In neutrophils, the phosphoinositide 3-kinase/Akt signaling cascade is involved in migration, degranulation, and O2− production. However, it is unclear whether the Akt kinase isoforms have distinct functions in neutrophil activation. Here we report functional differences between the 2 major Akt isoforms in neutrophil activation on the basis of studies in which we used individual Akt1 and Akt2 knockout mice. Akt2−/− neutrophils exhibited decreased cell migration, granule enzyme release, and O2− production compared with wild-type and Akt1−/− neutrophils. Surprisingly, Akt2 deficiency and pharmacologic inhibition of Akt also abrogated phorbol ester-induced O2− production, which was unaffected by treatment with the phosphoinositide 3-kinase inhibitor LY294002. The decreased O2− production in Akt2−/− neutrophils was accompanied by reduced p47phox phosphorylation and its membrane translocation, suggesting that Akt2 is important for the assembly of phagocyte nicotinamide adenine dinucleotide phosphate oxidase. In wild-type neutrophils, Akt2 but not Akt1 translocated to plasma membrane upon chemoattractant stimulation and to the leading edge in polarized neutrophils. In the absence of Akt2, chemoattractant-induced Akt protein phosphorylation was significantly reduced. These results demonstrate a predominant role of Akt2 in regulating neutrophil functions and provide evidence for differential activation of the 2 Akt isoforms in neutrophils.
Introduction
Akt, also known as protein kinase B (PKB), constitutes a family of serine/threonine kinases with a characteristic pleckstrin homology (PH) domain in the N-terminus followed by a catalytic domain and a C-terminal regulatory domain. Akt has 3 isoforms, Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ). Akt1 was initially identified through homologous DNA cloning in 1991 and was found as a cellular homologue of the viral oncogene v-akt from the acutely transforming retrovirus Akt8.1 Akt2 was identified as an overexpressed protein in ovarian carcinoma cell lines and primary ovarian tumors.2 Akt3 initially was cloned from the rat brain and testis.3 These Akt isoforms share a high degree of homology, especially in the catalytic domains (87%-90% identical amino acids). Walker et al,4 in an vitro study, found no discernable difference between Akt1, Akt2, and Akt3 in their ability to recognize and phosphorylate peptide substrate. In other studies,5,,–8 the individual Akt isoforms were found to distribute differently and have different functions despite the high level of homology between these isoforms. Akt1 is expressed in most tissues and promotes cell survival by inhibiting apoptosis. Akt1 also induces protein synthesis and is crucial to growth and development as shown in studies that used Akt1−/− mice.9,–11 Akt2 is expressed mainly in insulin-responsive organs, including liver, skeletal muscle, and adipose tissue. Consistent with its primary function in insulin signaling, the Akt2−/− mice display a type 2 diabetic phenotype.12,,–15 Akt3 is expressed at a lower level than the other 2 Akt isoforms in all adult tissues except the brain and testis, where it is abundant. Although the functions of Akt3 have not been well defined, mice lacking Akt3 have smaller brains.16,17
The phosphoinositide 3-kinase (PI3K)/Akt signaling pathway is critical to several neutrophil functions, including chemotaxis, degranulation, and O2− production.18 Akt proteins translocate to plasma membrane after agonist stimulation,19,20 which is similar to their activation process in other cells.21 Several isoforms of PI3K have been shown to mediate chemoattractant-stimulated generation of PtdIns(3,4,5)P3, to which the PH domain of Akt binds. Membrane targeting is critical to full activation of Akt, which involves phosphorylation at Thr308 and Ser473 (on the basis of the amino acid positions in human Akt1). Taking advantage of this feature, Servant et al22 used the PH domain of Akt as a probe to detect chemoattractant signaling in polarized neutrophils. The PI3K/Akt signaling pathway is one of the key components for the induced release of neutrophil granule contents,23 although the exact mechanisms remain unknown. Akt is one of the protein kinases that phosphorylate p47phox, resulting in its membrane translocation and activation of phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.24,–26 Akt phosphorylates the autoinhibitory region of p47phox at Ser304 and Ser328, and the authors of in vitro studies have shown that inhibition of this phosphorylation event reduces O2− generation.25,26
In all published studies of neutrophils, however, Akt has been treated as a single enzyme rather than a kinase family consisting of several isoforms. Therefore, it is unclear which Akt isoforms are expressed in neutrophils and whether these Akt isoforms have different functions in neutrophil activation. The lack of knowledge in Akt isoform signaling is in contrast to a much better understanding of different PI3K isoforms in neutrophil activation. To address the physiologic functions of the Akt isoforms in neutrophils, we have analyzed Akt-dependent activities in isogenic wild-type (WT) and knockout (KO) mouse neutrophils lacking the individual Akt isoforms. Our results have led to the discovery that Akt2 but not Akt1 is critical to chemoattractant-induced neutrophil O2− production, degranulation, and cell migration. Akt2 is also required for PMA-induced O2− production independent of PI3K.
Methods
Reagents
The phorbol ester phorbol 12-myristate 13-acetate (PMA), N-formyl-Met-Leu-Phe (fMLF), isoluminol, luminol, cytochalasin B, and zymosan were purchased from Sigma-Aldrich. The Akt inhibitor X (SH X), PI3K inhibitor LY294002, and protein kinase C (PKC) inhibitor GF109203X (GF) were purchase from Calbiochem. A detailed list of antibodies used in this study is provided in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article.
Animals
Akt1 gene deletion mice were generated in the laboratory of Nissim Hay as described.9 The generation of the Akt2 gene deletion mice has been described previously.27 These mice were backcrossed to C57BL/6 background. Mice that were 6 to 8 week of age were used in the study. All mouse procedures were conducted with the use of protocols approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Preparation of bone marrow-derived mouse neutrophil
Mice were killed with CO2 inhalation, followed by cervical dislocation. Femurs and tibias were isolated, and neutrophils were purified by the use of Nycoprep as described previously.28 The purity of neutrophils was typically 78% to 80%, with approximately 98% viable cells.
Reverse transcription PCR
Total RNA was extracted from mouse neutrophils with the use of TRIzol reagent (Invitrogen Life Technologies). Reverse transcription and polymerase chain reaction (PCR) amplification of the specific DNA fragments were conducted as described.23 PCR amplifications were performed for 30 cycles with denaturation at 94°C for 30 seconds, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute. PCR genotyping and primer sequence11 are detailed in supplemental Figure 1 legend.
Degranulation
The release of β-glucuronidase from stimulated neutrophils was determined by the use of procedures described previously,23 with the exception that fluorescence was measured on a FlexStation II (Molecular Devices) with excitation wavelength at 365 nm and emission wavelength at 460 nm. The percentage of release, relative to the total enzyme in neutrophils, was expressed.
Neutrophil polarization and migration
Freshly isolated neutrophils were suspended at a concentration of 5 × 106 cells/mL in modified Hanks balanced salt solution containing 150mM NaCl, 4mM KCl, 1.2mM MgCl2, 10 mg/mL glucose, 20mM HEPES, and 2% bovine serum albumin, pH 7.2. Cells (5 × 105 in 100 μL) were plated on fibrinogen-coated glass coverslips with 100 μg/mL fibrinogen. The coverslip was incubated in a humid chamber at 37°C with 5% CO2 for 15 minutes, and nonadherent cells were removed by 2 washes with modified Hanks balanced salt solution. Then cells were stimulated with a uniform concentration of fMLF. All images shown were captured with a 40×/1.3 NA oil objective with the use of an inverted microscope (Zeiss Axio Observer-D1) equipped with an Axiocam MR camera driven by Axiovision imaging software. For tracking cell migration, randomly selected cells were recorded in time-lapse mode. Images were taken at 10-second intervals for 10 minutes.
Immunofluorescence microscopy
Neutrophils on glass coverslips coated with fibrinogen were stimulated with or without agonists (fMLF or C5a as indicated in figure legends), washed once with ice-cold phosphate-buffered saline (PBS), and then fixed with 3% paraformaldehyde in PBS as described previously.29 The anti-Akt antibodies were used at 1:500 dilution, and cells were incubated with a rhodamine red-X–conjugated goat anti–mouse IgG (secondary antibody) at room temperature for 1 hour. After washing, the coverslips were mounted on glass slides with the use of ProLong Gold anti-fade reagent with DAPI (Molecular Probes). Fluorescence images were captured with a Zeiss LSM 510 confocal microscope equipped with helium-neon, argon, and krypton laser sources.
Superoxide generation
O2− generation was measured by the use of isoluminol or luminol-enhanced chemiluminescent method as described previously.30 Each sample contains 1 × 106 neutrophils, which were stimulated with PMA, fMLP, C5a, or serum-opsonized zymosan.
Membrane fractionation
Approximately 2.5 × 107 neutrophils in 500 μL of PBSG buffer were stimulated with 5μM fMLF for 2 minutes before 5 mL of ice-cold PBS was added. The cells were pelleted and suspended into 500 μL of HES I buffer (0.25M sucrose; 20mM Tris, pH 7.6; 1mM EDTA [ethylenediaminetetraacetic acid] plus protease inhibitor set I; Calbiochem). The cells were subjected to 3 cycles of freeze (in liquid nitrogen) and thaw (at 37°C). Samples were then centrifuged at 600g to clear unbroken cells. Total cell lysate was layered on 500 μL of HES II buffer (1.12M sucrose; 20mM Tris, pH 7.6; 1mM EDTA) and centrifuged at 16 000g for 60 minutes. The supernatant saved was designated as the cytosol fraction. The plasma membrane layers were removed from the sucrose cushion, suspended in HES I buffer, and centrifuged at 16 000g for 60 minutes. The resulting pellets were collected as plasma membrane.
Detection of p47phox phosphorylation
Enrichment and separation of phosphorylated proteins from neutrophil lysates was performed by the use of a phosphoprotein purification kit (QIAGEN), as described previously.23 Antibody detection of phosphorylated p47phox was conducted as detailed in a recent publication.28 The Western blot result was quantified with the use of ImageJ software (National Institutes of Health).
Detection of Akt phosphorylation
Bone marrow neutrophils (1 × 107) from WT, Akt1−/−, and Akt2−/− mice were stimulated with C5a (100nM) for 1 minute. Total cell lysate was prepared and resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and detected with specific anti–phospho-Akt antibodies that recognize phosphorylated Akt at S473 (Cell Signaling) and T308 (Calbiochem). For loading control, β-actin and total Akt in the cell lysate were blotted with the use of specific antibodies against these proteins.
Statistical analysis
Data were analyzed by the Student t test with the PRISM software (Version 4.0; GraphPad).
Results
Expression of Akt isoforms in neutrophils
To determine whether the neutrophil Akt isoforms function differently, we first examined their expression profile in mouse bone marrow–derived neutrophils. Using reverse transcription PCR with primers that bind to the coding sequence unique to each of the Akt isoforms, we found that the transcripts of both Akt1 and Akt2 are abundant in mouse neutrophils (supplemental Figure 1A). In contrast, the transcript of Akt3 is hardly detectable. On the basis of this result, we chose to focus on Akt1 and Akt2 in the present study. Genetically altered mice lacking the individual Akt isoform genes were prepared as described previously.9,11 Genotyping of the Akt1 and Akt2 KO mice confirmed the absence of the individual Akt isoform genes (supplemental Figure 1B). Antibodies that specifically recognize Akt1 or Akt2 were used for the detection of Akt protein expression in the KO neutrophils. As shown (supplemental Figure 1C), the absence of Akt1 and Akt2 proteins in the respective KO neutrophils was confirmed. Deletion of either Akt1 or Akt2 does not change the expression level of the other Akt isoform in peripheral blood neutrophils. Interestingly, Akt2 gene deletion resulted in an increase in the number of peripheral blood neutrophils (4.260 ± 0.534 × 103/μL compared with 1.285 ± 0.099 in WT and 1.735 ± 0.304 in Akt1−/− mice; P < .01 between Akt2−/− and WT or Akt1−/− mice; n = 4). There was also an increase in the percentage of neutrophils among total white blood cells (supplemental Figure 2A), a phenotype similar to that of the PI3K-γ KO mice.31 Neutrophils from the 2 Atk KO lines were morphologically indistinguishable from those from WT mice (supplemental Figure 2B). In subsequent assays, equal numbers of neutrophils from WT, Akt1−/−, and Akt2−/− mice were used.
Akt2, but not Akt1, is required for neutrophil degranulation and migration
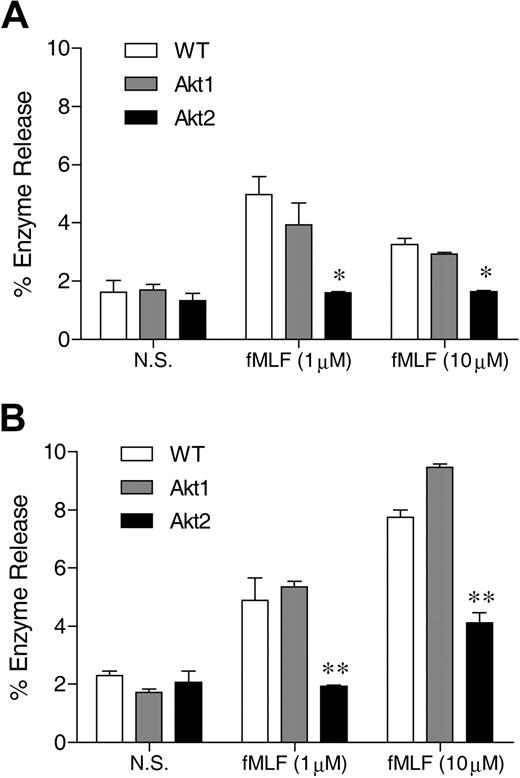
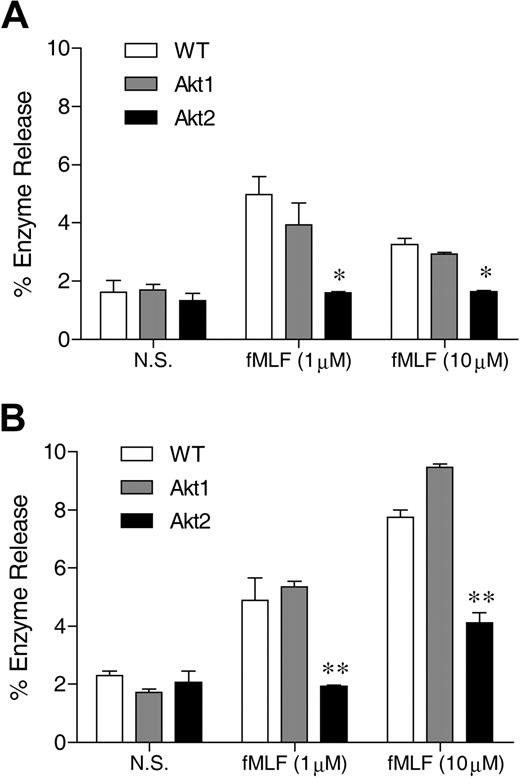
Neutrophils respond to chemoattractants, such as fMLF, with release of granule contents. The degranulation process is dependent on the PI3K-Akt pathway.23 The role of individual Akt isoforms in neutrophil degranulation is unclear because available pharmacologic inhibitors of Akt do not distinguish between different isoforms of Akt. We compared the ability of WT and Akt isoform KO neutrophils to release β-glucuronidase after fMLF stimulation. In WT neutrophils, fMLF induced maximal β-glucuronidase release at an agonist concentration of 1μM (Figure 1), consistent with its potency at the mouse formyl peptide receptors.32 In the presence of cytochalasin B, a greater concentration of fMLF (10μM) induced an additional increase in β-glucuronidase release (compare Figure 1B with 1A). Regardless of the presence of cytochalasin B, deletion of the Akt2 gene resulted in a significant reduction in β-glucuronidase release (P < .01 compared with WT and Akt1−/− neutrophils), although a significant amount of enzyme release over baseline (P < .05) remained when the Akt2−/− neutrophils were exposed to 10μM of fMLF. Degranulation induced by C5a (not shown) and PMA (Figure 5F) was similarly diminished by deletion of the Akt2 gene.
Akt2 is important for neutrophil release of β-glucuronidase. Bone marrow–derived neutrophils from wild-type (WT), Akt1−/−, and Akt2−/− mice were preincubated without (A) or with (B) cytochalasin B (10μM) for 15 minutes on ice and 15 minutes at 37°C. The cells were then stimulated with fMLF at indicated concentrations for 10 minutes. The released β-glucuronidase was quantified and expressed as percentage of total cellular β-glucuronidase. The results are shown as means ± SEM, on the basis of 3 independent experiments each conducted in duplicate. *P < .05, **P < .01, compared with WT in the same stimulation group.
Akt2 is important for neutrophil release of β-glucuronidase. Bone marrow–derived neutrophils from wild-type (WT), Akt1−/−, and Akt2−/− mice were preincubated without (A) or with (B) cytochalasin B (10μM) for 15 minutes on ice and 15 minutes at 37°C. The cells were then stimulated with fMLF at indicated concentrations for 10 minutes. The released β-glucuronidase was quantified and expressed as percentage of total cellular β-glucuronidase. The results are shown as means ± SEM, on the basis of 3 independent experiments each conducted in duplicate. *P < .05, **P < .01, compared with WT in the same stimulation group.
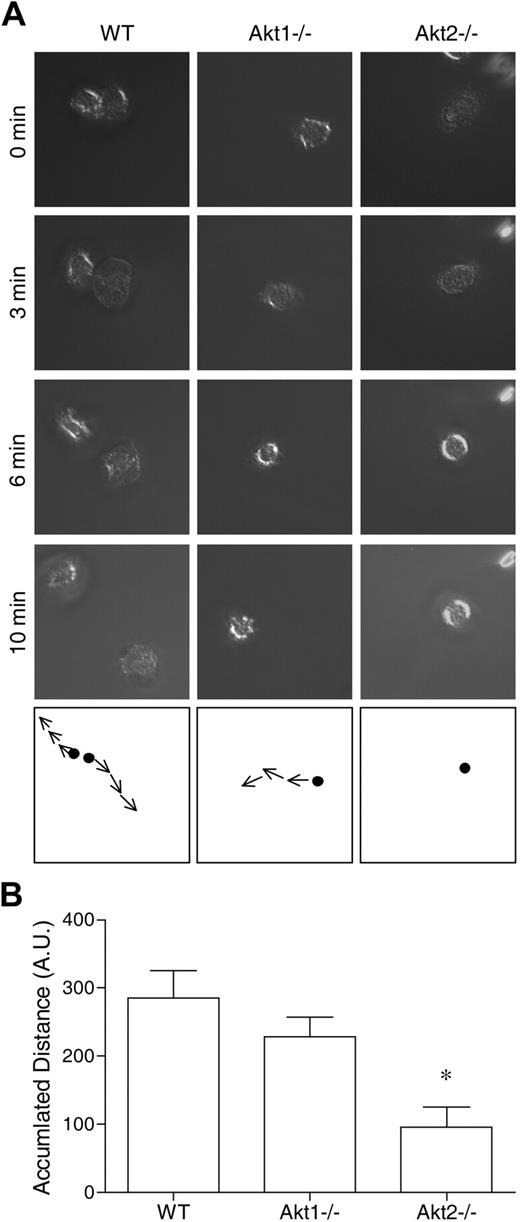
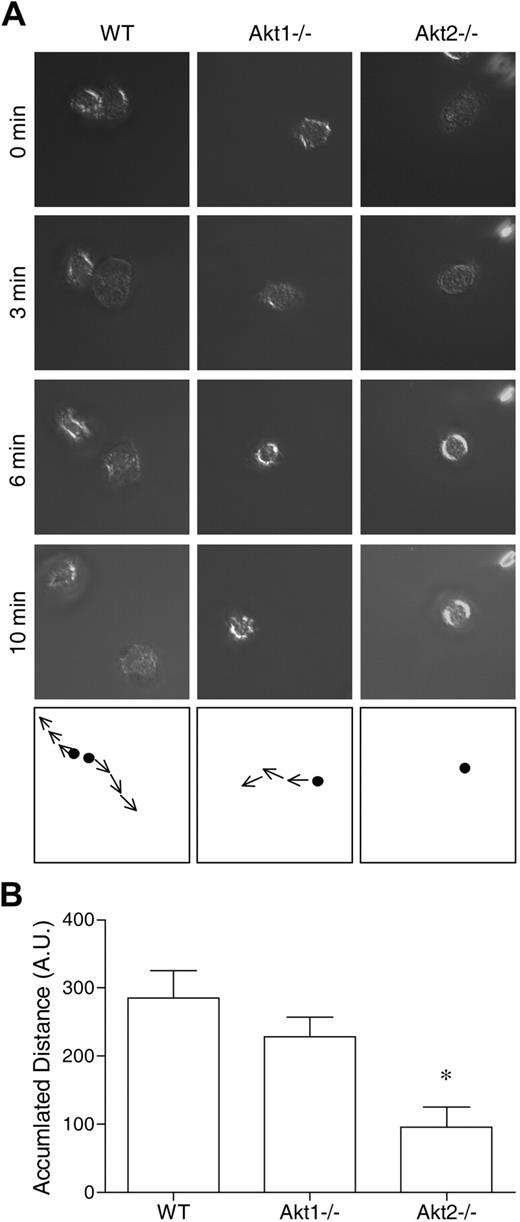
The aforementioned results suggest that Akt2 but not Akt1 is critical to the activation of neutrophils by at least 2 types of soluble stimuli: classic chemoattractants that triggers a G protein–coupled signaling pathway, and a diacylglycerol mimetic (PMA) that directly activates PKC. Because the PI3K-Akt pathway is known to play a role in chemoattractant-induced neutrophil activation,19,24,31,33 we next examined whether deletion of the individual Akt isoform genes has an impact on neutrophil migration. Both WT and the individual KO neutrophils were stimulated with fMLF (1μM), and neutrophil polarization, shape change, and movement were recorded as describe in “Neutrophil polarization and migration.” We found that Akt1 gene deletion had minimal impact on neutrophil migration, but Akt2−/− neutrophils barely moved when stimulated with fMLF (Figure 2). In video microscopy analysis, the Akt2−/− neutrophils failed to migrate upon chemoattractant stimulation (supplemental Video 6, compared with supplemental Video 4 and supplemental Video 5). No significant neutrophil migration was observed in the absence of fMLF (supplemental Videos 1-3).
Deficiency of Akt2 but not Akt1 impairs neutrophil migration. Bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice were seeded onto glass coverslips coated with fibrinogen and stimulated with 1μM fMLF. The migration of the neutrophils under a uniform fMLF concentration was monitored periodically, and video images were recorded. The path of cell migration over time is shown in the bottom panels. Representative images, collected from 3 independent experiments, are shown in panel A. In panel B, the distance traveled by the cells was calculated by use of the ImageJ software with manual tracking (n = 10 from each sample). *P < .05 compared with WT mouse neutrophils. Video clips for cell migration are shown in the supplemental Videos.
Deficiency of Akt2 but not Akt1 impairs neutrophil migration. Bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice were seeded onto glass coverslips coated with fibrinogen and stimulated with 1μM fMLF. The migration of the neutrophils under a uniform fMLF concentration was monitored periodically, and video images were recorded. The path of cell migration over time is shown in the bottom panels. Representative images, collected from 3 independent experiments, are shown in panel A. In panel B, the distance traveled by the cells was calculated by use of the ImageJ software with manual tracking (n = 10 from each sample). *P < .05 compared with WT mouse neutrophils. Video clips for cell migration are shown in the supplemental Videos.
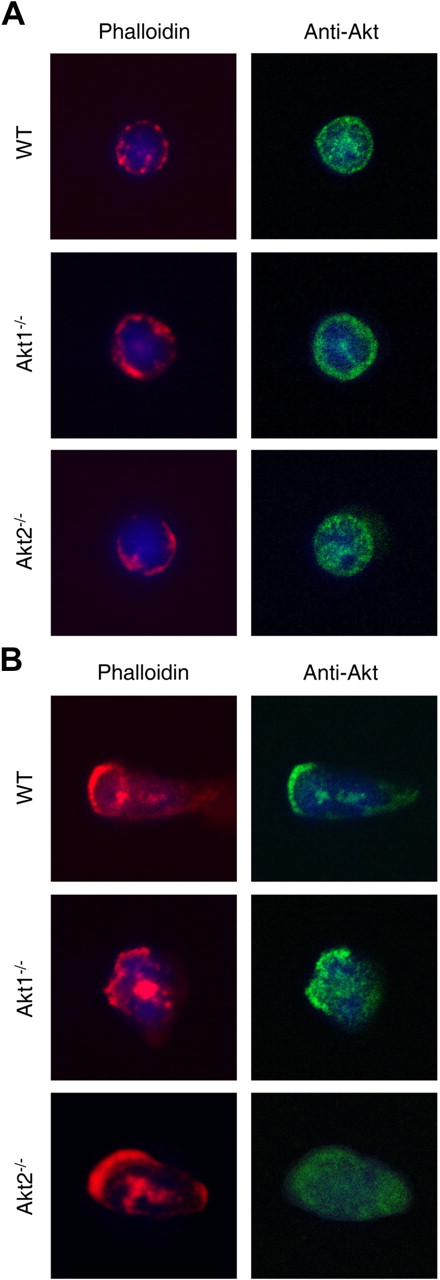
Servant et al22 have shown that Akt proteins translocate to the leading edge of neutrophils upon chemoattractant stimulation. We therefore examined Akt localization in the KO neutrophils before and after fMLF stimulation. In unstimulated WT and Akt KO neutrophils, the majority of Akt proteins were found in the cytoplasmic compartment (Figure 3A). After 2 minutes of fMLF stimulation, in the WT and Akt1−/− cells a significant amount of the cytoplasmic Akt redistributed to the leading edge, where F-actin was also present (Figure 3B top and middle panels). In contrast, in Akt2−/− neutrophils the remaining Akt protein (Akt1) was largely cytoplasmic, although polarization of the cells was evident on the basis of F-actin formation in the leading edge (Figure 3B bottom panels). These results demonstrate differences among the Akt isoforms in their membrane translocation upon fMLF stimulation.
Redistribution of Akt2 but not Akt1 in polarized neutrophils. Bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice were seeded onto glass coverslips and incubated with buffer (A) or 1μM fMLF (B) for 2 minutes at 37°C. Stimulation was terminated when 1 mL of ice-cold PBS was added. The cells were fixed with paraformaldehyde and stained first with a pan-Akt mouse mAb and then with an Alexa Fluor 488–conjugated goat anti–mouse IgG together with rhodamine phalloidin (1:500, to stain F-actin). 4′,6-Diamidino-2-phenylindole (in mounting solution) was used for nuclear staining. Representative images shown are selected from 20 images for each experimental condition from 2 independent experiments.
Redistribution of Akt2 but not Akt1 in polarized neutrophils. Bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice were seeded onto glass coverslips and incubated with buffer (A) or 1μM fMLF (B) for 2 minutes at 37°C. Stimulation was terminated when 1 mL of ice-cold PBS was added. The cells were fixed with paraformaldehyde and stained first with a pan-Akt mouse mAb and then with an Alexa Fluor 488–conjugated goat anti–mouse IgG together with rhodamine phalloidin (1:500, to stain F-actin). 4′,6-Diamidino-2-phenylindole (in mounting solution) was used for nuclear staining. Representative images shown are selected from 20 images for each experimental condition from 2 independent experiments.
Akt2 is required for neutrophil O2− production induced by soluble stimuli
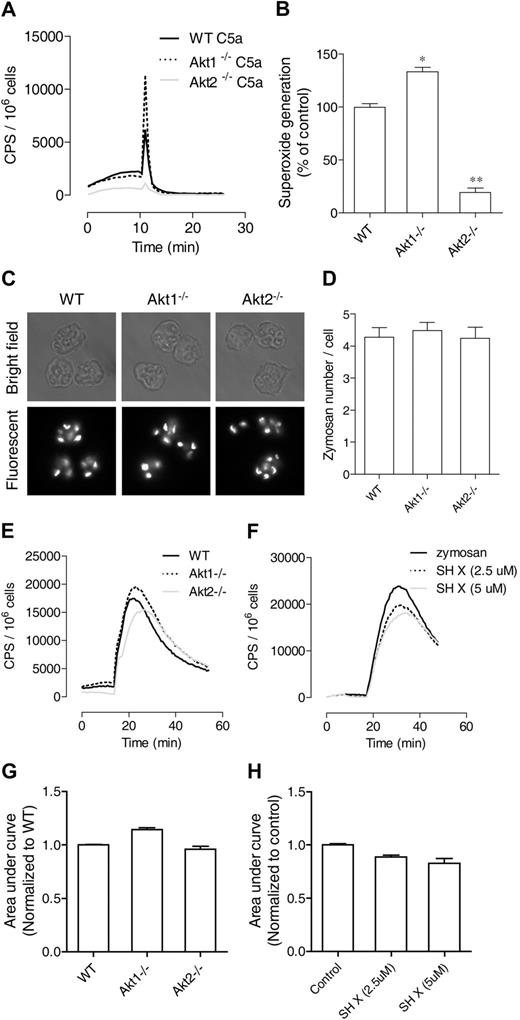
The PI3K-Akt pathway has been implicated in chemoattractant-induced neutrophil NADPH oxidase activation, primarily on the basis of studies in which the authors used pharmacologic inhibition of PI3K and genetic deletion of the PI3K isoforms. It is unclear whether neutrophil O2− production is dependent on Akt, although Akt has been identified as one of the kinases that phosphorylate p47phox.25,26 The availability of individual Akt1 and Akt2 KO mice has made it possible to determine whether Akt is critical to NADPH oxidase activation and which one of the Akt isoforms is required for neutrophil O2− production. We found that stimulation of neutrophils from these Akt KO mice produced drastically different results. The Akt2−/− neutrophils generated significantly less O2− when stimulated with C5a (Figure 4A) or fMLF (data not shown), whereas the Akt1−/− neutrophils responded to the same ligand with an increase in O2− generation. Quantification of O2− production verified that the reduction in Akt2−/− neutrophils was highly significant compared with WT and Akt1−/− neutrophils (Figure 4B). Moreover, the PMA-stimulated O2− production was also markedly reduced in Akt2−/− neutrophils (Figure 5A). However, a small but statistically significant increase (P < .05) was observed in Akt1−/− neutrophils (Figure 5A; quantification not shown).
Role of Akt2 in chemoattractant and particle-induced O2− generation. Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated with C5a (A) and O2− generation was recorded as counts per minute (CPS) on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 3 independent experiments. Because of the short duration of O2− production, peak values of chemiluminescence were quantified (B) and shown as percentage change (mean ± SEM) on the basis of 3 independent experiments. *P < .05, **P < .01 relative to WT neutrophils. (C) Uptake of fluorescein isothiocyanate (FITC)–labeled zymosan by bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice after a 45-minute incubation. Shown are images taken with bright field (top row) and fluorescent (bottom row, shown in black and white) microscopy. (D) The zymosan particles in 20 cells were counted and quantified, and no significant difference was seen among WT, Akt1−/−, and Akt2−/− neutrophils. Shown are mean ± SEM from 3 experiments. (E) Mouse neutrophils from WT, Akt1−/−, and Akt2−/− were stimulated with serum-opsonized, FITC-labeled zymosan (500 μg/mL), and O2− generation was recorded on the basis of luminol-enhanced chemiluminescence during the indicated time period. (F) WT neutrophils were pretreated with the Akt inhibitor X (SH X) at the indicated concentrations for 10 minutes and then stimulated with 500 μg/mL serum-opsonized, FITC-labeled zymosan. O2− generation was recorded during the time period as described previously. The traces shown in panels E and F are representative of 3 independent experiments. Quantifications of data in panels E and F are shown in panels G and H, respectively, as mean ± SEM, on the basis of 3 experiments.
Role of Akt2 in chemoattractant and particle-induced O2− generation. Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated with C5a (A) and O2− generation was recorded as counts per minute (CPS) on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 3 independent experiments. Because of the short duration of O2− production, peak values of chemiluminescence were quantified (B) and shown as percentage change (mean ± SEM) on the basis of 3 independent experiments. *P < .05, **P < .01 relative to WT neutrophils. (C) Uptake of fluorescein isothiocyanate (FITC)–labeled zymosan by bone marrow–derived neutrophils from WT, Akt1−/−, and Akt2−/− mice after a 45-minute incubation. Shown are images taken with bright field (top row) and fluorescent (bottom row, shown in black and white) microscopy. (D) The zymosan particles in 20 cells were counted and quantified, and no significant difference was seen among WT, Akt1−/−, and Akt2−/− neutrophils. Shown are mean ± SEM from 3 experiments. (E) Mouse neutrophils from WT, Akt1−/−, and Akt2−/− were stimulated with serum-opsonized, FITC-labeled zymosan (500 μg/mL), and O2− generation was recorded on the basis of luminol-enhanced chemiluminescence during the indicated time period. (F) WT neutrophils were pretreated with the Akt inhibitor X (SH X) at the indicated concentrations for 10 minutes and then stimulated with 500 μg/mL serum-opsonized, FITC-labeled zymosan. O2− generation was recorded during the time period as described previously. The traces shown in panels E and F are representative of 3 independent experiments. Quantifications of data in panels E and F are shown in panels G and H, respectively, as mean ± SEM, on the basis of 3 experiments.
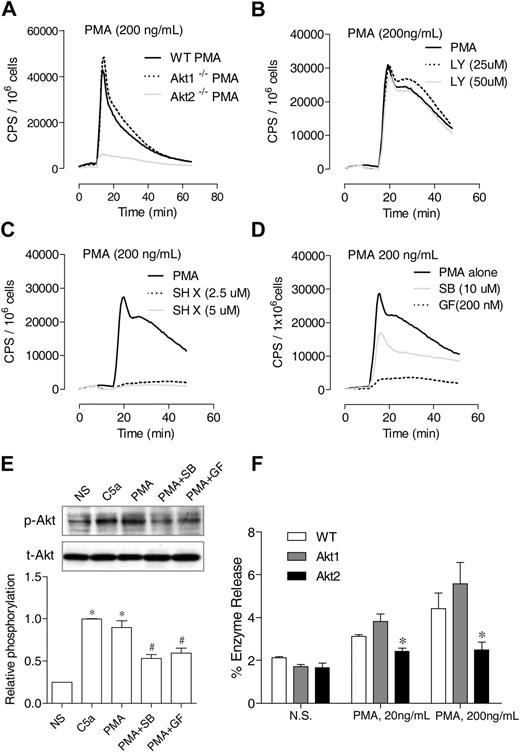
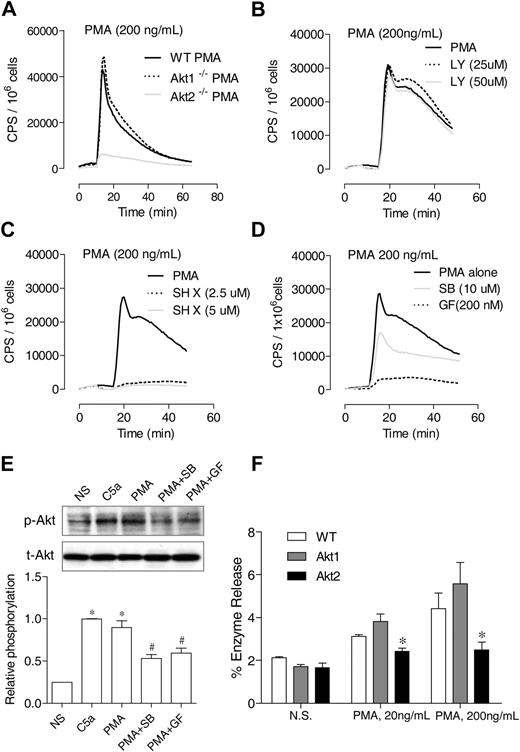
Differential requirements of PI3K and Akt for PMA-induced O2− generation. (A) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated with PMA (A), and O2− generation was recorded as counts per minute (CPS) on the basis of isoluminol-enhanced chemiluminescence. (B-C) WT mouse neutrophils were preincubated for 10 minutes with the PI3K inhibitor LY 294002 (B) or the Akt inhibitor SH X (C) at the indicated concentrations. The cells were then stimulated with PMA. O2− generation was recorded on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 2 independent experiments. (D) Neutrophils from WT mice were preincubated for 10 minutes with SB203580 (SB), GF109203x (GF), or vehicle (dimethyl sulfoxide, same concentration) and then stimulated with PMA. O2− generation was recorded on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 2 independent experiments. (E) The effect of SB203580 and GF109203x on PMA-induced Akt phosphorylation (top panel) was determined in neutrophils treated with the inhibitors described previously. PMA stimulation (200 ng/mL) was for 10 minutes. For comparison, neutrophils were also stimulated with C5a (100nM C5a). The relative level of Akt phosphorylation at Ser473 was determined on the basis of 3 experiments and are shown in the bar graph as mean ± SEM *P < .05 compared with the NS (no PMA or C5a stimulation) sample; #P < .05 compared with the PMA-stimulated sample. (F) Neutrophil from WT, Akt1−/−, and Akt2−/− mice were stimulated with different concentrations of PMA, and degranulation assay was performed as described in “Degranulation.” The percentage of released β-glucuronidase was determined and shown as mean ± SEM from 3 experiments, each in duplicate. *P < .05, compared to WT neutrophils.
Differential requirements of PI3K and Akt for PMA-induced O2− generation. (A) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated with PMA (A), and O2− generation was recorded as counts per minute (CPS) on the basis of isoluminol-enhanced chemiluminescence. (B-C) WT mouse neutrophils were preincubated for 10 minutes with the PI3K inhibitor LY 294002 (B) or the Akt inhibitor SH X (C) at the indicated concentrations. The cells were then stimulated with PMA. O2− generation was recorded on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 2 independent experiments. (D) Neutrophils from WT mice were preincubated for 10 minutes with SB203580 (SB), GF109203x (GF), or vehicle (dimethyl sulfoxide, same concentration) and then stimulated with PMA. O2− generation was recorded on the basis of isoluminol-enhanced chemiluminescence. The traces shown are representative of 2 independent experiments. (E) The effect of SB203580 and GF109203x on PMA-induced Akt phosphorylation (top panel) was determined in neutrophils treated with the inhibitors described previously. PMA stimulation (200 ng/mL) was for 10 minutes. For comparison, neutrophils were also stimulated with C5a (100nM C5a). The relative level of Akt phosphorylation at Ser473 was determined on the basis of 3 experiments and are shown in the bar graph as mean ± SEM *P < .05 compared with the NS (no PMA or C5a stimulation) sample; #P < .05 compared with the PMA-stimulated sample. (F) Neutrophil from WT, Akt1−/−, and Akt2−/− mice were stimulated with different concentrations of PMA, and degranulation assay was performed as described in “Degranulation.” The percentage of released β-glucuronidase was determined and shown as mean ± SEM from 3 experiments, each in duplicate. *P < .05, compared to WT neutrophils.
To identify the mechanism underlying deficient O2−production in Akt2−/− neutrophils, we first determined whether the phagocyte oxidase (phox) proteins were expressed normally in these cells. Western blotting analysis showed that the expression level of all phox components and the Rac small GTPase was unchanged in the Akt1 and Akt2 KO neutrophils (supplemental Figure 3). Next, we examined whether O2− production induced by other stimuli was affected. Deletion of the Akt1 and Akt2 genes did not affect neutrophil phagocytosis of serum opsonized zymosan particles (Figure 4C). No significant difference was observed between the individual KO neutrophils and WT neutrophils after quantification of the ingested particles in a 45-minute period (Figure 4D). The zymosan-induced superoxide generation was decreased in the Akt2−/− cells, but to a lesser extent than in the chemoattractant and PMA-stimulated neutrophils (Figure 4E), and the change was not statistically significant compared with WT neutrophils (Figure 4G; P > .05).
To further examine whether Akt is involved in phagocytosis-triggered O2− production, WT neutrophils were pretreated with the Akt inhibitor SH X and then incubated with serum-opsonized zymosan particles. A small but statistically significant reduction in O2− production (P < .05) was observed in WT neutrophils treated with 5μM SH X compared with vehicle-treated controls (Figure 4F,H). The inhibitory effect appears to be dose dependent. Likewise, a statistically significant decrease in O2− generation was observed when the Akt2−/− neutrophils were stimulated with IgG-coated zymosan, although no difference in the uptake of the IgG-coated zymosan particles was found (supplemental Figure 4). These findings indicate that Akt is involved in zymosan particle-induced O2− generation but is not critically required as in chemoattractant-induced O2− generation.
Differential requirement of PI3K and Akt for PMA-stimulated neutrophil activation
In neutrophils, Akt has been known as a downstream effector of the PI3Ks. It was reported that constitutive activation of PI3K leads to the continuous phosphorylation of p47phox by Akt.24 Unexpectedly, in this study we found that PMA-induced O2− production is differentially regulated by the PI3K inhibitor LY294002 and by the Akt inhibitor SH X. Although LY294002 had no inhibitory effect on the PMA-induced O2− generation in WT neutrophils (Figure 5B), SH X ablated neutrophil O2− production by PMA (Figure 5C). The PI3K-independent, Akt-dependent signaling suggests that the PMA-induced neutrophil O2− production involves an alternative pathway for Akt activation. Several kinases are reported to be downstream effectors of PMA and can phosphorylate p47phox, including p38 mitogen-activated protein kinase (MAPK)34 and selected isoforms of PKC.35
We therefore treated neutrophils with a pan-inhibitor of PKC (GF109203x) or a p38 MAPK inhibitor (SB203580) before the determination of PMA-induced O2− production and Akt phosphorylation. Both inhibitors reduced O2− generation (Figure 5D), with GF109203x being more potent, and they also inhibited Akt phosphorylation (Figure 5E). Thus, the PMA-induced, Akt-dependent O2− generation involves both the PKC isoforms found in neutrophils and, to a lesser extent, the p38 MAPK. The inhibition of PMA-induced O2− production by SH X is consistent with our finding that deletion of the Akt2 gene abrogates PMA-induced O2− production (Figure 5A). In addition to O2− generation, the PMA-induced β-glucuronidase release was reduced in neutrophils lacking Akt2 (Figure 5F).
Different membrane translocation of Akt1 and Akt2 correlates with their ability to mediate O2− production
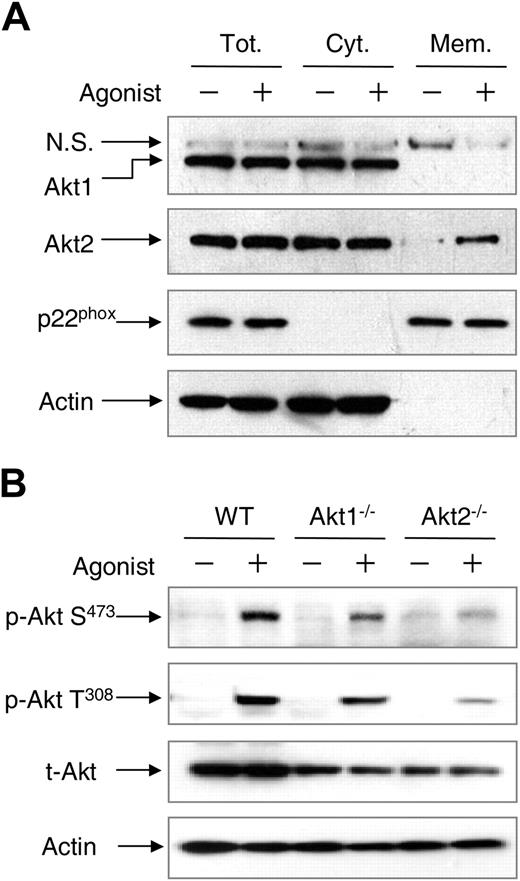
Results in Figure 3 show that Akt2 but not Akt1 translocates to the leading edge in polarized neutrophils upon chemoattractant stimulation. We examined whether the absence of one Akt isoform could affect the localization of the other Akt isoform. This was done by detecting the cellular distribution of Akt1 and Akt2 in WT neutrophils. Using cell fractionation and Western blotting, we found both Akt1 and Akt2 in the cytosolic fraction in resting neutrophils (Figure 6A). Upon fMLF stimulation, Akt2 but not Akt1 was detected in the membrane fraction, suggesting differential membrane translocation of the 2 Akt isoforms in WT neutrophils. A similar observation was made when the WT neutrophils were probed with the isoform-specific antibodies in imaging analysis (supplemental Figure 5). Akt translocation to the plasma membrane is required for the phosphorylation at both Ser473 and Thr308 in Akt1 and at similar amino acid positions in other Akt isoforms, as protein kinases critical to Akt phosphorylation are present in plasma membrane in activated cells.20,21
Different cellular distribution and phosphorylation of Akt1 and Akt2. (A) WT mouse neutrophils were stimulated with 5μM fMLF (+) or buffer (−) for 2 minutes. The membrane and cytosolic fractions were separated, resolved on SDS-PAGE, and detected with specific antibodies recognizing Akt1 or Akt2. N.S. indicates a nonspecific species recognized by the anti-Akt1 antibody. Anti-p22phox and anti–β-actin antibodies were used for detection of p22phox and β-actin as markers of the membrane and cytosolic fractions, respectively. Tot indicates total (membrane plus cytosolic fractions); Cyt, cytosolic fraction; and Mem, membrane fraction. (B) Chemoattractant-induced Akt phosphorylation. WT, Akt1−/−, and Akt2−/− neutrophils (1 × 107) from WT, Akt1−/−, and Akt2−/− mice were stimulated with C5a (+, 100nM) or buffer (−) for 1 minute. The samples were then resolved on SDS-PAGE and detected with specific anti–phospho-Akt antibodies recognizing phosphorylated Akt at Ser473 (Cell Signaling) and Thr308 (Calbiochem). β-actin and total Akt were detected for equal loading controls. Representative blots from 3 independent experiments are shown.
Different cellular distribution and phosphorylation of Akt1 and Akt2. (A) WT mouse neutrophils were stimulated with 5μM fMLF (+) or buffer (−) for 2 minutes. The membrane and cytosolic fractions were separated, resolved on SDS-PAGE, and detected with specific antibodies recognizing Akt1 or Akt2. N.S. indicates a nonspecific species recognized by the anti-Akt1 antibody. Anti-p22phox and anti–β-actin antibodies were used for detection of p22phox and β-actin as markers of the membrane and cytosolic fractions, respectively. Tot indicates total (membrane plus cytosolic fractions); Cyt, cytosolic fraction; and Mem, membrane fraction. (B) Chemoattractant-induced Akt phosphorylation. WT, Akt1−/−, and Akt2−/− neutrophils (1 × 107) from WT, Akt1−/−, and Akt2−/− mice were stimulated with C5a (+, 100nM) or buffer (−) for 1 minute. The samples were then resolved on SDS-PAGE and detected with specific anti–phospho-Akt antibodies recognizing phosphorylated Akt at Ser473 (Cell Signaling) and Thr308 (Calbiochem). β-actin and total Akt were detected for equal loading controls. Representative blots from 3 independent experiments are shown.
Therefore, it was predicted that failure of Akt1 to translocate to the plasma membrane would have a negative impact on its activation. This was investigated by Western blotting analysis of Akt phosphorylation in the individual Akt KO neutrophils, and the result was compared with that of WT neutrophils. No significant Akt phosphorylation was detected at resting state. Chemoattractant stimulation of WT neutrophils led to Akt phosphorylation that was readily detectable with antibodies recognizing the phosphorylated Akt at Ser473 and Thr308, respectively (Figure 6B). Deletion of the Akt1 gene had minimal effect on Akt phosphorylation at Ser473 (Figure 6B) and Thr308 (data not shown) when normalized against the total Akt proteins loaded. In contrast, deletion of the Akt2 gene essentially abolished chemoattractant-induced Akt phosphorylation at both residues. These results suggest that Akt2 is the predominant isoform activated in chemoattractant-stimulated neutrophils.
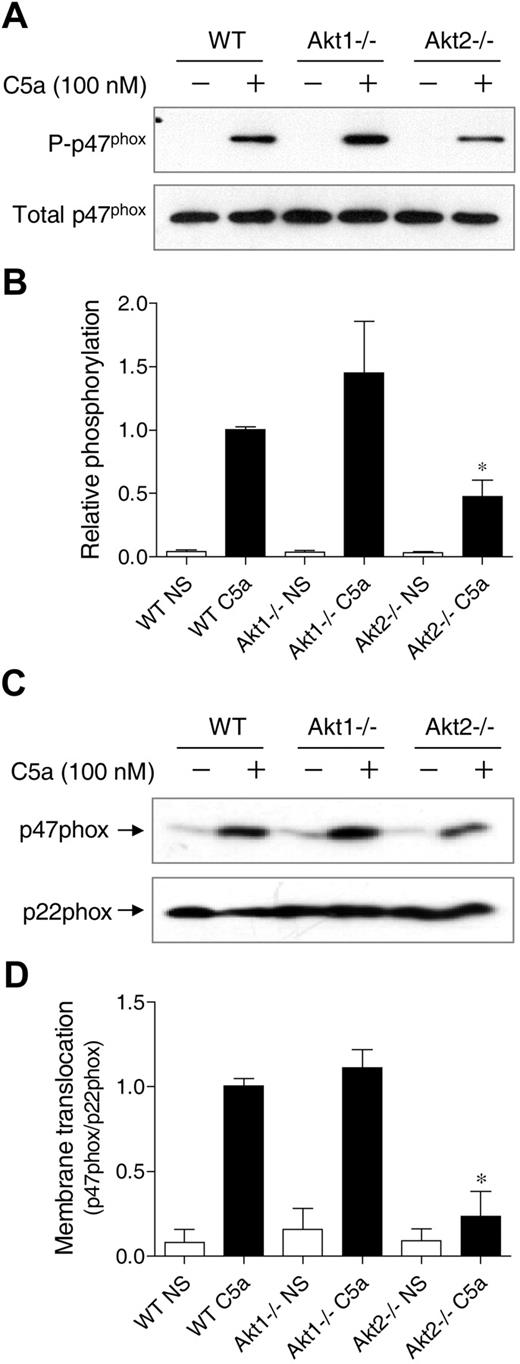
Decreased p47phox phosphorylation and membrane translocation in Akt2−/− mice
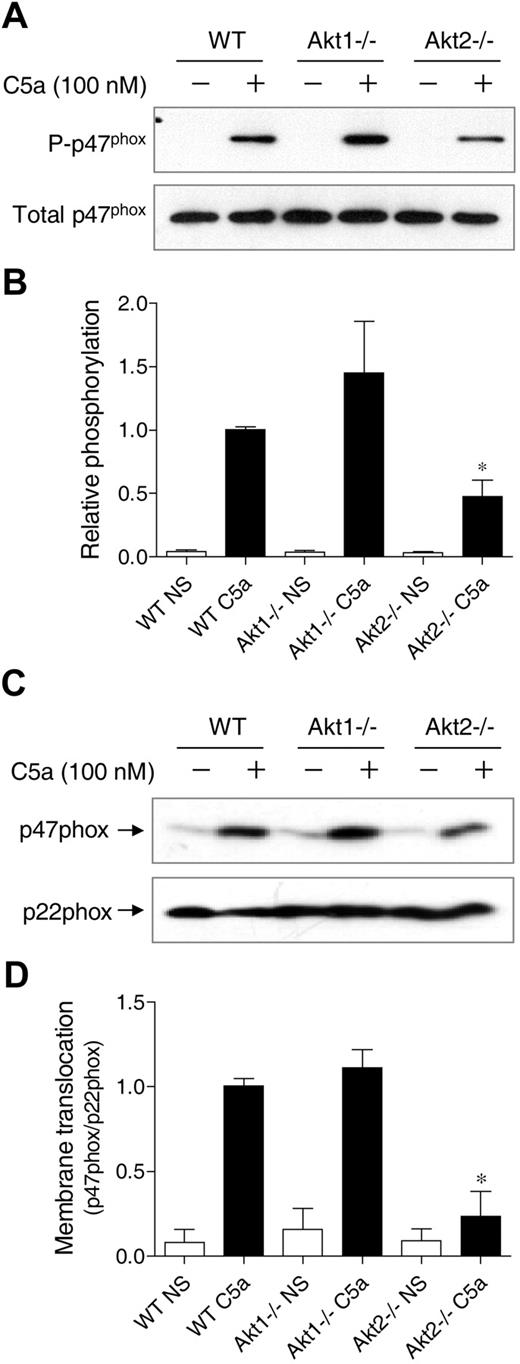
p47phox phosphorylation and membrane translocation is a key event leading to the activation of NADPH oxidase.36 Phosphorylation of p47phox triggers conformational changes of the protein to allow its membrane translocation and docking to the membrane-bound p22phox. Akt is one of the protein kinases that phosphorylate p47phox in its autoinhibitory region.25,26 To determine whether individual Akt gene deletion affects p47phox phosphorylation, phosphoproteins were collected from C5a-stimulated neutrophils, and the resulting products were separated on SDS-PAGE and blotted with an anti-p47phox antibody (Figure 7A-B). In Akt1−/− neutrophils, there was a visible increase in p47phox phosphorylation after C5a stimulation, although the change was not statistically significant (P > .05).
Effect of Akt gene knockout on p47phox phosphorylation and membrane translocation. (A) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated for 1 minute with C5a (100nM). Total phosphoproteins were prepared by the use of antiphosphoprotein affinity chromatography as described in “Detection of p47phox phosphorylation.” The p47phox proteins in total lysate (total p47phox) and in the phosphoprotein fraction (P-p47phox) were detected by the use of an anti-p47phox antibody. The relative intensity of the Western blot bands (phosphorylated p47phox/total p47phox) was quantified and shown in panel B; *P < .05; NS, no agonist stimulation. (C) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated for 1 minute with C5a (100nM). Membrane fractions were prepared, separated and resolved on SDS-PAGE, and detected with specific antibodies recognizing p47phox and p22phox (a membrane protein used as membrane marker for loading control). Western blot bands (p47phox/p22phox) was quantified and shown in panel D. *P < .05; NS, no agonist stimulation.
Effect of Akt gene knockout on p47phox phosphorylation and membrane translocation. (A) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated for 1 minute with C5a (100nM). Total phosphoproteins were prepared by the use of antiphosphoprotein affinity chromatography as described in “Detection of p47phox phosphorylation.” The p47phox proteins in total lysate (total p47phox) and in the phosphoprotein fraction (P-p47phox) were detected by the use of an anti-p47phox antibody. The relative intensity of the Western blot bands (phosphorylated p47phox/total p47phox) was quantified and shown in panel B; *P < .05; NS, no agonist stimulation. (C) Neutrophils from WT, Akt1−/−, and Akt2−/− mice were stimulated for 1 minute with C5a (100nM). Membrane fractions were prepared, separated and resolved on SDS-PAGE, and detected with specific antibodies recognizing p47phox and p22phox (a membrane protein used as membrane marker for loading control). Western blot bands (p47phox/p22phox) was quantified and shown in panel D. *P < .05; NS, no agonist stimulation.
In contrast, the phosphorylation of p47phox in Akt2−/− neutrophils was significantly reduced compared with the WT neutrophils. Thus, although the basal expression level of p47phox (unphosporylated) was not changed (Figure 7A bottom panel and supplemental Figure 2), the extent of p47phox phosphorylation was altered. It was not unexpected that Akt2 deficiency did not completely abolish p47phox phosphorylation because other C5a-activated kinases such as PKC and selected MAP kinases could also phosphorylate p47phox in activated neutrophils. Consistent with the reduced p47phox phosphorylation, there was a significant decrease in p47phox translocation to the plasma membrane in Akt2−/− neutrophils (Figure 7C-D). The same was also observed in PMA-stimulated cells (supplemental Figure 6). These findings further demonstrate that Akt2 plays an important role in neutrophil O2− production under the experimental conditions.
Discussion
Results from this study show for the first time that the 2 major Akt isoforms in neutrophils, Akt1 and Akt2, act differently in regulating major neutrophil functions. Although the Akt1 gene KO did not appear to have a negative impact on neutrophil migration, degranulation, and O2− production, the loss of Akt2 had a negative impact on these functions in neutrophils. The Akt kinase is known as an important constituent of neutrophil activation pathways,19 but its exact role in regulating neutrophil functions has not been fully understood. One of the characterized functions of Akt is the phosphorylation of p47phox, which contributes to p47phox membrane translocation and O2−generation.24,–26 Therefore, in the present study we focused primarily on the role of Akt isoforms in NADPH oxidase activation by the use of individual Akt KO mice. Loss of Akt2, but not Akt1, abrogated O2− production in neutrophils treated with the soluble stimuli C5a, fMLF, and PMA. The functional difference between Akt1 and Akt2 is not the result of defective expression of NADPH oxidase components. Moreover, the ability of the Akt1−/− and Akt2−/− neutrophils to generate O2− after phagocytosis of zymosan particles is mostly retained.
These findings indicate that Akt2 deficiency selectively incapacitates signaling pathways initiated by soluble stimuli such as chemoattractants. Particle-induced O2− production uses a different set of signaling molecules, including kinases of the Src and Syk/Zap70 families. Therefore, their signaling pathways and dependence on Akt are different. At present it is unclear whether the Akt2 KO mice are defective in host defense because the mice were kept in an environment free of bacteria and fungi. In vitro assay did not find difference in bacterial killing by the Akt isoform-deficient neutrophils (data not shown). The increase in blood neutrophil count in Akt2−/− mice could be a compensatory mechanism for functional defects resulting from the lack of this Akt isoform.31 The in vivo effect of Akt2 deficiency in host defense has yet to be determined in future studies.
Our results have shown that difference of these 2 Akt isoforms in membrane translocation underlies their functional disparity. Activation of Akt requires the phosphorylation at Thr308 and Ser473 or equivalent positions. Therefore, membrane targeting of Akt is a necessary step for its full phosphorylation.20,21 In this study, we found that Akt1 is localized in the cytosolic fraction in resting neutrophils. Stimulation with C5a, a potent anaphylatoxin and chemoattractant that induces activation of neutrophils, does not significantly alter the cellular distribution pattern of Akt1 in cells lacking Akt2. In contrast, Akt2 responds well to C5a stimulation with rapid translocation to the leading edge of polarized neutrophils. By conducting the Akt localization analysis in WT neutrophils with specific anti-Akt1 and anti-Akt2 antibodies, we obtained similar results and excluded the possibility that deletion of one Akt isoform affects the cellular localization of another.
Failure of Akt1 translocation to the plasma membrane was paralleled by its reduced phosphorylation, suggesting that Akt1 is minimally activated upon chemoattractant stimulation and could not compensate for the functional loss resulting from Akt2 gene deletion. At present, it remains unclear what structural features dictate the localization of the Akt isoforms in neutrophils. The PH domain, known to bind PtdIns(3,4)P2 and PtdIns(3,4,5)P3, is a highly conserved structure in all Akt isoforms. In adipocytes, insulin induces plasma membrane accumulation of Akt2 but not Akt1, and the difference could be attributed to the PH domain of these 2 Akt isoforms.37 However, the opposite was observed in another study, in which deficiency of Akt1 but not Akt2 led to reduced migration of mouse embryonic fibroblasts.38 Therefore, membrane localization of the Akt isoforms may be cell-type specific and possibly depends on the type of agonist stimulation as well.
Our results demonstrate that Akt2 is required for O2− production in neutrophils treated with soluble stimuli, whereas a role for Akt1 in O2− production remains unclear. In several experiments, we found a small but significant increase in O2− production in Akt1−/− cells. It is possible that Akt1 has a regulatory function that is different from the function of Akt2 in neutrophils. The underlying mechanism is currently unclear, although a visible (but statistically insignificant) increase in p47phox phosphorylation was observed in the Akt1−/− neutrophils. In recent literature, there is a growing body of evidence demonstrating physiologic functions of Akt1 in addition to its well-established function in cell growth and survival. Apart from the study showing a role for Akt1 in fibroblast migration,38 a recent report identifies Akt1 as being critical to the increased vascular permeability in response to histamine, bradykinin and carrageenan.39 The same study also showed that Akt1, but not Akt2, regulates microvascular leakage through endothelial nitric oxide synthase and nitric oxide synthase-dependent nitric oxide production. In macrophages, Akt1 has been recently identified as a key molecule in regulating different microRNAs, thereby affecting LPS tolerance.40 Therefore, although additional studies will be necessary to investigate whether and how Akt1 regulates neutrophil O2− production, it is evident that the Akt isoforms are not redundant but have their specific functions in different types of cells.
Using a green fluorescent protein construct containing the PH domain of Akt as a probe for PtdIns(3,4,5)P3, investigators have shown localization of the PH-Akt-green fluorescent protein construct to the leading edge of chemoattractant-stimulated leukocytes.22,41 This finding was confirmed in our study showing that Akt2, but not Akt1, translocates to the leading edge in chemoattractant-stimulated neutrophils. Our video microscopy results suggest that Akt2 is important for neutrophil migration upon chemoattractant stimulation. Therefore, it is likely that Akt2 plays an important role in the proximal signaling of chemoattractant receptors. Notably, a similar phenotype has been observed in PI3K-γ−/− neutrophils, which lack normal response to chemoattractants in cell migration assays.
Moreover, deletion of the Akt2 gene abrogates chemoattractant-induced degranulation and O2− production, suggesting that Akt is an upstream signaling molecule that regulates the major functions of neutrophils. Most published studies have shown that Akt activation is dependent on PI3K19,20,24 and that inhibition of PI3K abrogates Akt phosphorylation and Akt-mediated cell functions.26,31 Our observation that the PMA-induced O2− production is PI3K independent but Akt2 dependent shows that Akt could function separately from PI3K. Although the underlying mechanism remains to be identified, we observed that neutrophils exposed to either the p38 MAPK inhibitor SB203580 or the broad PKC inhibitor GF109203x exhibited decreased Akt phosphorylation, a finding that places Akt downstream of PKC and p38 MAPK. PI3K-independent activation of Akt has been suggested in recent studies, and the involvement of conventional PKC isoforms as well as other protein kinases is implicated.42,–44 Our finding that Akt2 is a required signaling molecule for PMA-induced NADPH oxidase activation provides a new lead to an as-yet unidentified mechanism by which Akt isoforms regulate neutrophils functions.
Our results challenge the existing paradigm that all Akt proteins have the same or similar PI3K-dependent functions in neutrophil activation. These findings are potentially significant in guiding future studies of the Akt kinases in neutrophils. On the basis of data obtained from the present study, it is apparent that many of the chemoattractant-induced activities may be attributed to Akt2 and not Akt1. Moreover, the observation that Akt2 is also necessary for PMA-induced O2− production but is independent of PI3K suggests a new function of this serine/threonine kinase that is distal from the activated receptor. Additional investigations into this property of Akt2 will likely uncover the mechanisms by which PMA activates neutrophils. As for Akt1, its role in neutrophil activation has yet to be defined. Another possible function of Akt1 is in the regulation of neutrophil lifespan, and it may be activated by agonists other than chemoattractants. Investigation into these possibilities with the use of isoform KO mice is expected to extend our understanding of the Akt kinases in neutrophil biology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kelly O'Brien for assistance in establishing Akt knockout breeding colonies. We also thank Feng Qian, Benjamin Gantner, and Ni Cheng for helpful discussions.
This work was supported in part by National Institutes of Health grants AI033503 and HL077806 (to R.D.Y.). J.C. was supported by a predoctoral fellowship from the American Heart Association, Midwest Chapter.
National Institutes of Health
Authorship
Contribution: J.C. developed the concept, designed and conducted experiments, analyzed data, and prepared the manuscript; H.T. conducted neutrophil migration assay; N.H. prepared knockout mice and participated in discussions; J.X. advised on neutrophil migration experiments and participated in discussions; and R.D.Y. developed the concept, designed experiments, coordinated research collaboration, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard D. Ye, Department of Pharmacology, University of Illinois at Chicago, 835 South Wolcott Ave, Chicago, IL 60612; e-mail: yer@uic.edu.