Abstract
The molecular basis of the HNA-3a/b (5b/a) leukocyte antigen system has not yet been defined despite evidence that HNA-3a–specific antibodies are particularly prone to cause severe, often fatal, transfusion-related lung injury. We used genome-wide single nucleotide polymorphism scanning and sequencing of DNA from persons of different HNA-3a/b phenotypes to identify a single single nucleotide polymorphism in exon 7 of the CLT2 gene (SLC44A2) that predicts an amino acid substitution in the first extracellular loop of choline transporter-like protein 2, a member of the choline transporter-like protein family of membrane glycoproteins, and correlates perfectly with HNA-3a/b phenotypes (R154 encodes HNA-3a; Q154 encodes HNA-3b). Mass spectrometric analysis of proteins immunoprecipitated from leukocytes by anti–HNA-3a provided direct evidence that anti–HNA-3a recognizes choline transporter-like protein 2. These findings will enable large-scale genotyping for HNA-3a/b to identify blood donors at risk to have HNA-3a–specific antibodies and should facilitate development of practical methods to detect such antibodies and prevent transfusion-related lung injury.
Introduction
The leukocyte antigens 5a and 5b (now designated HNA-3b and HNA-3a) constitute a biallelic antigen system identified by van Leeuwen et al in 1964 using antibodies from multiparous women that agglutinated leukocytes of normal persons.1 Despite the passage of more than 40 years, molecular properties of the HNA-3 antigens have not been defined. Characterization of HNA-3a has become particularly important with the recognition that HNA-3a–specific antibodies are prone to cause severe, often fatal, transfusion-associated acute lung injury (TRALI).2-5 Here, we provide evidence that HNA-3a is carried on choline transporter-like protein 2 (CTL2), a member of the choline transporter–like family of membrane glycoproteins, and that the antigen results from a single nucleotide polymorphism (SNP) in exon 7 of the CTL2 gene (SLC44A2) encoding R154 (HNA-3a) or Q154 (HNA-3b) in the first extracellular loop of the mature protein.
Methods
Antibodies and antibody detection
HNA-3a–specific antibodies were from donors implicated in TRALI reactions.3,6 Isolation of neutrophils, T and B lymphocytes, and platelets from normal donor blood, detection of antibodies reactive with these cells by flow cytometry and agglutination, and immunoprecipitation of membrane proteins have been described previously.7-9
SNP analysis
Genotyping of DNA from 8 unrelated HNA-3a–negative persons was performed using the Human Genome-Wide SNP Array 6.0 (Affymetrix). Genotypes were assigned using Birdseed 2.0.10 Quality control studies, including assessment of call rate, investigation of potential genetic relationships among samples, and examination of data to identify potential departures from expected levels of heterozygosity, were completed using plink.11 Eigenstrat was used to assess geographic ancestry in combination with HapMap CEU, YRI, JPT, and CHB samples.12 All samples had high call rates (> 98%) with high-quality genotype data. The 8 HNA-3a–negative persons appeared to be of recent European descent and were quite similar in ancestry to the HapMap CEU samples. Association studies were performed using the 8 HNA-3a–negative samples as cases and unrelated European samples from HapMap3 as controls, with significance assessed using 100 000 permutations; thus, the smallest possible P value in permutation analysis was P = 1 × 10−6.
DNA sequencing
Genomic DNA was amplified by the polymerase chain reaction using primers designed to amplify exon 7 of the CTL2 gene SLC44A2 (reference sequence National Center for Biotechnology Information database; www.ncbi.nlm.nih.gov/nucore/NM_001145056.1). Primer sequences are available on request. Automated sequence analysis of polymerase chain reaction products was performed in both directions with the Big Dye Terminator version 3.1 Cycle Sequencing kit on an ABI 3130XL genetic analyzer (Applied Biosystems).13
Mass spectrometry
HNA-3a–specific antibody was incubated with HNA-3a–positive leukocytes and subjected to immunoprecipitation.7 Immunoprecipitated proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with Coomassie blue. Candidate protein bands were excised, prepared, and analyzed by mass spectrometry (MS) as previously described.14,15
Research approval
Studies were approved by the Institutional Review Board, BloodCenter of Wisconsin.
Results and discussion
Serologic studies
Although HNA-3a is widely considered to be a neutrophil antigen, in flow cytometric studies we found that lymphocytes carry HNA-3a in quantities equal to those found on neutrophils and that lymphocytes from obligate HNA-3a heterozygotes (parents of HNA-3a–negative persons) possess 50% as much antigen as homozygotes (data not shown). Because lymphocytes are easier to manipulate than neutrophils, they were used in subsequent studies.
Genome-wide SNP analysis
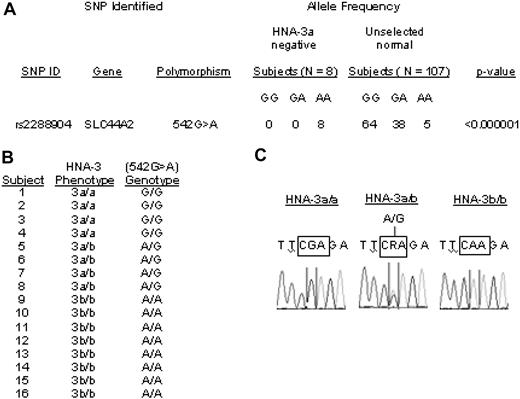
The 1964 van Leeuwen report indicated that gene frequencies of HNA-3a (5b) and HNA-3b (5a) are 0.82 and 0.18, respectively.1 Less than 5% of the general population is HNA-3a–negative (homozygous for the low-frequency allele). Therefore, interrogation of genome-wide scan results was focused on SNPs for which each of the 8 unrelated HNA-3a–negative subjects was homozygous at an allele relatively rare in controls. Results are shown in Figure 1A. All the HNA-3a–negative subjects, but fewer than 5% of unrelated European controls, were homozygous for a low-frequency allele at only 2 SNPs, rs2288904 and rs10420809. None of the 100 000 permutations generated associations as strong as those observed at these 2 SNPs (both P < 1×10−6 corrected for the number of SNPs analyzed). Both are located in SLC44A2 on chromosome 19 encoding the membrane glycoprotein CTL2. However, only SNP rs2288904 (G>A542), in exon 7 of SLC44A2, predicts an amino acid substitution (R>Q154). Gene frequencies of the alleles of rs2288904 are 0.82 and 0.18, identical to those expected for HNA-3a/b. The findings suggested that the high-frequency allele of SNP (rs2288904) encoding R154 might encode HNA-3a.
THE HNA-3a/b polymorphism is closely associated with a mutation in the CTL2 gene (SLC44A2). (A) Genome-wide SNP analysis of DNA from 8 unrelated HNA-3a–negative and 107 random persons. Each of the HNA-3a–negative subjects possessed AA at SNP rs2288904, whereas the distribution of A and G at the same position in random subjects corresponded to that expected for Hardy-Weinberg distribution of 2 alleles with gene frequencies of 0.21 and 0.79, respectively. (B) HNA-3a/b phenotypes and G>A542 genotypes in 16 subjects. Without exception, the HNA-3a/a, HNA-3a/b, and HNA-3b/b phenotypes correlated with the presence of GG, GA, and AA, respectively, at SNP rs2288904. (C) Representative DNA sequencing results for the G>A542 SNP in persons with phenotypes HNA-3a/3a, HNA-3a/3b, and HNA-3b/3b.
THE HNA-3a/b polymorphism is closely associated with a mutation in the CTL2 gene (SLC44A2). (A) Genome-wide SNP analysis of DNA from 8 unrelated HNA-3a–negative and 107 random persons. Each of the HNA-3a–negative subjects possessed AA at SNP rs2288904, whereas the distribution of A and G at the same position in random subjects corresponded to that expected for Hardy-Weinberg distribution of 2 alleles with gene frequencies of 0.21 and 0.79, respectively. (B) HNA-3a/b phenotypes and G>A542 genotypes in 16 subjects. Without exception, the HNA-3a/a, HNA-3a/b, and HNA-3b/b phenotypes correlated with the presence of GG, GA, and AA, respectively, at SNP rs2288904. (C) Representative DNA sequencing results for the G>A542 SNP in persons with phenotypes HNA-3a/3a, HNA-3a/3b, and HNA-3b/3b.
DNA sequencing
To further characterize the association between SNP rs2288904 and HNA-3a, we sequenced exon 7 of SLC44A2 in 8 HNA-3b/b, 4 HNA-3a/b, and 4 HNA-3a/3a subjects and found that, without exception, G542 and A542 predicted the HNA-3a and HNA-3b phenotypes, respectively (Figure 1B). Together with the genome-wide SNP analysis, the findings provide strong evidence that the HNA-3a/b antigens are determined by a G>A SNP at nt542 of the SLC44A2 gene, encoding an amino acid switch from arginine to glutamine at position 154 in the first extracellular loop of the CTL2 protein. When G is present at nt542, amino acid 154 is arginine, creating the HNA-3a antigen.
MS
To obtain direct evidence that HNA-3a is carried on CTL2, we subjected proteins immunoprecipitated by anti–HNA-3a to gel electrophoresis liquid chromatography (GeLC)–MS/MS under reducing and nonreducing conditions.14,15 The method used is not biased toward a predefined band and enabled characterization of most components of the immunoprecipitate. A second sample was analyzed using in-solution digestion, a complementary approach that allows single LC-MS/MS analysis of the complete sample. In addition to the proteins usually found in coimmunoprecipitates, such as immunoglobulins, cytoskeletal and plasma proteins, CTL2 was identified in each of 3 independent studies with statistical significance (P < .0001; supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article). As shown in Figure 2, 7 CTL2 peptides identified spanned the entire length of the protein, suggesting that CTL2 in lymphocytes is similar to that identified in the inner ear.16
CTL2 peptides identified by mass spectrometry in protein immunoprecipitated from leukocytes with anti–HNA-3a. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with Coomassie blue. Bands of interest were excised and processed for analysis by mass spectrometry as described in “Mass spectrometry.” CTL2 was identified in each of 3 independent studies with statistical significance (P < .0001; Supplemental data). The 7 CTL2 peptides identified (shaded text) spanned almost the entire length of the protein.
CTL2 peptides identified by mass spectrometry in protein immunoprecipitated from leukocytes with anti–HNA-3a. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with Coomassie blue. Bands of interest were excised and processed for analysis by mass spectrometry as described in “Mass spectrometry.” CTL2 was identified in each of 3 independent studies with statistical significance (P < .0001; Supplemental data). The 7 CTL2 peptides identified (shaded text) spanned almost the entire length of the protein.
These observations provide strong evidence that the HNA-3a antigen results from substitution of arginine for glutamine at position 154 in CTL2. CTL2 is a member of the choline transporter–like family of proteins16-18 predicted to have 10 trans-membrane domains and 5 extracellular peptide loops.16 Position 154 is located in the first loop, consisting of 178 amino acids starting at residue 55. Cysteine residues at positions 139 and 158 could be disulfide-linked to produce a cyclic structure stabilizing the HNA-3 epitope, explaining our finding that HNA-3a is abolished by reduction with dithiothreitol (data not shown). Potential N-glycosylation sites at positions 187 and 200 could also influence antigenicity. The function of CTL2 is as yet unknown.16,18-20 The finding that cross-linking of HNA-3a on neutrophils is a potent trigger for activation21 suggests that CTL2 is functionally important in this cell type and might explain the severity of TRALI reactions induced by HNA-3a–specific antibodies.2,3,5,21 Remarkably, autoantibodies specific for CTL2 have been implicated as a cause of autoimmune deafness.16,19 Formal confirmation that HNA-3a is determined by the rs2288904 SNP in SLC44A2 will require a demonstration that introducing R at position 154 in the expressed protein creates the HNA-3a epitope. These studies are in progress.
Current methods used to reduce the risk of TRALI include the transfusion of male plasma only and screening of multiparous female blood donors for human leukocyte antigen class I and II antibodies. Screening for antibodies against HNA-3a, despite their known clinical significance, is not performed because practical methods are not available. Elucidation of the molecular properties of the HNA-3a/b alloantigens should facilitate detection of anti–HNA-3a in blood donors using natural or recombinant CTL2 or a CTL2 fragment as a target for antibody. Our findings also show that the HNA-3a–negative genotype can be identified by typing DNA for rs2288904, enabling rapid identification of HNA-3a–negative persons as part of a diagnostic assessment for TRALI and to identify persons at risk to produce anti–HNA-3a.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff at the Ulrich Broeckel Lab, Medical College of Wisconsin, and Children's Research Institute for processing the genome-wide SNP chips; Ashley Zurawel, University of Colorado Denver, for her assistance with the MS analysis; and the staff at the Molecular Diagnostics and Product Development Labs, BloodCenter of Wisconsin for their technical advice on DNA sequencing.
B.R.C. is a PhD candidate at the University of Wisconsin–Milwaukee, and this work is submitted in partial fulfillment of the requirements for the PhD degree.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant HL-13629).
National Institutes of Health
Authorship
Contribution: B.R.C. obtained reagents and samples, designed and performed experiments, analyzed data, and wrote and reviewed the manuscript; N.J.C. designed SNP studies, analyzed and assisted in interpretation of SNP data, and contributed to the writing of the paper; M.J.S. performed experiments and collected data; A.K. analyzed SNP data; K.B. performed experiments; K.H. helped design MS experiments, performed MS analysis, analyzed and interpreted MS data, and contributed to the writing of the paper; and R.H.A. helped with design of experiments, assisted in the writing of the paper, and provided other advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian R. Curtis, Platelet & Neutrophil Immunology Laboratory, BloodCenter of Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: brian.curtis@bcw.edu.