Abstract
Complement receptor 2–negative (CR2/CD21−) B cells have been found enriched in patients with autoimmune diseases and in common variable immunodeficiency (CVID) patients who are prone to autoimmunity. However, the physiology of CD21−/lo B cells remains poorly characterized. We found that some rheumatoid arthritis (RA) patients also display an increased frequency of CD21−/lo B cells in their blood. A majority of CD21−/lo B cells from RA and CVID patients expressed germline autoreactive antibodies, which recognized nuclear and cytoplasmic structures. In addition, these B cells were unable to induce calcium flux, become activated, or proliferate in response to B-cell receptor and/or CD40 triggering, suggesting that these autoreactive B cells may be anergic. Moreover, gene array analyses of CD21−/lo B cells revealed molecules specifically expressed in these B cells and that are likely to induce their unresponsive stage. Thus, CD21−/lo B cells contain mostly autoreactive unresponsive clones, which express a specific set of molecules that may represent new biomarkers to identify anergic B cells in humans.
Introduction
Random V(D)J recombination generates a large number of autoreactive B cells, which can be silenced in the bone marrow by 3 main tolerance mechanisms: deletion, receptor editing, and anergy.1-3 Deletion results in the removal of autoreactive clones by apoptosis, whereas receptor editing allows autoreactive B cells to alter their self-reactive B-cell receptor (BCR). This process may rescue immature B-cell clones from deletion and allow their differentiation to resume. In contrast to deletion and receptor editing, anergy does not remove autoreactive B-cell clones from the total B-cell population but renders them irresponsive to antigenic stimulation.4-7 Anergic autoreactive B cells remain in the periphery but they have a short life span, which ultimately results in their elimination.8,9
Initial reports have demonstrated that deletion is used mainly to eliminate B cells, which express highly autoreactive BCRs against membrane-bound antigens.10,11 However, receptor editing has since been shown to be the major B-cell tolerance mechanism against these antigens, and clonal deletion appears to be a default mechanism when receptor editing fails to silence autoreactive B cells.12 Alternatively, anergy appears to be preferentially induced in B cells that express moderately autoreactive BCRs toward soluble antigens.11 Using transgenic mouse models, anergic B cells have been described as unable to become activated, proliferate, or secrete antibodies upon BCR triggering (reviewed in Cambier et al7 ). Indeed, BCR signaling is abnormal in these cells and BCR aggregation fails to induce an increased concentration of intracellular calcium [Ca2+]i or tyrosine phosphorylation cascades. It is believed that this irresponsive state results from chronic BCR exposure to self-antigens, which desensitizes BCR signaling abilities.13,14 The characterization of unresponsive B cells in unmanipulated mice and in humans showed that anergic B cells represent a small percentage of circulating B cells.15,16
We report here that an unusual B-cell population, which down-regulates the complement receptor CR2/CD21 and was previously reported in systemic lupus erythematosus (SLE) and common variable immunodeficiency disease (CVID) patients, develops in some rheumatoid arthritis (RA) patients.17-23 These CD21−/lo B cells are enriched in autoreactive clones that are refractory to most stimulation, suggesting that human CD21−/lo B cells use an anergic mechanism to be tolerized.
Methods
Patients and healthy donor controls
CVID and RA patients are described in supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Healthy donors include a 36-year-old white male (HD10) and 24-year-old white female (HD11). Additional blood leukocyte preparations from control donors were obtained from the New York Blood Center. Samples were collected after persons signed informed consent in accordance with Hospital for Special Surgery institutional review board–approved protocols and the Declaration of Helsinki.
B-cell purification, single-cell sorting, cDNA, and reverse-transcription PCR
Peripheral B cells were purified from the blood of patients and control donors by negative selection using RosetteSep procedure (StemCell Technologies). Alternatively, mature naive B cells were enriched from peripheral blood mononuclear cells using the Naive B Cell Isolation Kit II (Miltenyi). B cells were stained with fluorescein isothiocyanate (FITC) anti–human CD27, phycoerythrin (PE) anti–human CD10, and either anti–human immunoglobulin M (IgM) biotin and allophycocyanin (APC) anti–human CD19 or PE–cyanin 7 (Cy7) anti–human CD19 and APC anti–human CD21 (Pharmingen, Becton Dickinson). Biotinylated antibodies were revealed using streptavidin–PE-Cy7 (Caltag Laboratories). Single CD21loCD10+IgMhiCD27− new emigrant, CD19+CD10−CD21+CD27− conventional mature naive, and CD19+CD10−CD21−/loCD27− B cells from patients and control donors were sorted on a FACSVantage (Becton Dickinson) into 96-well polymerase chain reaction (PCR) plates containing 4 μL of lysis solution (0.5× phosphate-buffered saline containing 10 mM dithiothreitol, 8 U RNAsin [Promega], and 0.4 U 5′-3′ RNase Inhibitor [Eppendorf]) and immediately frozen on dry ice. All samples were stored at −70°C. RNA from single cells was reverse-transcribed in the original 96-well plate in 12.5-μL reactions containing 100 U of Superscript II RT (Gibco BRL) for 45 minutes at 42°C. Reverse-transcription polymerase chain reaction reactions and primer sequences were as described.24 Immunoglobulin sequences and mutation status were analyzed by Ig BLAST comparison with GenBank using the National Center for Biotechnology Information IgBlast server (http://www.ncbi.nlm.nih.gov/igblast/). Heavy chain complementarity determining region 3 was defined as the interval between the conserved cysteine at position 92 in the VH framework 3 and the conserved tryptophan at position 103 in JH segments.
Antibodies
Additional flow cytometric analyses were performed using anti–B cell–activating factor receptor (BAFF-R)–FITC, CD86-FITC, major histocompatibility complex (MHC) class II/HLA-DR–FITC, CD44-FITC, CD62L-FITC, CD25-FITC, Fas/CD95-FITC, ICOSL-PE, TACI-PE, CD80-PE, MHC class I/HLA-A,B,C–PE, CD11c-PE, CD32-PE, CD93-PE, CD40–Alexa Fluor 647, CXCR4-APC (Biolegend), CD22-FITC, CD72-FITC, CD21-PE, CD38-PE, CD69-PE, CD85j-PE, IgD-PE, CD19–PE-Cy7, CD21-APC (Pharmingen, Becton Dickinson), CCR7-FITC, interleukin-4 receptor (IL-4R)–PE (R&D Systems), and CD23-FITC (Serotec). Unconjugated anti-CD58 (Pharmingen, Becton Dickinson) and CD85d (Abcam) mouse monoclonal antibodies were coupled using the Alexa Fluor 488 Monoclonal Antibody Labeling Kit (Invitrogen).
Microarray gene expression profile analysis
RNA was extracted from 1 × 105 to 3 × 105 batch-sorted CD19+CD10−CD21+CD27− conventional mature naive and CD19+CD10−CD21−/loCD27− B cells using the Absolutely RNA microprep kit (Stratagene). RNA (100-200 ng per sample) was obtained, and the quality of the purified RNA was assessed by the Bioanalyzer from Agilent. Using the Ovation biotin system kit from Nugen, 30 to 50 ng of RNA was amplified and labeled to produce cDNA. Labeled cDNA was hybridized on chips containing the whole human genome (Human Genome U133 2.0 from Affymetrix). The data discussed in this article have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus and are accessible through GEO Series accession number GSE13917 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=hzgjvycoecikyrc&acc=GSE13917).25
B-cell activation, proliferation, and survival
Naive B cells, which contained CD21+ and CD21−/lo B cells from control donors and patients were plated at 150 000 to 200 000 cells per well in a 96-well plate in RPMI 10% serum and 20 μg/mL polyclonal F(ab)′2 rabbit anti–human IgM (Jackson ImmunoResearch Laboratories), 1 μg/mL multimeric soluble recombinant human CD40L (Alexis Biochemicals), and/or 1 μg/mL CpG (Invivogen) for 48 hours. To assess B-cell proliferation, naive B cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen), plated, and stimulated as described in this section. The cells were harvested on day 3 and labeled for CD19 and CD21 expression before determining the division number by decreasing CFSE fluorescence in flow cytometric experiments. The proportions of apoptotic and dead cells were assessed by flow cytometry to measure binding with annexin V and propidium iodide using the Annexin V-FITC Apoptosis Detection Kit I (BD Pharmingen).
Calcium mobilization
To measure free intracellular calcium concentration [Ca2+]i, naive B cells were first labeled with anti-CD19 and anti-CD21 antibodies, and loaded with 5 mM Fluo-3 AM (Molecular Probes–Invitrogen) in the presence of 0.2% Pluronic F-127 (Sigma-Aldrich) for 30 minutes at room temperature. Cells were washed and resuspended at 106 cells/mL, and [Ca2+]i was monitored over time by flow cytometry on gated CD19+CD21+ and CD19+CD21−/lo B cells. After the baseline was established, cells were stimulated with 20 μg/mL F(ab′)2 mouse anti–human IgM. Mean [Ca2+]i was analyzed using the FCS Assistant 1.2.9 software (BD Biosciences).
Antibody production, ELISAs, and Immunofluorescence assays
Cloning strategy, expression vectors, and antibody reactivity against specific antigens were as described.24 Highly polyreactive ED38 was used as positive control in HEp-2 reactivity and polyreactivity enzyme-linked immunosorbent assays (ELISAs).24 Antibodies were considered polyreactive when they recognized at least 2 and usually all of the 4 analyzed antigens that include single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), insulin, and lipopolysaccharide. For indirect immunofluorescence assays, HEp-2 cell–coated slides (Bion Enterprises Ltd) were incubated in a moist chamber at room temperature with purified recombinant antibodies at 50 to 100 μg/mL. FITC-conjugated goat anti–human IgG was used as detection reagent. Pictures were taken with an Axioskop (Zeiss) using a Plan-Neofluar 40×/.75 objective and Axiovision 3.1 acquisition software.
Statistics
Differences between healthy donor and patient populations were analyzed for statistical significance with unpaired Student t tests, using SigmaPlot software (Systat). A P value of less than .05 was considered significant.
Supporting information
Supplemental material includes 8 figures and 19 tables.
Results
CVID and RA patients may display unusual peripheral CD21−/lo B cells
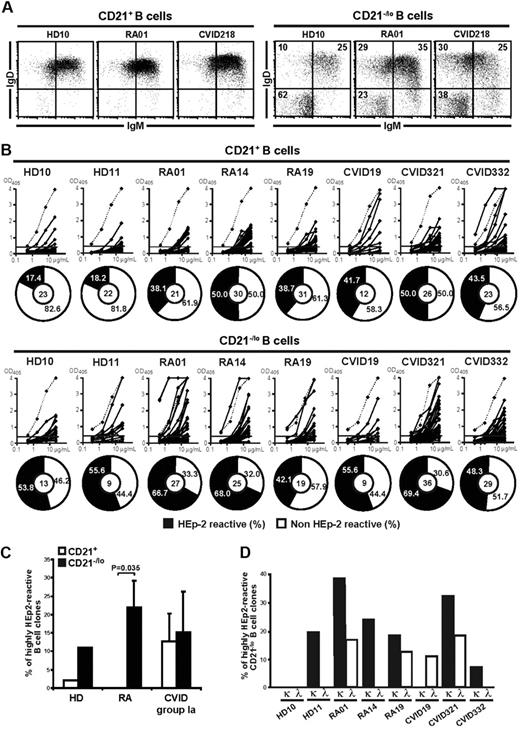
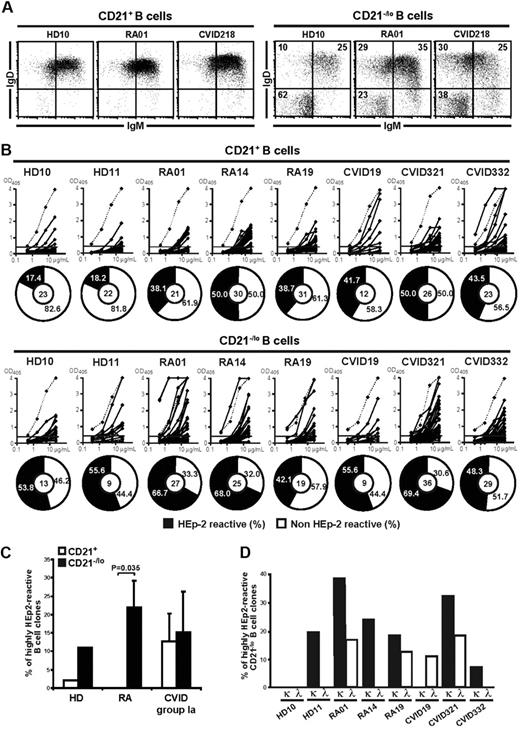
CVID comprises a heterogeneous group of patients suffering from reduced serum levels of several Ig isotypes resulting in an increased susceptibility to bacterial infections (supplemental Table 1).26 Indeed, CVID patients frequently show severely decreased frequencies of CD27+ memory B cells, especially those expressing switched isotypes.21,27,28 In addition, some CVID patients display an unusual population of B cells that lack complement receptor 2 (CR2/CD21).21-23 The presence of 20% or more CD21−/lo B cells in the blood of CVID patients was proposed to be a criteria for the classification of these patients as CVID group Ia who are prone to develop autoimmunity (Figure 1A-B).21,22 Because CD21−/lo B cells were also reported in the blood of patients with autoimmune diseases including SLE,17-20 we analyzed the frequency of CD19+CD27−CD21−/lo B cells in healthy donors and untreated active RA patients (supplemental Table 2). We found that the proportion of CD21−/lo B cells was low in peripheral CD27-depleted B cells from healthy donors (Figure 1C-D).17 In contrast, a subgroup of RA patients displayed a high frequency of CD21−/lo B cells (Figure 1C-D). Similarly to CVID patients, CD19+CD27−CD21−/lo B cells in RA patients were not new emigrant/transitional B cells because they expressed neither CD10 nor CD38 (data not shown). Moreover, the frequency of CD19+CD10−CD27−CD21−/lo (from now on referred to as CD21−/lo) B cells was stable in the same RA patients over a 4 year-period. Thus, both CVID and RA patients may display an increased proportion of CD21−/lo B cells in their blood.
CVID and RA patients may display an increased frequency of CD21−/lo B cells in their blood. (A) CVID group Ia patients were previously defined as patients displaying a frequency of CD21−/lo naive B cells > 20% of total B cells.21 Each diamond represents a person and (B) dot plots show CD19 and CD21 expression on CD27−-gated peripheral B cells from representative CVID group Ia patients. (C) Increased CD21−/lo B-cell frequency in RA patients. Dot plots show CD19 and CD21 expression on CD27-depleted peripheral B cells from a representative healthy donor (HD10) and RA patients. (D) The frequencies of CD21−/lo naive B cells are compared between healthy donors and RA patients. Each diamond represents a person and statistically significant differences are indicated.
CVID and RA patients may display an increased frequency of CD21−/lo B cells in their blood. (A) CVID group Ia patients were previously defined as patients displaying a frequency of CD21−/lo naive B cells > 20% of total B cells.21 Each diamond represents a person and (B) dot plots show CD19 and CD21 expression on CD27−-gated peripheral B cells from representative CVID group Ia patients. (C) Increased CD21−/lo B-cell frequency in RA patients. Dot plots show CD19 and CD21 expression on CD27-depleted peripheral B cells from a representative healthy donor (HD10) and RA patients. (D) The frequencies of CD21−/lo naive B cells are compared between healthy donors and RA patients. Each diamond represents a person and statistically significant differences are indicated.
CD21−/lo B cells express highly autoreactive antibodies including ANAs
The physiology of CD21−/lo B cells has not been thoroughly investigated and the involvement of these B cells in the development of autoimmunity is unknown. BCR expression in CD21−/lo B cells was assessed by flow cytometry after depletion of CD27+ memory B cells. In agreement with previous reports, we found that the majority of CD21−/loCD27− B cells from healthy donors expressed isotype-switched B cells (Figure 2A).29-31 In contrast, CD21−/loCD27− B cells from RA and CVID patients preferentially expressed IgM and/or IgD (Figure 2A). Indeed, patients' CD21−/lo B cells were enriched in IgMloIgD+ B cells, which represented approximately 30% of their CD21−/lo B cells, whereas this population accounted for only 10% of CD21−/lo B cells in healthy donors (Figure 2A).
CD21−/lo naive B cells express autoreactive BCRs. (A) A majority of CD21−/lo B cells from RA and CVID patients express IgM and IgD. Dot plots show IgM and IgD expression gated on CD19+CD21+(left) and CD19+CD21−/lo (right) CD27-depleted B cells. (B) Most CD21−/lo naive B cells express autoreactive antibodies. Antibodies cloned from single CD21+CD27−CD10− and CD21−/loCD27−CD10− B cells from 2 healthy donors (HD10 and 11), 3 RA, and 3 CVID patients were tested by ELISA for reactivity against HEp-2 lysates. Dotted lines show ED38-positive control.24 Horizontal lines show cutoff OD405 for positive reactivity. For each B-cell population, the frequency of HEp-2–reactive and non–HEp-2–reactive clones is summarized in pie charts, with the number of antibodies tested indicated in the centers. The frequency of HEp-2–reactive B cells was higher in CD21−/lo than in CD21+ B cells in all persons. (C) CD21−/lo B cells express highly autoreactive antibodies. The frequency of highly HEp-2–reactive antibodies, arbitrarily defined by ELISA OD405 > 2, expressed by CD21+ and CD21−/lo B cells from HD, RA, and CVID patients is represented. A large fraction of CD21−/lo B cells from most persons was highly HEp-2 reactive. P values calculated using a paired t test are indicated when significant. (D) A majority of highly autoreactive antibodies from CD21−/lo B cells express kappa light chains. The frequency of highly HEp-2–reactive CD21−/lo B cells from HD, RA, and CVID patients is represented subdivided among kappa and lambda clones. Many highly HEp-2–reactive CD21−/lo B cells express kappa light chains, suggesting that receptor editing using lambda light chains was not induced to silence these clones.
CD21−/lo naive B cells express autoreactive BCRs. (A) A majority of CD21−/lo B cells from RA and CVID patients express IgM and IgD. Dot plots show IgM and IgD expression gated on CD19+CD21+(left) and CD19+CD21−/lo (right) CD27-depleted B cells. (B) Most CD21−/lo naive B cells express autoreactive antibodies. Antibodies cloned from single CD21+CD27−CD10− and CD21−/loCD27−CD10− B cells from 2 healthy donors (HD10 and 11), 3 RA, and 3 CVID patients were tested by ELISA for reactivity against HEp-2 lysates. Dotted lines show ED38-positive control.24 Horizontal lines show cutoff OD405 for positive reactivity. For each B-cell population, the frequency of HEp-2–reactive and non–HEp-2–reactive clones is summarized in pie charts, with the number of antibodies tested indicated in the centers. The frequency of HEp-2–reactive B cells was higher in CD21−/lo than in CD21+ B cells in all persons. (C) CD21−/lo B cells express highly autoreactive antibodies. The frequency of highly HEp-2–reactive antibodies, arbitrarily defined by ELISA OD405 > 2, expressed by CD21+ and CD21−/lo B cells from HD, RA, and CVID patients is represented. A large fraction of CD21−/lo B cells from most persons was highly HEp-2 reactive. P values calculated using a paired t test are indicated when significant. (D) A majority of highly autoreactive antibodies from CD21−/lo B cells express kappa light chains. The frequency of highly HEp-2–reactive CD21−/lo B cells from HD, RA, and CVID patients is represented subdivided among kappa and lambda clones. Many highly HEp-2–reactive CD21−/lo B cells express kappa light chains, suggesting that receptor editing using lambda light chains was not induced to silence these clones.
To determine whether unswitched CD21−/lo B cells from RA and CVID patients, as well as from healthy donors, expressed autoreactive BCRs, we compared the reactivity of antibodies expressed by single CD21−/lo and single conventional CD19+CD10−CD27−CD21+ (referred to as CD21+) mature naive B cells from the same persons. Most Ig genes expressed by CD21−/lo B cells from RA and CVID patients were devoid of somatic hypermutations, thereby demonstrating that these B cells belonged to the naive compartment. IgM genes amplified from CD21−/lo B cells from 2 healthy donors displayed unmutated and mutated sequences, revealing that these cells contain both naive and memory B cells (supplemental Tables 3-18 and data not shown). Therefore, we expressed only BCRs encoded by germline Ig genes to compare naive B-cell populations. We found that the frequency of polyreactive and HEp-2–reactive clones was increased in CD21−/lo compared with CD21+ B cells in all persons (Figure 2B and supplemental Figure 1). Differences in HEp-2 reactivity between CD21−/lo and CD21+ B cells reached significance for both healthy donors and patients (supplemental Figure 2). In addition, CD21−/lo B cells often expressed highly HEp-2–reactive antibodies (arbitrarily defined by ELISA optical density at 405 nm [OD405] > 2) compared with their CD21+ B-cell counterparts (Figure 2C). Highly HEp-2–reactive antibodies often displayed heavy chain complementarity determining regions containing many positive charges favoring autoreactivity and were mostly kappa clones, which suggested that receptor editing using lambda light chains was not induced to silence these B cells (Figure 2D and supplemental Tables 3-18).24,32,33 Indeed, CD21−/lo B cells showed a statistically significant decrease in upstream Vκ gene usage combined with an increase in Jκ1 gene usage compared with conventional CD21+ B cells, further suggesting decreased secondary recombination events in CD21−/lo B cells despite their autoreactive features (supplemental Figure 3). Thus, CD21−/lo B cells are enriched in polyreactive and strongly autoreactive clones, which are normally silenced during B-cell development in the bone marrow.
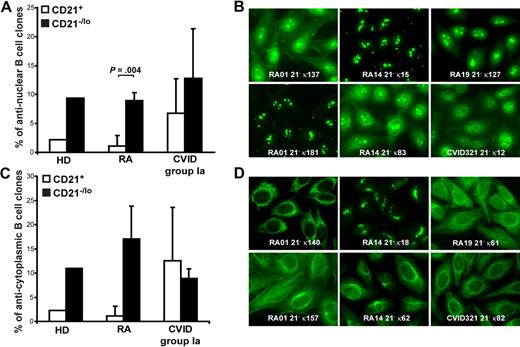
Immunofluorescence assays revealed that 5.6% to 22.2% of naive CD21−/lo B cells expressed antinuclear antibodies (ANAs; Figure 3A-B). ANAs expressed by CD21−/lo B cells showed a diversity of nucleolar and speckled antinuclear staining patterns (Figure 3B). In contrast, ANAs were rarely detected in CD21+ B cells from healthy donors and RA patients but could be fairly frequent in CD21−/lo B cells from CVID patients (Figure 3A and data not shown). In addition, CD21−/lo B cells expressed antibodies that often recognized specific cytoplasmic structures, which included mitochondria, the Golgi apparatus, and cytoskeleton components (Figure 3C-D, and supplemental Tables 3-18). These antibodies corresponded mostly to the highly HEp-2–reactive clones identified by ELISA. In contrast, antibodies expressed by CD21+ B cells rarely recognized well-defined structures in HEp-2 cells. We conclude that CD21−/lo B cells differ from CD21+ conventional B cells in that they often express autoreactive antibodies that may recognize nuclear or cytoplasmic antigens.
CD21−/lo B cells contain antinuclear and anticytoplasmic clones. The frequencies of antinuclear (A) and anticytoplasmic (C) clones in CD21+ and CD21−/lo B cells from HD, RA, and CVID patients are shown. P values calculated using a paired t test are indicated when significant. (B) ANAs expressed by CD21−/lo B cells showed a diversity of nucleolar (RA01 21− κ181, RA01 21− κ137, RA14 21− κ15, and RA14 21− κ83), nucleolar and speckled (RA19 21− κ127), and speckled (CVID 21− κ12) antinuclear staining patterns. (D) Anticytoplasmic CD21−/lo B cells can react with well-defined intracellular structures, which include mitochondria (RA01 21− κ140, and CVID321 21− κ82), the Golgi (RA14 21− κ18), and cytoplasmic fibers (RA19 21− κ61, RA14 21− κ62, and RA01 21− κ157 that is likely to recognize actin).
CD21−/lo B cells contain antinuclear and anticytoplasmic clones. The frequencies of antinuclear (A) and anticytoplasmic (C) clones in CD21+ and CD21−/lo B cells from HD, RA, and CVID patients are shown. P values calculated using a paired t test are indicated when significant. (B) ANAs expressed by CD21−/lo B cells showed a diversity of nucleolar (RA01 21− κ181, RA01 21− κ137, RA14 21− κ15, and RA14 21− κ83), nucleolar and speckled (RA19 21− κ127), and speckled (CVID 21− κ12) antinuclear staining patterns. (D) Anticytoplasmic CD21−/lo B cells can react with well-defined intracellular structures, which include mitochondria (RA01 21− κ140, and CVID321 21− κ82), the Golgi (RA14 21− κ18), and cytoplasmic fibers (RA19 21− κ61, RA14 21− κ62, and RA01 21− κ157 that is likely to recognize actin).
CD21−/lo B cells fail to get activated through BCR and CD40 triggering
We analyzed the physiology of CD21−/lo B cells by studying the induction of CD25, CD69, CD23, TACI, ICOS-L, and Fas by flow cytometry on CD21+ and CD21−/lo gated B cells after BCR, CD40, and Toll-like receptor 9 (TLR) stimulations for 2 days. CD21+ B cells from RA01, RA19, CVID218, and CVID321 patients up-regulated CD69 and CD25 after BCR, CD40, or TLR9 triggering (Figure 4A-B and data not shown). In contrast, CD21−/lo B cells from the same patients failed to properly induce cell surface expression of CD25 and CD69 after BCR and CD40 triggering (Figure 4A-B). The induction of CD25 and CD69 in CD21−/lo B cells by TLR9 stimulation with CpG, although decreased compared with conventional B cells, was less affected than those induced by BCR and/or CD40 triggering (Figure 4A-B). Defects in the induction of CD23 on CD21−/lo compared with CD21+ B cells were also observed after BCR, TLR9, or especially CD40 stimulations (supplemental Figures 4-5). These defects were not the result of a global inability of CD21−/lo B cells to get activated, because CD21−/lo B cells up-regulated cell surface expression of ICOS-L, TACI, and Fas after BCR, CD40, or TLR9 stimulation (supplemental Figures 4-5). In addition, similar results were obtained when CD21+ and CD21−/lo B cells were initially fractionated and then stimulated in vitro for 2 days (data not shown). Thus, CD21+ and CD21−/lo B cells display distinct B-cell responses after BCR, CD40, or TLR9 stimulation.
CD21−/lo naive B cells fail to properly respond to BCR triggering. CD21−/lo naive B cells from (A) RA01 and (B) CVID321 patients fail to up-regulate CD69 and CD25 expression after stimulation with F(ab′2) anti-IgM, recombinant human CD40L, and TLR9 agonist CpG. Total naive B cells containing both CD21−/lo and CD21+ B cells were plated and dot plots show CD69 and CD25 expression after 2 days according to gated CD21 expression. (C) BCR triggering in CD21−/lo naive B cells does not properly increase [Ca2+]i. [Ca2+]i was assessed by flow cytometry in CD21−/lo and CD21+ naive B cells from a healthy donor (HD) and RA01 and CVID321 patients. Both B-cell populations were loaded with Fluo-3 and expressed similar levels of surface IgM expression when experiments were performed. Arrow indicates when F(ab′)2 anti-IgM was added. Data from each group of persons are representative of 2-3 independent experiments.
CD21−/lo naive B cells fail to properly respond to BCR triggering. CD21−/lo naive B cells from (A) RA01 and (B) CVID321 patients fail to up-regulate CD69 and CD25 expression after stimulation with F(ab′2) anti-IgM, recombinant human CD40L, and TLR9 agonist CpG. Total naive B cells containing both CD21−/lo and CD21+ B cells were plated and dot plots show CD69 and CD25 expression after 2 days according to gated CD21 expression. (C) BCR triggering in CD21−/lo naive B cells does not properly increase [Ca2+]i. [Ca2+]i was assessed by flow cytometry in CD21−/lo and CD21+ naive B cells from a healthy donor (HD) and RA01 and CVID321 patients. Both B-cell populations were loaded with Fluo-3 and expressed similar levels of surface IgM expression when experiments were performed. Arrow indicates when F(ab′)2 anti-IgM was added. Data from each group of persons are representative of 2-3 independent experiments.
BCR triggering does not induce calcium flux in CD21−/lo B cells
The inability of CD21−/lo B cells to up-regulate some activation markers upon BCR triggering could result from an inability to properly induce intracellular calcium flux. We analyzed [Ca2+]i after BCR stimulations in these B cells compared with conventional CD21+ B cells. BCR triggering induced a strong [Ca2+]i increase in CD21+ conventional B cells from healthy donors as well as RA and CVID patients (Figure 4C and data not shown). In contrast, CD21−/lo B cells from RA01, RA19, RA26, CVID321, and CVID218 patients induced only a weak elevation of [Ca2+]i in response to BCR stimulation (Figure 4C and data not shown). We conclude that CD21−/lo B cells do not exhibit a proper calcium response after BCR stimulation.
CD21−/lo B cells do not proliferate after BCR and CD40 triggering
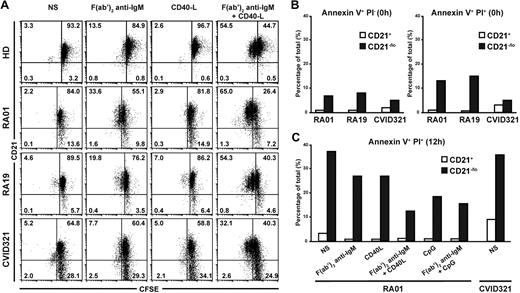
Because CD21−/lo B cells showed an impaired activation compared with CD21+ B cells in response to several stimulations, we investigated their ability to proliferate after antigenic stimulation. Carboxyfluorescein succinimidyl ester (CFSE)–labeled CD21+ and CD21−/lo B cells from 5 RA and 4 CVID patients and healthy donor controls were stimulated through their BCR or CD40 for 3 days, and CFSE dilution, which reflects B-cell proliferation, was then assessed by flow cytometry (Figure 5A and data not shown). Anti-IgM F(ab′)2 stimulation induced the proliferation of a few CD21+ B cells in all persons, whereas CD40L did not (Figure 5A). BCR and CD40 cotriggering resulted in the proliferation of a majority of CD21+ B cells from both healthy donors and patients, and some of these B cells underwent up to 2 divisions (Figure 5A). In contrast, RA and CVID CD21−/lo B cells failed to proliferate after BCR and CD40 costimulation. We conclude that unlike CD21+ conventional B cells, CD21−/lo B cells are unable to proliferate after antigenic stimulation, further suggesting their unresponsive stage.
CD21−/lo naive B cells do not proliferate after BCR and CD40 cotriggering and are prone to cell death. (A) Total naive B cells from healthy donors (HD), and RA and CVID patients containing both CD21−/lo and CD21+ B cells were labeled with CFSE and stimulated with F(ab′2) anti-IgM and/or recombinant human CD40L. Dot plots show CD21 expression according to CFSE dilution after 3 days. CFSE dilution in CD21+ but not in CD21−/lo B cells stimulated with F(ab′2) anti-IgM and recombinant human CD40L reveals that some CD21+ B cells underwent up to 2 divisions, whereas CD21−/lo B cells did not divide. (B) Increased frequency of annexin V+ propidium iodide–negative (PI−) early apoptotic, and annexin V+ PI+ late apoptotic and dead cells in freshly isolated CD21−/lo naive B cells from RA and CVID patients compared with their CD21+ B-cell counterparts. (C) BCR, CD40, and/or TLR9 triggering do not prevent the apoptosis and cell death of CD21−/lo naive B cells. Total naive B cells containing both CD21−/lo and CD21+ B cells from RA01 or CVID321 patients were plated, and annexin V and PI staining was assessed after 12 hours. BCR, CD40, and TLR9 stimulation prevents late apoptosis and cell death in CD21+ but not in CD21−/lo B cells.
CD21−/lo naive B cells do not proliferate after BCR and CD40 cotriggering and are prone to cell death. (A) Total naive B cells from healthy donors (HD), and RA and CVID patients containing both CD21−/lo and CD21+ B cells were labeled with CFSE and stimulated with F(ab′2) anti-IgM and/or recombinant human CD40L. Dot plots show CD21 expression according to CFSE dilution after 3 days. CFSE dilution in CD21+ but not in CD21−/lo B cells stimulated with F(ab′2) anti-IgM and recombinant human CD40L reveals that some CD21+ B cells underwent up to 2 divisions, whereas CD21−/lo B cells did not divide. (B) Increased frequency of annexin V+ propidium iodide–negative (PI−) early apoptotic, and annexin V+ PI+ late apoptotic and dead cells in freshly isolated CD21−/lo naive B cells from RA and CVID patients compared with their CD21+ B-cell counterparts. (C) BCR, CD40, and/or TLR9 triggering do not prevent the apoptosis and cell death of CD21−/lo naive B cells. Total naive B cells containing both CD21−/lo and CD21+ B cells from RA01 or CVID321 patients were plated, and annexin V and PI staining was assessed after 12 hours. BCR, CD40, and TLR9 stimulation prevents late apoptosis and cell death in CD21+ but not in CD21−/lo B cells.
CD21−/lo B cells are prone to die by apoptosis
We assessed the survival potential of CD21−/lo B cells by analyzing the binding of annexin V to their surface, which along with propidium iodide (PI) staining can define annexin V+PI− early apoptotic cells and annexin V+PI+ late apoptotic and dead cells. We found that freshly isolated CD21−/lo B cells from RA and CVID patients contained a higher frequency of both annexin V+PI− early apoptotic and annexin V+PI+ late apoptotic and dead B cells compared with conventional CD21+ B cells (Figure 5B). When naive B cells from RA and CVID patients were left unstimulated for 12 hours, CD21−/lo B cells contained approximately 35% of annexin V+PI+ dying cells, whereas there were less than 10% in CD21+ B cells (Figure 5C). Moreover, triggering through BCR, CD40, and/or TLR9 only partially rescued these B cells from cell death. In contrast, stimulated CD21+ B cells contained virtually no annexin V+PI+ B cells (Figure 5C). In agreement with their increased susceptibility to die, CD21−/lo B cells also contained a higher proportion of annexin V+PI− early apoptotic cells than CD21+ B cells, regardless of the stimulation (supplemental Figure 6). Hence, CD21−/lo B cells are more susceptible to apoptosis and cell death than CD21+ B cells, and their ability to be eliminated is poorly rescued by BCR stimulation in combination with either CD40 or TLR9 triggering.
The transcriptome of CD21−/lo B cells shows an inhibitory gene signature
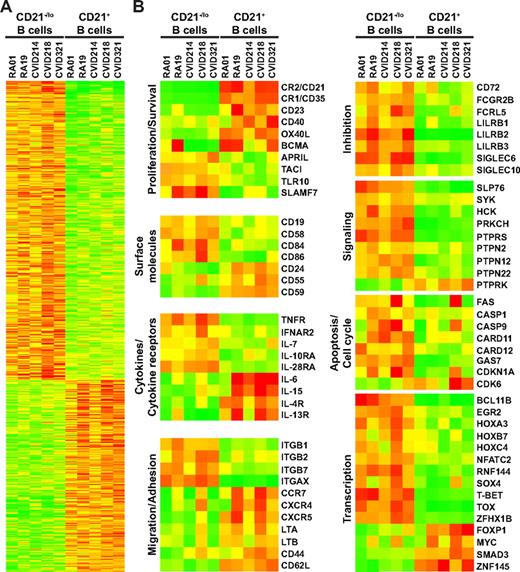
We intended to further characterize CD21−/lo autoreactive unresponsive B cells by performing gene array profiling and compare transcripts expressed in CD21−/lo versus CD21+ mature naive B cells isolated from 2 RA and 3 CVID patients (Figure 6). A total of 1079 transcripts were found expressed with a greater than 2-fold statistically significant difference by the 2 B-cell subpopulations and included 656 up-regulated and 423 down-regulated transcripts in CD21−/lo B cells (Figure 6A). Some of those differentially expressed genes, and a few others of specific interest, are shown on Figure 6B and supplemental Table 19. Correlating with a decreased expression of CD21 on their cell surface, we found that CD21−/lo B cells down-regulated the transcription of CR2/CD21, as well as other complement binding molecules CR1/CD35, CD55, and CD59 (Figure 6B). Many genes encoding survival and activation molecules such as CD40, OX40L, and BCMA, as well as the cytokine receptors IL-4R and IL-13R that stimulate B-cell proliferation, were all down-regulated in CD21−/lo B cells (Figure 6B). In contrast, CD21−/lo B cells up-regulated the transcription of a whole set of immunoreceptor tyrosine–based inhibition motif (ITIM) receptor genes likely to inhibit B-cell activation and proliferation. They included CD72, Fc receptors CD32/FCGR2B and FCRL5/IRTA2, as well as LILRB and SIGLEC genes (Figure 6B). Genes encoding phosphatases potentially mediating the inhibitory effect of these ITIM receptors were also found up-regulated in the CD21−/lo B cells (Figure 6B). In addition, these B cells down-regulated transcripts encoding the chemokine receptors CCR7, CXCR4, and CXCR5 as well as lymphotoxin A, which regulates B-cell trafficking (Figure 6B). Lymphocyte homing capacities are also likely to differ between CD21−/lo and CD21+ B cells because transcripts for many integrins (ITG), including ITGAX, which encodes CD11c molecules, were up-regulated in CD21−/lo B cells, whereas the transcripts for selectin CD62L were down-regulated (Figure 6B). The increased amounts of growth arrest–specific 7 (GAS7) and CDKN1A/p21 combined with decreased cyclin-dependent kinase 6 (CDK6) and MYC transcripts in CD21−/lo B cells are likely to result in the observed defective proliferation of these cells. In addition, the up-regulation of Fas/CD95 and caspase genes in CD21−/lo B cells could be responsible for their increased susceptibility to cell death. Finally, we found that some transcription factor genes were up-regulated in CD21−/lo B cells compared with CD21+ B cells, such as BCL11B, EGR2, HOXA3, HOXB7, HOXC4, NFATC2, SOX4, TBET, and TOX, whereas a few transcription factor genes, such as FOXP1, were found down-regulated (Figure 6B). In conclusion, the transcriptome of CD21−/lo B cells is characterized by the up-regulation of many genes encoding molecules likely to inhibit B-cell activation, proliferation, and survival and the down-regulation of B-cell activating genes, supporting the observation that CD21−/lo B cells are refractory to antigenic stimulation.
Gene array comparisons of CD21−/lo and CD21+ naive B cells from RA and CVID patients using the Affymetrix Human Genome U133 Plus 2.0 Array. (A) Transcripts differentially expressed by CD21−/lo and CD21+ naive B cells from 2 RA and 3 CVID group Ia patients are shown. (B) Selected transcripts well known to B-cell biology or of potential interest are presented. Up- and down-regulated transcripts are indicated in red and green, respectively. The magnitude of expression is depicted by the color bar.
Gene array comparisons of CD21−/lo and CD21+ naive B cells from RA and CVID patients using the Affymetrix Human Genome U133 Plus 2.0 Array. (A) Transcripts differentially expressed by CD21−/lo and CD21+ naive B cells from 2 RA and 3 CVID group Ia patients are shown. (B) Selected transcripts well known to B-cell biology or of potential interest are presented. Up- and down-regulated transcripts are indicated in red and green, respectively. The magnitude of expression is depicted by the color bar.
CD21−/lo B cells display a specific B-cell phenotype
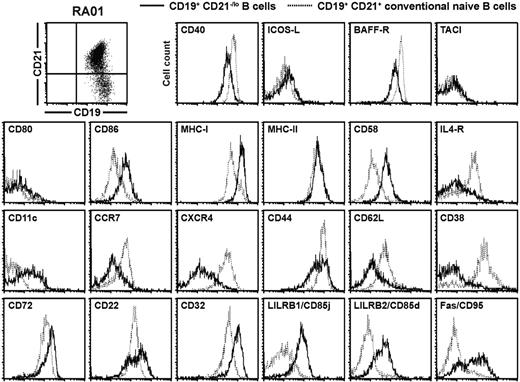
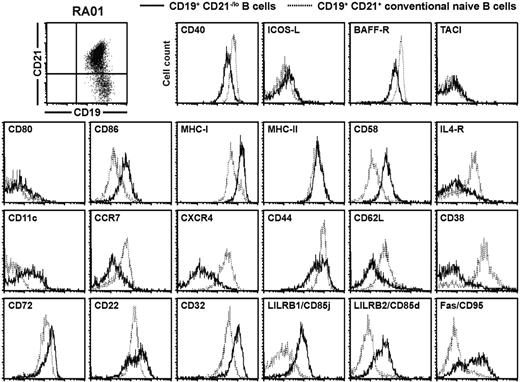
The expression of many genes found either up-regulated or down-regulated in gene array experiments was validated by flow cytometry in RA01, RA19, CVID214, CVID218, and CVID321 after B-cell surface staining with specific monoclonal antibodies. Representative results for RA patients (Figure 7) as well as CVID patients and healthy controls (supplemental Figures 7-8) are shown. In agreement with gene array analysis, CD21−/lo B cells were CD19hiCD40loCD86+CD58hiIL-4RloCD11c+CCR7−/loCXCR4loCD44loCD62L−/loCD72hiCD32hiCD85j+CD85d+Fas+ (Figure 7 and supplemental Figures 7-8). Moreover, we also found that BAFF-R was down-regulated and MHC class I and ITIM receptor CD22 were up-regulated in CD21−/lo B cells, whereas changes in their gene expression were not detected in gene array experiments (Figure 7 and supplemental Figures 7-8). Human CD21+ and CD21−/lo B cells expressed TACI, ICOS-L, CD80, and MHC class II at similar levels and did not express CD93, which was found on the cell surface of mouse anergic transitional 3 B cells (Figure 7 and supplemental Figures 7-8, and data not shown). Hence, CD21−/lo B cells display a unique phenotype, which is characterized by the down-regulation of receptors that normally favor B-cell survival and/or activation, and the up-regulation of molecules inhibiting B-cell functions.
CD21−/lo B cells from RA and CVID patients display a phenotype reflecting gene array profiling data. Overlays for the indicated markers were obtained when CD27-depleted B cells isolated from the blood of patient RA01 were gated on either CD19+CD21−/lo (bold line) or CD19+CD21+ (dashed line) B cells. The similar phenotypes of CD21−/lo B cells from CVID patients and healthy donors are shown in supplemental Figures 7 and 8.
CD21−/lo B cells from RA and CVID patients display a phenotype reflecting gene array profiling data. Overlays for the indicated markers were obtained when CD27-depleted B cells isolated from the blood of patient RA01 were gated on either CD19+CD21−/lo (bold line) or CD19+CD21+ (dashed line) B cells. The similar phenotypes of CD21−/lo B cells from CVID patients and healthy donors are shown in supplemental Figures 7 and 8.
Discussion
We report herein the identification in humans of a unique autoreactive B-cell population, which lacks CD21 expression and is refractory to B-cell stimulation. Indeed, naive CD21−/lo B cells express a diverse germline BCR repertoire, which is enriched in autoreactive clones. In addition, CD21−/lo B cells showed impaired calcium-mediated signaling, did not up-regulate some activation markers, and did not proliferate in response to BCR triggering. CD21−/lo B cells were also prone to die faster than CD21+ conventional B cells, suggesting they have a shorter half-life. Because most of these features are characteristic of mouse anergic B cells, autoreactive CD21−/lo B cells, which escaped central B-cell tolerance deletion and remain in the periphery in an irresponsive stage, therefore represent a population of anergic B cells in humans.4,14,15,34-38
B cells that express intrinsically autoreactive BCRs encoded by the VH4-34 gene have been suggested to be anergic because they fail to induce normal calcium flux after BCR triggering.39 In addition, VH4-34 B cells are enriched in mature naive B cells and remain excluded from germinal centers in healthy donors.39,40 However, we did not find any increase in VH4-34 gene use in CD21−/lo B cells, which expressed a very diverse immunoglobulin repertoire. The abundance of self-antigens expressed on erythrocytes and recognized by VH4-34 BCRs may induce a unique anergic program in these cells that differs from that of CD21−/lo B cells, which may recognize a broader set of self-antigens. Another population of irresponsive B cells that down-regulated IgM expression was recently reported in humans.16 These B cells also express autoreactive antibodies and fail to induce calcium flux and tyrosine phosphorylation cascade after IgD triggering but could get activated and proliferated in vitro.16 By analogy to anergic mouse models, which express different autoreactive transgenic antibodies and develop different anergic B-cell programs, we would propose that VH4-34–expressing, IgM-negative, and CD21−/lo B cells represent different populations of anergic B cells in humans.
The existence of some CD21−/lo B cells have been previously reported in CVID and SLE patients, in persons infected with either human immunodeficiency virus (HIV) or hepatitis C virus combined with mixed cryoglobulinemia, as well as in healthydonors.17-23,29-31,41 All these CD21−/lo B cells, with the exception of those in CVID patients, belong to the memory B-cell compartment because they frequently express mutated isotype switched antibodies.29-31 In agreement with these reports, we found that memory B cells represented the majority of CD21−/lo B cells in healthy donors. In contrast, CD21−/lo B cells from RA and CVID patients were often naive B cells because they produced germline-encoded IgM and IgD antibodies. However, both naive and memory CD21−/lo B cells displayed a similar gene expression profile and expressed common cell surface molecules, which differed from conventional CD21+ B cells.29,31 It is unknown whether memory CD21−/lo B cells from healthy donors are anergic, but CD21−/lo B cells from HIV-infected viremic persons and CVID patients poorly respond to antigenic stimulation.23,29 Chronic antigen exposure, such as in HIV-infected viremic persons, may lead antigen-reactive B cells to an anergic phenotype similar to CD21−/lo naive B cells and disable them to secrete antibodies.
The phenotype of CD21−/lo B cells revealed the modulation of the expression of many molecules that may prevent the activation of these B cells. The down-regulation of the complement receptor CR2/CD21 previously reported on mouse anergic B cells is likely to result in an increased BCR signaling threshold and compromise the ability of CD21−/lo B cells to be activated.35,37 In addition, CD21 is able to break anergy in mouse B cells, further suggesting that its down-regulation contributes to an anergic stage in mice and humans.35,38,42 The down-regulation of CD40, OX40L, IL-4R, and IL-13R, which play an important role in B-cell activation and proliferation, may also be responsible for the anergic responses of CD21−/lo B cells. Moreover, increased amounts of many ITIM receptors on CD21−/lo B cells, including CD22, FcγR2B/CD32, and CD72 that was already reported in mouse anergic B cells, can further dampen the activation of these B cells.43-46 The overexpression of many phosphatases in CD21−/lo B cells is also likely to decrease their antigenic responses. Chemokine receptors including CCR7, CXCR4, and CXCR5 as well as selectin CD62L have been shown to play a major role in B-cell trafficking in secondary lymphoid organs and germinal centers.47,48 The down-regulation of these receptors on CD21−/lo B cells is likely to exclude them from B-cell follicles and being recruited in germinal centers.9,49 In addition, the up-regulation of many integrins also involved in B-cell homing may direct these autoreactive B cells to specific compartments where they may be eventually eliminated. Moreover, the increased expression of molecules favoring cell death and blocking cell cycle entry may further prevent the expansion of CD21−/lo B cells and favor their removal. Altogether, CD21−/lo B cells express a specific set of molecules that may prevent the activation of these B cells. Some of these molecules may also serve as biomarkers to identify human unresponsive autoreactive naive B cells and distinguish them from the other CD21−/lo B cells belonging to the memory compartment.
Unresponsive autoreactive CD21−/lo B cells express a specific B-cell phenotype, which may be regulated by transcription factors. However, transgenic mouse models did not identify a specific transcription factor whose expression would be responsible for the establishment of an anergic B-cell program.43 Indeed, it has been postulated that because the maintenance of an anergic stage requires constant BCR triggering by self-antigens, the anergic phenotype may not depend on a genetic program ensured by transcription factors.13,14 Gene array comparisons between CD21−/lo and CD21+ naive B cells revealed the induction of a set of transcription factors in human unresponsive autoreactive CD21−/lo B cells. It is unknown whether this transcription factor signature is dependent on constant BCR triggering by self-antigens, but it unveils new leads in the identification of factors that may control the expression of the molecules resulting in the inability of CD21−/lo B cells to respond to antigenic stimulations.
Three mechanisms normally ensure the silencing of developing autoreactive B cells. Receptor editing mediated by secondary recombination has been reported to be very efficient at silencing autoreactive B cells reacting against membrane-bound antigens and deletion seems to be used when receptor editing failed to silence autoreactive B cells.12 Anergy may be preferentially induced in B cells that express moderately autoreactive BCRs toward soluble antigens.11 In agreement with this hypothesis, we found that CD21−/lo B cells that express unmutated IgM in healthy donors display weakly autoreactive BCRs and may represent anergic B cells. Healthy donors also showed low frequencies of CD21−/lo B cells, suggesting that anergy is rarely used to silence developing autoreactive B cells in physiological conditions. Factors that favor the development or the maintenance of CD21−/lo B cells in the blood of some RA and CVID patients remain to be determined. The serum of CVID group Ia but not RA patients contains elevated concentrations of B cell–activating factor (BAFF), which is known to inhibit the counterselection of anergic B cells in mice and likely to contribute to the large accumulation of CD21−/lo B cells in these CVID patients50 (and data not shown).
The induction of an unresponsive program in highly autoreactive B cells may represent risks to develop autoimmunity. Indeed, elevated numbers of highly autoreactive unresponsive CD21−/lo B cells remain in the blood of RA and CVID patients instead of being eliminated, and immune reactions may create a favorable environment to break tolerance and eventually activate these CD21−/lo B cells. In RA, all patients develop autoimmunity, whether or not they display CD21−/lo B cells, and it is unclear how these B cells contribute to the initiation and/or progression of the disease. However, CVID group Ia patients who display high frequencies of CD21−/lo B cells are more susceptible to develop autoimmune syndromes, suggesting that CD21−/lo B cells may contribute to the development of autoimmunity in humans.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are very much indebted to the patients and their families. We thank Dr S. Rudchenko and S. Semova for help with the single-cell sorter, Drs D. Orange, D. Goldenberg, and K. Warnatz for providing RA and CVID patient samples, and Dr G. Charvin for microscopy analysis.
This study was supported by grant AI061093 and AI071087 from National Institutes of Health/National Institute of Allergy and Infectious Diseases.
National Institutes of Health
Authorship
Contribution: I.I. performed the experiments, with help from E.M. for cell isolation and sorting and from Y.-S.N., L.M., G.M., D.S., and I.S. for antibody cloning and reactivity testing; E.M. initiated the collaboration with J.S., J.B., J.H.B., and C.C.-R., who provided patient blood samples; and I.I. and E.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.-S.N., L.M., G.M., and E.M. is Department of Immunobiology, Yale University School of Medicine, New Haven, CT. The current affiliation for J.S. is Division of Rheumatology, Hospital for Joint Diseases, NYU Langone Medical Center, New York, NY.
Correspondence: Eric Meffre, Department of Immunobiology, Yale University School of Medicine, 10 Amistad St, New Haven, CT 06520-8089; e-mail: eric.meffre@yale.edu.

![Figure 4. CD21−/lo naive B cells fail to properly respond to BCR triggering. CD21−/lo naive B cells from (A) RA01 and (B) CVID321 patients fail to up-regulate CD69 and CD25 expression after stimulation with F(ab′2) anti-IgM, recombinant human CD40L, and TLR9 agonist CpG. Total naive B cells containing both CD21−/lo and CD21+ B cells were plated and dot plots show CD69 and CD25 expression after 2 days according to gated CD21 expression. (C) BCR triggering in CD21−/lo naive B cells does not properly increase [Ca2+]i. [Ca2+]i was assessed by flow cytometry in CD21−/lo and CD21+ naive B cells from a healthy donor (HD) and RA01 and CVID321 patients. Both B-cell populations were loaded with Fluo-3 and expressed similar levels of surface IgM expression when experiments were performed. Arrow indicates when F(ab′)2 anti-IgM was added. Data from each group of persons are representative of 2-3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/24/10.1182_blood-2009-09-243071/4/m_zh89991052690004.jpeg?Expires=1769244769&Signature=eGXXbMKdzmoyAkZ00qZWkMlu1kbvbJ5rfYEWYN4svirzxTSHKR0iWX3kNlbnt13iSl491U70kBg1JDMOcwO6WTLpRjWwRgMe0b0vCpbRxhw9J~aop~GeLQRFRzoKcmvcOo67-W15AyD7ADdGYGwAzc72wyb1xEP4Vk3mXzFzlLAN0V6NRhNdI6xnYKBNDR5~8G5NfIUc33rAvpO1D5IP7TYv8o3sMClCADJIymlFwJw-Hd9FYjrmkrEYzbE6pnwSxzS0H6-Ml0dn5zfKuhOgRmn9UHzeTX2VdnvdCkp7-iBykBwkRZhr-d8P~dp3vEPfa24cD9FKLLbcHcqdZpNNvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 4. CD21−/lo naive B cells fail to properly respond to BCR triggering. CD21−/lo naive B cells from (A) RA01 and (B) CVID321 patients fail to up-regulate CD69 and CD25 expression after stimulation with F(ab′2) anti-IgM, recombinant human CD40L, and TLR9 agonist CpG. Total naive B cells containing both CD21−/lo and CD21+ B cells were plated and dot plots show CD69 and CD25 expression after 2 days according to gated CD21 expression. (C) BCR triggering in CD21−/lo naive B cells does not properly increase [Ca2+]i. [Ca2+]i was assessed by flow cytometry in CD21−/lo and CD21+ naive B cells from a healthy donor (HD) and RA01 and CVID321 patients. Both B-cell populations were loaded with Fluo-3 and expressed similar levels of surface IgM expression when experiments were performed. Arrow indicates when F(ab′)2 anti-IgM was added. Data from each group of persons are representative of 2-3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/24/10.1182_blood-2009-09-243071/4/m_zh89991052690004.jpeg?Expires=1769244770&Signature=iFuMU8EQWqu62w1sCR0~mG2682UGpanb9~2~glXgc0Ldy2Nq-8i60epGQ8ZxakU6qBREmt6uCMg6n-30XyfqjtIugfkt89OqFM3SBlQp1TogfgND2ntpWCbxr0Zvw~jkgcUsIGRl5hiF-inZOGMFZnva-SV4L6wS54J3KpBG0pO8gwSbxELVPFcsXtFkh5oXLbnyMvNAKpSISkJLJ1p1X6yh2hXN5OAX4RXSX-nQXDrtFxpVGzL33u55csHh7kbALlWFmM8FjLW~MO4QR9J2vyYRSf~ysBSGhc~kr~n1W~SZoYJi5PQfDtYivXAogG4HtKfLXxCUZ6T2DcHRlf~s0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)