 gene therapy study, tyrosine phosphorylation of STAT1 was detected in B-CLL cells in the majority of patients 24 hours after treatment and correlated with the decreased circulating leukemia cell counts.37 These clinical observations agree with our findings that tyrosine phosphorylation of STAT1 promotes apoptosis in B-CLL cells.
There are several advantages supporting the development of TLR9-targeted immunotherapy for CLL. First, triggering TLR9 with CpG-B ODN induces apoptosis of B-CLL cells. This finding indicates that CpG-B ODN have a direct antileukemia effect as immunotherapeutic drugs for CLL. Although CLL is a heterogeneous B-cell malignancy with frequent and various cytogenetic abnormalities and defects in individual patients, the results from our study and another group38 show that CpG ODN can induce apoptosis in most B-CLL samples. Second, TLR9-targeting of B-CLL cells with CpG-B ODN activates leukemia B cells and induces dramatic changes in morphology and phenotype. The resulting leukemia blasts might be more sensitive to chemotherapy drugs and more susceptible to killing by NK and T cells. Third, B-CLL cells are known to express tumor-associated antigens (eg, survivin) that are recognized by T cells39,40 but are poor antigen-presenting cells (APCs) because of low expression of costimulatory molecules essential for T-cell activation.32,33 CLL cells with poor APC function can also result in a contact-dependent impairment of immunologic synapse formation with T cells in CLL patients.41,42 These defects can probably be overcome by treating B-CLL cells with TLR9 agonists. Fourth, B-CLL cells produce immunosuppressive cytokines that contribute to the defects in T-cell responses.13 Identifying TLR agonists that could preferentially alter B-CLL cell cytokine production to polarize a Th1 response may reverse T-cell abnormalities and enhance antileukemia responses in CLL patients. Fifth, dendritic cells (DCs) in CLL patients are dysfunctional and unable to stimulate an effective T-cell response,43 which has prompted the use of in vitro generated DC vaccines against CLL.40,44 CpG ODNs and CpG-induced cytokines can potently enhance DC maturation and their APC function, which may reverse the dysfunction of endogenous DCs. In addition, CpG-induced apoptotic B-CLL cells will be readily taken up by DCs to present tumor antigens to elicit T-cell antitumor responses. The further development of CpG-based immunotherapeutic approaches for CLL requires better understanding in the interactions of CpG-treated CLL cells with various immune effecter cells. Our studies in murine leukemia and brain glioma models have shown that administration of CpG ODNs induced in vivo activation of DCs to activate NK and T cell-mediated antitumor responses with potent therapeutic efficacy.45,46 Adding CpG ODN to PBMCs from lymphoma patients induced activation of blood DCs to enhance T-cell and NK-cell function.47 Besides the immune-enhancing effects, CpG ODNs are stable, chemically well defined, inexpensive, and safe agents with clinical drugs available for studies. These points support the development of TLR9-targeted therapy with CpG-B ODN as a therapeutic agent for CLL. Whether CpG-induced IL-10 production by B-CLL cells may alter serum IL-10 levels in CLL patients and affect the microenvironment with potential immunosuppresive effect on host immune responses is subject to further investigation in clinical trials.
Our results show that human B-CLL cells express TLR1, TLR6, TLR7, TLR9, and TLR10, with significant higher TLR9 expression in B-CLL cells than in normal B cells. Given that human naive B cells express undetectable levels of TLR9, triggering the B-cell receptor up-regulates the expression of TLR9 in naive B cells, and that memory B cells express high levels of TLR9,48 our finding of high TLR9 expression in fresh B-CLL cells from patients indicates that B-CLL cells are probably derived from an “experienced” mature B cell or a memory B cell. Among the leukocyte populations examined to date,28,49,50 TLR9 is expressed on human B cells and plasmacytoid DCs only. Therefore, TLR9-targeted immunotherapy of CLL is relatively specific for B-CLL cells. At least 3 distinct classes of CpG ODN with structural and functional differences have been identified.31 CpG-B ODNs are more potent TLR9 agonists than CpG-A ODNs for stimulating normal human B cells. Our results further demonstrated that CpG-B ODNs, but not CpG-A ODNs, induced significant activation and apoptosis of leukemic B cells. Given that B-CLL progresses slowly and can be treated to achieve a period of clinical remission, it is an ideal disease model for allowing the application of TLR agonists to manipulate host immune responses against leukemia cells in a minimum residual disease setting.
In conclusion, our results demonstrate that TLR9 targeting of B-CLL cells with CpG-B ODN induced NF-κB-dependent production of autocrine IL-10, which induces tyrosine phosphorylation of STAT1, thereby leading to apoptosis of B-CLL cells. This new understanding supports the development of TLR9-targeted therapy with CpG ODN as a therapeutic agent for treating human CLL. Both CpG 2006 (also known as CpG 7909 and PF-3512676) and CpG 685 (also known as GNKG168) are being tested in phase 1 clinical trials as monotherapy in patients with relapsed or refractory B-CLL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukemia & Lymphoma Society (research grants 6188-05 and R6241-08), Randy Shaver Cancer Research and Community Fund, and the Leukemia Research Fund (W.C.); National Institutes of Health (AI050737) and Leukemia & Lymphoma Society (Scholar award; M.A.F.); and National Institutes of Health (R01 CA72669; B.R.B.).
National Institutes of Health
Authorship
Contribution: X.L. and E.A.M. performed research, analyzed data, and wrote the paper; V.B. and D.J.W. provided blood samples and clinical information of the CLL patients; M.A.F. and B.R.B. designed research and revised the paper; and W.C. designed and directed the overall study, analyzed data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wei Chen, University of Minnesota Cancer Center, MMC 806, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: chenw@umn.edu.

![Figure 2. TLR9 signaling by CpG-B ODN induces apoptotic death of B-CLL cells. (A) B-CLL cells were cultured in media with or without CpG 2216 or CpG 2006 for 9 days. Annexin V/PI-positive, TMRE-negative apoptotic (R1 gate) and annexin V/PI-negative, TMRE-positive viable (R2 gate) B-CLL cells in cultures were determined at the indicated time points. Flow cytometry data shown are representative for day 7 culture with indicated number (%) of viable B-CLL cells. Kinetic changes of viable B-CLL cell number in cultures are summarized in the adjacent bar graph. Data are the mean ± SD from 5 independent experiments with B-CLL cells from different CLL patients. *P < .01, viable B-CLL cells in media with versus without CpG ODN at each time point. (B[b]) B-CLL cells were cultured in media with or without the indicated CpG ODNs. Kinetic changes of annexin V/PI-negative, TMRE-positive B-CLL cells in cultures were determined. Data are representative results from 1 of 5 independent experiments with B-CLL cells from different CLL patients. The number (%) of viable B-CLL cells is indicated. (C) Representative results showing the addition of CpG-B ODNs to unpurified patient PBMCs containing different percentages (96%, 79%, or 45%) of B-CLL cells. (D) Data are results of day 5 cultures from independent experiments with purified B-CLL cells from the 23 CLL patients listed in Table 1. Each dot represents an individual patient, and the horizontal bar represents the median level. *P < .01, B-CLL cell numbers in cultures with versus without CpG ODNs. Annexin V/PI staining and TMRE staining were concurrently performed to determine the number of viable B-CLL cells in cultures with B-CLL cells from 10 CLL patients, and the results were comparable (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/24/10.1182_blood-2009-03-213363/4/m_zh89991053370002.jpeg?Expires=1769092340&Signature=tPUsCCOo-CuWDSNAlhrvG3LSodH-y1Nz8wWUvzs9KgVUiIopEZCi0REIcNqWZFRZ9rHo~ZGJLpAdXB5t~tFf25z98QJUyJZ~kt6VYS3M1~TEPtMGrRcnZJ7LA9-I9aElnjpOCjkz78z2GwT8p7Okcq18~Qqn0qC2hKfeJwWCk-P2IewpuSxcoU3tB6p~V~vyRjDzlPql5Hd536YF28dcekHvlKwyW0sxfuebZfT01hhFy4G01aVg7lRFGFwlIixNuej-oxUA294NciXMiHfwc8Em-7pvf866zA0tiNw2iVH3KnGntxEIItQwhsr8XNZ~CQlkmj0PkIYltHi6rusFNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CpG ODN-induced apoptosis of B-CLL cells depends on NF-κB and JAK/STAT signaling pathways and tyrosine phosphorylation of STAT1. (A) Cleavage of caspase-9, caspase-3, and PARP in B-CLL cells cultured with CpG 2006 were determined by Western blots at the indicated time points. Data are representative results from 2 of 3 independent experiments with B-CLL cells from different CLL patients. (B) B-CLL cells were cultured with or without CpG 2006 for 5 days in the presence or absence of pan-caspase, caspase-3, or caspase-9 inhibitor. Data are the mean ± SD from 3 independent experiments. *P < .01, B-CLL cell numbers versus CpG 2006 only group. (C) B-CLL cells with or without CAPE and/or AG490 pretreatment were cultured in media with or without CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in CAPE and/or AG490 pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 5 independent experiments. Western blots of AG490 pretreatment on CpG 2006-induced activation and cleavage of caspase-9, caspase-3, and PARP in B-CLL cells at day 5 cultures. (D[b]) Western blot time course of CpG 2006-induced tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells from 5 patients. Data are densitometrically assessed and presented as the mean ± SD in the adjacent bar diagram on the right. *P < .01, STAT1 expression in B-CLL cells before versus at each time point after CpG 2006 stimulation. (E) Western blots of tyrosine- or serine-phosphorylated forms of STAT1 expression in B-CLL cells with or without CAPE or AG490 pretreatment and cultured in media with or without CpG 2006 stimulation were determined at the indicated time points. Data are representative results of 3 independent experiments. (F) Western blots of STAT1 antisense ODN on total, tyrosine-, or serine-phosphorylated forms of STAT1 expression in B-CLL cells cultured with or without CpG 2006 for 2 days. Data are representative results of 3 independent experiments. (G) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured in the presence or absence of CpG 2006 for 5 days. *P < .01, the number of viable B-CLL cells in STAT1 antisense ODN or STAT1 sense ODN pretreated group versus B-CLL cells cultured with CpG 2006 only. Data are the mean ± SD from 3 independent experiments. (H) B-CLL cells with or without STAT1 antisense ODN or STAT1 sense ODN pretreatment were cultured with CpG 2006 for 3 days. Expression of surface markers (shaded histogram) on fresh or cultured B-CLL cells was analyzed by flow cytometry, indicated with MFI number, and overlaid with isotype control (unshaded histogram). Data are representative results from 1 of 5 independent experiments. The adjacent diagrams on the right are MFI fold increases of the indicated surface molecules on day 3 cultured versus uncultured B-CLL cells from 5 CLL patients. *P < .01. The horizontal bar represents the median level with the indicated fold increase number.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/24/10.1182_blood-2009-03-213363/4/m_zh89991053370005.jpeg?Expires=1769092340&Signature=2QI1FYkNcxbzzspprON7l0Ici-Fg4ZXXUdAtKPfsxWi7WH7~4bWlX-j07cXTBvi4J4mbN9tALqa9hwsxe~69~yAolNrnIzhXdmP41z~z8y7ux8kglRLUxY5pKEKVvW3AFp2NtA-nD2U2CUwpey6evV3PHw~1sRh6zP1tHVBgr0Kab3GQ3BR6yUI2r~AewcbTemF2EJAebBG14A5LytoUfxXRw~j50GgPOndQXlIUQzQ9ezy9yYUHc8SYdt67I5znWGiOYXGvJy0UGuwwyznFMIdZcCxUKCvHBdVft5ThyQNtFGIDQXuM3efP2WZeGFt8FbdfbdTSDvKnk5C6STVabA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal