In this issue of Blood, Isnardi and colleagues describe a phenotypically distinct population of autoreactive B cells that have become functionally limited upon stimulation, or “anergic.”1 Importantly, these cells are found at increased frequency in some rheumatoid arthritis patients and in patients with autoimmune-associated CVID.
The concept of clonal anergy was first proposed by Beverly Pike and Gustov Nossal in 1982 when they found that B-cell precursors cultured with high levels of antibody against surface IgM (anti-Cμ) impeded any B-cell development.2 In contrast, low concentrations of anti-Cμ resulted in normal numbers of B cells. However, unlike B cells that emerged in the absence of any anti-Cμ, these cells could no longer be activated by polyclonal stimulation to proliferate or to secrete antibody. Clonal anergy was proposed as a way to inactivate B cells stimulated early in development when only autoantigens would be presented. Experiments by the Goodnow group elegantly demonstrated clonal anergy in vivo using mice with transgenic B-cell receptors (BCR) against hen-egg lysozyme (HEL).3 When crossed to mice expressing a soluble form of HEL, the transgenic B cells became anergic. In the more than 20 years since these first papers were published, there have been many informative studies describing anergic B cells in unmanipulated and various transgenic mouse models,4 but only now are we beginning to characterize anergic B cells in humans.
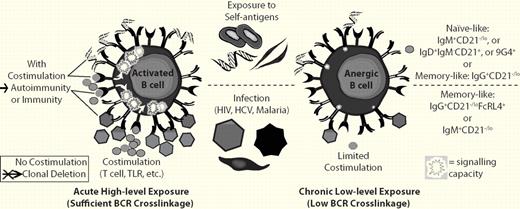
Chronic low-level BCR cross-linkage and a lack of sufficient costimulation are believed to result in B-cell anergy, whereas high BCR cross-linkage with costimulation leads to activation or without proper costimulation leads to clonal deletion. B cells of several types in humans have been demonstrated to be autoreactive and anergic (top). In addition, memory B cells (bottom) from patients with HIV have been shown to be “exhausted,” which is similar to anergy. B cells with a similar phenotype have also been found in other chronic infectious diseases including HCV8 and malaria.7
Chronic low-level BCR cross-linkage and a lack of sufficient costimulation are believed to result in B-cell anergy, whereas high BCR cross-linkage with costimulation leads to activation or without proper costimulation leads to clonal deletion. B cells of several types in humans have been demonstrated to be autoreactive and anergic (top). In addition, memory B cells (bottom) from patients with HIV have been shown to be “exhausted,” which is similar to anergy. B cells with a similar phenotype have also been found in other chronic infectious diseases including HCV8 and malaria.7
The anergic B cells described by Isnardi et al in this issue of Blood were naive-like B cells identified primarily by down-regulation of cell-surface complement receptor 2 (or CD21−/lo).1 Because chronic infection and other autoimmune diseases such as lupus have been previously reported to also cause a CD21−/lo (and in some instances, anergic) phenotype, Isnardi et al propose that this phenotype results from the common feature of being chronically stimulated by B-cell receptor engagement leading to an eventual reduction in signaling capacity1 (see figure). Induction of anergy may also depend on the context of BCR stimulation invoking the long-held central theory of 2-signal stimulation for immunity versus single-signal induction of tolerance first proposed by Bretscher and Cohn some 40 years ago.5 In modern terms, the chronic stimulation likely occurs without sufficient costimulation via T-cell help or pattern-recognition receptors (ie, Toll-like receptor engagement). In the absence of sufficient costimulation, high BCR cross-linkage leads to clonal deletion whereas low BCR cross-linkage results in anergy (see figure).
What is striking is that “the anergic B cell” appears to take multiple forms. Phenotypically, the various anergic B cells found in humans are quite divergent. The RA and common variable immunodeficiency (CVID) “anergic” cells are predominantly CD21−/lo naive B cells that have never been involved in an immune response, whereas the anergic cells isolated from healthy people or from people with chronic infectious diseases look predominantly like CD21−/lo IgG memory B cells. For example, Fc-receptor-like-4 (FCRL4)–expressing IgG memory B cells that are also CD21−/lo and have become functionally inert are expanded in HIV patients.6 A similar population was reported to be expanded in malaria patients7 and an IgM memory-like CD21−/lo B-cell population is expanded in hepatitis C virus (HCV) patients.8 In these instances of chronic infection, functional inactivation of lymphocytes is referred to as “exhaustion” rather than anergy. The Sanz laboratory reported that human B cells known to be naturally autoreactive to polylactosamine glycans that can be identified by the 9G4 anti-idiotypic antibody are anergic and strictly naive.9 Zheng et al also identified 9G4+IgM+ marginal zone–like memory and plasma cells, suggesting a complex regulation of 9G4+ B cells.10 In addition, Duty et al recently identified a subset of naive B cells that is common in healthy people that is anergic and naturally autoreactive to diverse self-antigens.11 Similar to the anti-HEL transgenic mouse model of anergy, these anergic B cells have down-regulated IgM on the cell surface but maintain IgD and, in contrast to the cells reported by Isnardi et al1 and exhausted memory B cells,6-8 they are CD21+ and are negative for FCRL4 (S.F.A. and P.C.W., unpublished data, 2010). In mice, a number of laboratories have identified a similar diversity of B-cell phenotypes that are anergic, ranging from transitional-like to memory cell–like and with diverse self-antigen specificities (reviewed in Cambier et al4 ). Early on there was hope that we might identify an anergic B-cell type in humans that might be diagnostic of autoimmune diseases or targeted in some fashion for the treatment of disease. In the end, it seems that the anergic phenotype is likely a common phenomenon that can affect various types of B cells.
When B cells recognize self, induction of anergy can help to avoid autoimmune reactivity. However, there is evidence in both humans9-11 and mice4 that anergic, autoreactive B cells can become activated with sufficient stimulation. Thus, since their discovery,2 these cells have posed a risk and a conundrum: why are they maintained at all? One possibility is that anergic B cells are simply being detected as they are honed from the repertoire and therefore pose no risk. The examples of exhaustion in B cells associated with chronic infectious diseases suggests that rescue from anergy by proper stimulation may in some cases be a necessary measure to fight infections. Another intriguing possibility is that they are the precursors of regulatory B cells or are themselves able to induce tolerance of autoreactive T-cell populations via HLA-II presentation of self-peptides. Examples of regulatory B cells have been recently identified in both mice12 and humans.13 A role for anergy or a lack thereof in causing or exacerbating either autoimmunity or chronic infectious disease has not been formally established, likely because of the complicated nature of this phenotype. The hope is that by furthering our understanding of the causes and function of anergy, we may manipulate this phenotype in the treatment of diseases.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■