Beginning with initial observations a century ago,1 the complex events involved in platelet production from megakaryocytes have been characterized with increasing precision during the past decade.2-4 In this issue of Blood, Schwertz and colleagues add surprising new findings that elucidate the probable terminal event of thrombopoiesis, that is, the duplication of human blood platelets.5 Their experiments were conducted ex vivo in microdrops, as well as in suspension and whole blood cultures. The implication of their report is that platelet replication may also occur in the circulation and during platelet storage under blood-banking conditions.
Multinucleated megakaryocytes in the bone marrow stroma extend long, tubular cytoplasmic projections (“proplatelets”) between endothelial cells into the lumina of marrow sinusoids. While anchored to the megakaryocyte within the sinusoids, or after shear-induced breakage, the proplatelets use microtubular rearrangements at their tips to produce and release mature platelets into the circulation.1-4 These platelets normally circulate for about 10 days.
Although devoid of a nucleus and the capacity to transcribe new mRNA, each platelet produced by this mechanism retains a robust capacity to cleave and activate precursor forms of mRNA. These precursor mRNAs, previously transcribed in the polyploid nuclei of a megakaryocyte progenitor, are then transported through the megakaryocyte's extended cytoplasmic proplatelet stalks to nascent platelet “buds” at the tips of the stalks.2-4 Each mature platelet that escapes into the circulation continues to process its composition of precursor mRNAs and to translate the resulting functional mRNAs into proteins.6,7 Included among the platelet-translated proteins identified so far are glycoprotein αIIbβ3, cyclooxygenase-1 and -2 isoforms, components of the spliceosome complex involved in precursor mRNA intron removal, proapoptosis and antiapoptosis factors, plasminogen activator inhibitor-1, P-selectin, and tissue factor.5-7
In their report, Schwertz et al demonstrate that human platelets also retain the essential molecular components and synthetic capacity that enables them to replicate within a few hours (in suspension culture).5 A platelet does this by extending a thin cytoplasmic projection with a newly forming platelet at the tip of the projection. Using this maneuver, a circulating platelet can form a functioning offspring. The new platelets are equipped, through the connecting cytoplasmic projection from the parent, with organelles (including mitochondria and its DNA) and α-granules—and respond to thrombin and adenosine diphosphate (ADP).
It is an initially disconcerting concept that platelets, unequipped with nuclear DNA, can produce functional replicas of themselves. This phenomenon becomes biologically reasonable, however, because circulating platelets (1) remain active in protein synthesis and (2) contain the same molecular machinery (dynamic microtubules, actin, and myosin) that allows proplatelets (derived from megakaryocyte cytoplasm) to pinch platelet “buds” from the tips of proplatelet projections.
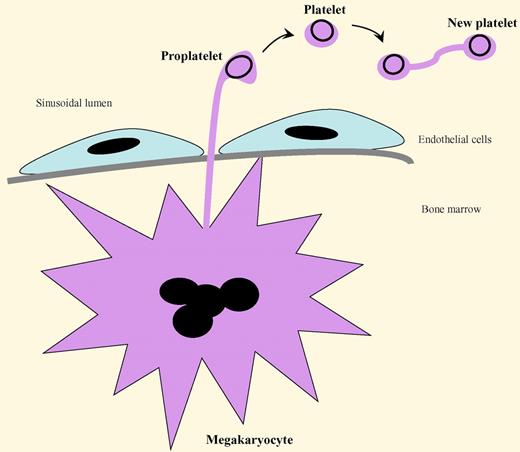
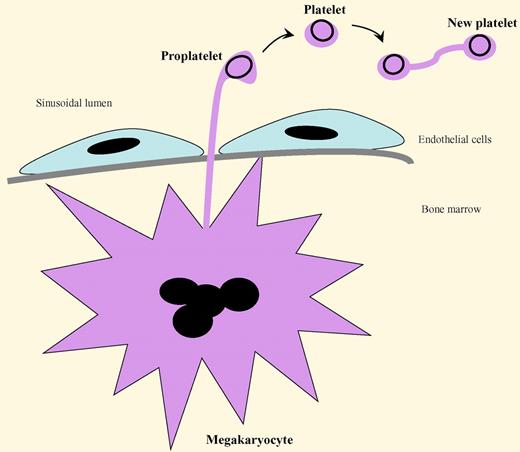
A proposed, expanded model of platelet production that includes the findings of Schwertz et al is presented in the figure.5
The “budding” of platelets from proplatelets, and then from mature platelets, during thrombopoiesis. Megakaryocytes in the bone marrow extend cytoplasmic projections (proplatelets) between endothelial cells into the sinusoidal lumen. The proplatelets pinch off their tips to form platelets. In a similar process, a circulating platelet is capable of extending a thin cytoplasmic projection and transferring some of its metabolic, granular, and organelle contents into an attached cell body to construct a duplicated new platelet. The dark circles in the proplatelets and platelets represent microtubules. (For an example of the platelet replication process, see Figure 1A in the article by Schwertz et al on page 3801.)
The “budding” of platelets from proplatelets, and then from mature platelets, during thrombopoiesis. Megakaryocytes in the bone marrow extend cytoplasmic projections (proplatelets) between endothelial cells into the sinusoidal lumen. The proplatelets pinch off their tips to form platelets. In a similar process, a circulating platelet is capable of extending a thin cytoplasmic projection and transferring some of its metabolic, granular, and organelle contents into an attached cell body to construct a duplicated new platelet. The dark circles in the proplatelets and platelets represent microtubules. (For an example of the platelet replication process, see Figure 1A in the article by Schwertz et al on page 3801.)
How will the study of Schwertz et al relate to clinical practice? The authors suggest that their observations may explain why, in some patients, platelet counts are higher than would be predicted when only few megakaryocytes are seen in bone marrow samples. It is also possible that augmentation of platelet “budding” (by methods yet to be defined) could be a novel therapeutic approach to some types of thrombocytopenia.
The work of Schwertz et al may be useful even sooner in blood banking. Platelets ex vitro continue the synthesis of proteins for as long as 10 days.8 Schwertz et al report that the numbers of platelets also increase over time, as a consequence of ex vitro platelet “budding,” and suggest that platelet numbers may increase during platelet storage under blood banking conditions.5 The authors' experimental findings and provocative suggestions are likely to reinvigorate investigation of methods to optimize the preparation and “budding” of stored platelet concentrates.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■