Abstract
Platelets originate from megakaryocytes (MKs) by cytoplasmic elongation into proplatelets. Direct platelet release is not seen in bone marrow hematopoietic islands. It was suggested that proplatelet fragmentation into platelets can occur intravascularly, yet evidence of its dependence on hydrodynamic forces is missing. Therefore, we investigated whether platelet production from MKs could be up-regulated by circulatory forces. Human mature MKs were perfused at a high shear rate on von Willebrand factor. Cells were observed in real time by videomicroscopy, and by confocal and electron microscopy after fixation. Dramatic cellular modifications followed exposure to high shear rates: 30% to 45% adherent MKs were converted into proplatelets and released platelets within 20 minutes, contrary to static conditions that required several hours, often without platelet release. Tubulin was present in elongated proplatelets and platelets, thus ruling out membrane tethers. By using inhibitors, we demonstrated the fundamental roles of microtubule assembly and MK receptor GPIb. Secretory granules were present along the proplatelet shafts and in shed platelets, as shown by P-selectin labeling. Platelets generated in vitro were functional since they responded to thrombin by P-selectin expression and cytoskeletal reorganization. In conclusion, MK exposure to high shear rates promotes platelet production via GPIb, depending on microtubule assembly and elongation.
Introduction
Megakaryocyte (MK) differentiation is a continuous process characterized by sequential steps. MK ploidy increases through endomitosis, with parallel increase in cell size. Synthesis of storage organelles is enhanced and plasma membrane invaginates, resulting in the formation of demarcation membranes. Finally, mature MKs undergo complete cytoskeleton reorganization with microtubule involvement, to induce pseudopodial elongations called proplatelets.1,2 Platelets are released from the tips of proplatelets that contain the organelles.3 In static conditions, platelets are formed from mature MKs cultured in the presence of thrombopoietin (TPO).3 MK fragmentation into platelets does not occur in the extravascular, hematopoietic space in normal bone marrow. To achieve platelet formation, MKs need to migrate into the sinusoid capillaries and mature MKs are generally located in the vicinity of sinusoids, more precisely on their abluminal side.4 MK fragmentation occurs only in the intravascular space, and mature MKs have been identified in the blood circulation where they may interact via their surface receptors with proteins expressed on endothelial cells.4–6 Adhesion of MKs to endothelial cells is essential for robust platelet production in vitro, a process that is believed to mimic what occurs in vivo when MKs interact with bone marrow endothelial cells within the vascular niche.7 Recently, the process of proplatelet formation was examined in mouse skull bone marrow in vivo: the authors observed large MK fragments resembling immature proplatelets protruding into the intravascular luminal side of bone marrow sinusoids and entering flowing blood.8
Circulating platelets are essential for primary hemostasis by their capacity of adhesion to the subendothelial matrix and aggregation.9 Among the constituents of vascular endothelial cells, von Willebrand factor (VWF) allows translocation of circulating platelets in conditions of high shear rates (> 1000 s−1) through binding of their GPIb receptor.10 GPIb-mediated signaling occurs progressively during tethering of platelets and allows activation of the αIIbβ3 integrin and platelet aggregation. GPIb is crucial for cytoskeletal reorganization by linking the actin-binding protein filamin.11 The Bernard-Soulier syndrome (BSS) is a bleeding disorder characterized by severe thrombocytopenia and giant platelets.12 It is due to genetic abnormalities of the GPIb-IX-V complex, in particular of the GPIbα subunit that contains the VWF and thrombin binding sites. Normal numbers of MKs are found in the bone marrow of BSS patients, suggesting that the macrothrombocytopenia is related to defective platelet formation from MKs. It is intriguing that in optimal culture conditions, the yield of shed platelets in vitro is far below what could be expected from the large daily platelet production in vivo.13 Many steps of platelet shedding remain elusive, in particular a role of shear forces on platelet formation has never been demonstrated. In the present report, we examined the role of high shear rates on platelet release from mature MKs adherent to a vascular matrix in flow conditions and found that it required both intact microtubules and the GPIb receptor.
Methods
Proteins
VWF was a gift of Laboratoire Français du Fractionnement et des Biotechnologies. Fibrinogen was from Hyphen BioMed. Human VWF and fibrinogen were purified and depleted of contaminant fibronectin and of fibrinogen and VWF, respectively.14 Equine tendon collagen was from Nycomed, and human fibronectin was from VWR. Wild-type and mutated (V1316M type 2B) recombinant VWF (rVWF) were obtained as described elsewhere.15
Antibodies
Monoclonal antibody (MoAb) 713, which blocks VWF binding to GPIbα, was a kind gift of Dr Girma (Inserm Unit 770).15 Polyclonal anti-glycocalicin (the extracellular domain of GPIbα containing the VWF binding site) and anti-CD62P antibodies were a kind gift of Dr Berndt (Monash University).16 Phycoerythrin (PE)–conjugated anti-CD62P (P-selectin), fluorescein isothiocyanate (FITC)–conjugated anti-CD41a (αIIb), anti-CD42b (GPIbα) and nonimmune antibodies were from Beckman Coulter. Anti–α-tubulin MoAb was from Sigma-Aldrich. The anti-β3 integrin P37 MoAb was a gift of Dr Gonzalez-Rodriguez (CSIC).17 The anti-αIIbβ3 Fab (C7E3) abciximab was from Lilly.
Cell culture
Human MKs used in this study were cultured from precursor cells isolated either from umbilical cord blood or from bone marrow. Human bone marrow samples (harvested during hip surgery) and cord blood samples were obtained after informed consent and approval from our Institute Ethics Committee (Assistance Publique des Hôpitaux de Paris) and in accordance with the Declaration of Helsinki. Human umbilical cord blood and bone marrow CD34+ cells were separated by an immunomagnetic technique (StemCell Technologies) as previously described.18 The cells were grown in Iscove modified Dulbecco medium (IMDM; Gibco-Invitrogen) supplemented with 15% BIT 9500 serum substitute (StemCell Technologies) and 20 nM TPO peptide agonist AF13948 (Sigma-Aldrich). Stem cell factor (10 ng/mL; Amgen) was added on day 1 of culture. MKs were cultured for 10 to 16 days in the presence of TPO. Cells were shown by flow cytometry to express GPIb and αIIbβ3 (60%-70% positive cells). For shear experiments, cells were used between days 10 and 16. Human endothelial cells were isolated from umbilical veins and grown to confluence on coverslips as reported.19 Cells from a first to third passage were used. Stimulation with 10 μM forskolin and 100 μM IBMX was performed for 20 minutes before perfusion with MKs.
Human platelets
Blood was obtained from healthy persons who had not ingested any medication during the previous 2 weeks. Blood was drawn into 15% (vol/vol) acid citrate dextrose, pH 5.8. Washed platelets were prepared from isolated platelet-rich plasma in the presence of apyrase (1 U/mL; Sigma-Aldrich) and acid citrate dextrose (1 mL for 40 mL).15 Briefly, after washing, platelets were resuspended in Hepes buffer (10 mM Hepes, N-[2-hydroxyethyl]piperazine-N′-[ethanesulfonic acid], 136 mM NaCl, 2.7 mM KCl, and 2 mM MgCl2), pH 7.5, containing 0.15% bovine serum albumin (BSA). Platelets were counted with an electronic particle counter (Model Z1; Coulter Electronics), and concentration was adjusted to 1.5 × 108 platelets/mL.
Perfusion studies
Perfusion studies were performed using a previously published flow chamber obtained from Maastricht Instrumentation.14 The flow chamber consisted of a rectangular cavity 0.05 mm high, 29 mm long, and 5 mm wide, carved in a Plexiglas block. The bottom of the chamber was lined with a glass coverslip coated overnight at 4°C with VWF (20 μg/mL) diluted in Tris-buffered saline (TBS; 25 mM Tris-HCl, pH 7.4, 150 mM NaCl). In some experiments, another purified protein (fibronectin, fibrinogen, collagen, or type 2B-rVWF) was tested. MKs in suspension in IMDM (2 mL) at a concentration of 0.8 to 1.5 × 106 cells/mL were drawn in a 5-mL glass gas-tight microsyringe (Exmine; Ito Corporation) connected to the chamber with an extension set (Steritex; Codan). A flow rate of 225 μL/min was applied with an electric pump, generating a shear rate of 1800 s−1. The cells were perfused for 10 minutes, followed by IMDM perfusion at the same shear rate for 10 minutes. All experiments were performed at 37°C maintained with a Minitüb heating system (Minitüb Abfüll und Labortechnik). In some experiments, cells or coverslips were preincubated with antibodies for 10 minutes before perfusion. After the end of the perfusion, the coverslip was fixed with ice-cold methanol for 5 minutes, washed with distilled water, dried, and stained with Romanovsky staining. In some experiments, cells were fixed with a perfusion of 2% paraformaldehyde (Carlo Erba) in PBS directly in the flow chamber and processed for immunofluorescence as described in “Confocal immunofluorescence.” Effluents were collected at the exit of the chamber for further studies, including thrombin activation.
Videomicroscopy system
The perfusion chamber was positioned on the stage of an inverted microscope (Axiovert 135; Zeiss). A CCD camera (Sony) was used to visualize cells, using a 20× Hoffman modulation contrast objective. Continuous recording was performed with a digital image recorder (Replay Software; Microvision Instruments) connected to a videotimer (VTG) at 0.25 image/second. For visualization, using “Archimed” software (Microvision Instruments), frames were read at a velocity of 10 images/second (40-fold acceleration). Image frames were analyzed with Histolab or Videomet quantification software (Microvision Instruments).
Cell activation studies and flow cytometry
Effluent cell suspensions were collected and centrifuged in the presence of 0.5 mM EDTA at 700g for 12 minutes, and the pellets were resuspended in IMDM. Aliquots were incubated in the absence or presence of thrombin (0.5 U/mL) for 10 minutes at 37°C. Cells in suspension (100 μL) were incubated for 20 minutes at room temperature with FITC–anti-CD62P and PE–anti-CD41a (5 μL of each) or nonimmune-conjugated control antibody, before adding 300 μL PBS. The cells were then analyzed with a FACSort flow cytometer (Becton Dickinson); 10 000 events were acquired with settings on the platelet region identified by their characteristic profile on a right-angle scatter (SSC) and forward-angle scatter (FSC) plot determined in separate experiments with unstimulated washed platelets.
Static adhesion
Aliquots (150 μL) of unactivated and thrombin-activated cells were added to fibrinogen-coated glass Lab-Tek chamber slides (Nunc) incubated for 30 minutes at 37°C in static conditions. Nonadherent cells were removed and the slides were washed 3 times with PBS, before fixation with 2% paraformaldehyde in PBS for 30 minutes and storage at 4°C for confocal immunofluorescence the next day.
Confocal immunofluorescence
Paraformaldehyde-fixed cells were permeabilized for 5 minutes with 0.1% Triton X-100 in PBS, nonspecific binding was blocked in PBS-BSA 2%, and cells were incubated 90 minutes at 37°C with the anti-β3 P37 MoAb (10 μg/mL), anti–α-tubulin MoAb (2 μg/mL), or polyclonal anti-CD62P (1/100) in PBS-BSA, washed, and incubated with 1:200 dilution of a secondary antibody conjugated to AlexaFluor 488 or AlexaFluor 546 (Molecular Probes). The negative control used purified IgG at the same concentration. For actin labeling, permeabilized cells were incubated at room temperature for 30 minutes with 50 μL of 30 nM AlexaFluor 546–phalloidin (Molecular Probes) in PBS containing 2% BSA. The coverslips were then washed twice and incubated with TOPRO3 before mounting in Vectashield mounting solution (Vector Laboratories). AlexaFluor 488/AlexaFluor 546 staining was analyzed with a Leica TCS SP2 AOBS confocal microscope.
Electron microscopy
Cells present on the coverslips, or alternatively effluent cell suspensions after passage over the various matrices, were fixed in glutaraldehyde fixative (1.5% final concentration in phosphate buffer 0.1 M, pH 7.4) and were then processed for electron microscopy as previously described.20 Cell monolayers were detached from the coverslips with liquid nitrogen before thin sectioning. Some cells in suspension were activated by thrombin. Examination was performed on a Jeol 10-11 electron microscope (Jeol Ltd).
Statistics
We used the Student unpaired t test for statistical analysis; P values less than .05 were considered significant. Error bars represent the standard error of the mean (SEM).
Results
Deformations of MKs under shear
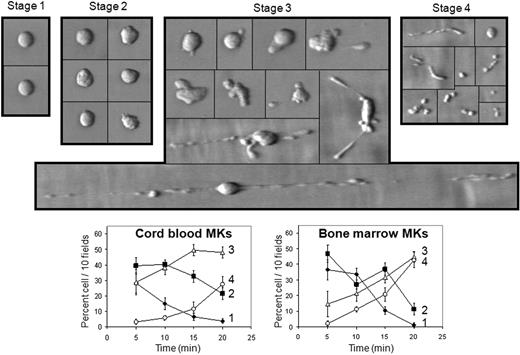
Cord blood mature MKs were perfused at varying shear rates on a surface coated with purified VWF. At 1800 s−1, MKs established transient interactions with VWF, slowing down the velocity of MKs rolling on VWF and progressively leading to profound morphologic changes that finally led to platelet shedding from MKs. The different steps of platelet formation are shown in supplemental Videos 1 and 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article) and summarized in Figure 1. The process starts with roughly spherical undeformed MKs (stage 1); then the cell periphery deforms and pseudopods become visible on adherent MKs (stage 2). Elongation of the cytoplasm then takes place, organized along the flow, at the rear and/or the front of the cell body (stage 3). Intermediate swellings with a characteristic beads-on-a-thread aspect start to appear (supplemental Video 1). Elongation occurred rapidly, at a velocity of 21 μm/min, thus 25-fold higher than reported in static conditions.3 Interestingly, fragmentation occurred not only at the tip of these extensions, but also between the swellings, in particular in thinner constrictions, releasing fragments of various sizes, and most often several beads still attached together, but devoid of the main cell body (Figure 1, supplemental Video 2). Finally, these fragments were further broken down into smaller particles, the size of a platelet, clearly visible during late time points (Figure 1, supplemental Video 2). Cord blood– and bone marrow–derived MKs produced a similar proportion of fragmented proplatelets and platelets, reaching 27.6% (± 4.9%) of adherent cord blood MKs and 42.8% (± 3.7%) of adherent bone marrow MKs at 20 minutes (Figure 1). This effect was dependent on the extent of shear rate and on cell concentration (not shown). Shear exposure of immature MKs, cultured less than 9 days, did not allow translocation and fragmentation.
MK deformation on a VWF surface at high shear rate. Cells suspended in IMDM were perfused on VWF at 1800 s−1 for 10 minutes; then IMDM alone was perfused for 10 minutes. Adhesion led to major cell shape changes and to proplatelet formation. Microphotographs during perfusion indicate 4 different stages starting from undeformed MKs (stage 1) to early deformation characterized by loss of cell sphericity (stage 2); stage 3 includes later deformation of MKs with cytoskeletal reorganization at a cell pole or both ends, also a long thin filament of successive “beads on a thread”; stage 4 comprises fragmentation of proplatelets and cleavage from the nucleus, as well as platelet formation and release. Proportion of cells relative to the total number of cells counted in 10 fields was plotted as a function of perfusion time classified according to these 4 stages for cord blood MKs (bottom left panel) or bone marrow MKs (bottom right panel) perfused on VWF. Photographs are representative of 15 experiments, curves are mean (± SEM) of 8 experiments. The bar represents 10 μm. Black lines have been inserted to indicate repositioned images.
MK deformation on a VWF surface at high shear rate. Cells suspended in IMDM were perfused on VWF at 1800 s−1 for 10 minutes; then IMDM alone was perfused for 10 minutes. Adhesion led to major cell shape changes and to proplatelet formation. Microphotographs during perfusion indicate 4 different stages starting from undeformed MKs (stage 1) to early deformation characterized by loss of cell sphericity (stage 2); stage 3 includes later deformation of MKs with cytoskeletal reorganization at a cell pole or both ends, also a long thin filament of successive “beads on a thread”; stage 4 comprises fragmentation of proplatelets and cleavage from the nucleus, as well as platelet formation and release. Proportion of cells relative to the total number of cells counted in 10 fields was plotted as a function of perfusion time classified according to these 4 stages for cord blood MKs (bottom left panel) or bone marrow MKs (bottom right panel) perfused on VWF. Photographs are representative of 15 experiments, curves are mean (± SEM) of 8 experiments. The bar represents 10 μm. Black lines have been inserted to indicate repositioned images.
Effect of high shear rate and VWF on proplatelet and platelet formation
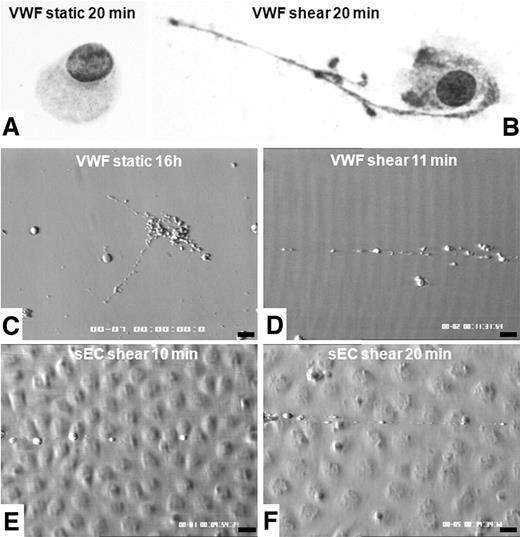
The morphology of MKs incubated in static conditions for 20 minutes on VWF remained unchanged with respect to that of the cells before contact with VWF (Figure 2A). As proplatelet formation was recently shown to occur within 16 hours after MK plating on VWF,20 we reproduced these findings in static conditions, demonstrating that MKs were fully mature and equipped for platelet biogenesis (Figure 2C). When exposed to a shear rate of 1800 s−1, these MKs formed long extensions and rapidly released proplatelets and platelets within 10 to 20 minutes (Figure 2B,D). To study shear-induced proplatelet formation on a physiologically relevant surface, we used VWF released from stimulated endothelial cells in inflammatory conditions.21 We obtained evidence that high-molecular-weight multimers of VWF supported MK rolling, adhesion, and proplatelet formation within 20 minutes (Figure 2E-F).
Comparison of shear or static conditions of MK deformations on a VWF-containing surface. Coverslips were removed from the flow chamber and rinsed with PBS before fixation in ice-cold methanol and subsequent Romanovsky staining (A-B, magnification ×500): during static 20-minute adhesion, cells displayed a spherical shape, without any proplatelet extension (A), but after exposure to high shear rate for 20 minutes, MKs extended long filopods at their tip and beaded platelet-like spikes were formed along the shaft (B). In separate experiments, images were recorded on coverslips coated with VWF (C-D) or on stimulated HUVECs (E-F) during real-time perfusion. Panel C shows proplatelet formation during 16 hours on VWF-coated slides in static conditions extending in different directions (C). In shear conditions, after 20 minutes, proplatelet formation is bipolar and organized along the flow (D). Similar organization of proplatelet formation is seen on UL-VWF released by HUVECs stimulated by IBMX and forskolin (E-F). Bars on panels C through F represent 10 μm.
Comparison of shear or static conditions of MK deformations on a VWF-containing surface. Coverslips were removed from the flow chamber and rinsed with PBS before fixation in ice-cold methanol and subsequent Romanovsky staining (A-B, magnification ×500): during static 20-minute adhesion, cells displayed a spherical shape, without any proplatelet extension (A), but after exposure to high shear rate for 20 minutes, MKs extended long filopods at their tip and beaded platelet-like spikes were formed along the shaft (B). In separate experiments, images were recorded on coverslips coated with VWF (C-D) or on stimulated HUVECs (E-F) during real-time perfusion. Panel C shows proplatelet formation during 16 hours on VWF-coated slides in static conditions extending in different directions (C). In shear conditions, after 20 minutes, proplatelet formation is bipolar and organized along the flow (D). Similar organization of proplatelet formation is seen on UL-VWF released by HUVECs stimulated by IBMX and forskolin (E-F). Bars on panels C through F represent 10 μm.
α-Granule proteins and microtubule network in shear-induced proplatelets and platelets
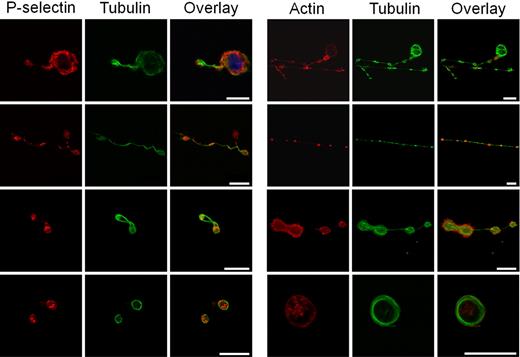
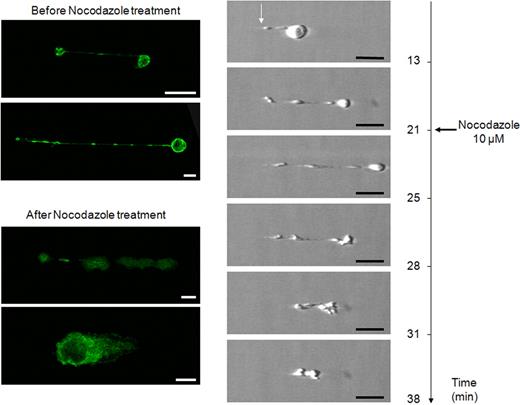
Proplatelets transport individual α-granules on their microtubule tracks.2 To identify the presence of α-granule protein, coverslips were fixed at the end of perfusion on VWF at high shear rate, and punctuate positive staining with anti–human P-selectin antibody was visualized along the microtubule tracks (Figure 3), as previously reported.22 Proplatelet elongation requires the sliding of overlapping microtubules that stain for α- and β1-tubulin.3 We found that MK extensions were stained by an anti-tubulin antibody in different territories from those staining for actin, confirming that these extensions displayed a similar structure as the one identified in proplatelet-forming MKs, by the work of Patel et al1,3 and Italiano et al.23,24 Actin labeling showed diffuse staining in cytoplasmic areas, as previously shown,3 whereas tubulin labeling followed the proplatelet shaft in elongated MKs and displayed the specific ring pattern on platelets (Figure 3). The importance of microtubules in shear-induced proplatelet formation was confirmed using nocodazole, an inhibitor of microtubule assembly, in 2 ways. As expected, preincubation with the inhibitor completely prevented MK elongation and proplatelet formation in flow conditions (data not shown). To demonstrate that nocodazole was not acting on preformed platelets in cell suspensions, the microtubule inhibitor (10 μM) was added after the elongation process had started: the effect visualized in real time showed that nocodazole could revert the shear-induced elongation when added after it had occurred (Figure 4, supplemental Video 3). The specificity of reversal was confirmed by tubulin staining. Interestingly, following nocodazole action at 25 minutes, tubulin was no longer organized in proplatelet-forming MKs, thus indicating that the elongated processes were different from tethers (Figure 4). Indeed, membrane tethers are dynamic structures extending from small, localized adhesion contacts under the influence of flow, and are induced by VWF-GPIb interaction, but they do not display any tubulin staining.25 Taken together, these experiments demonstrate that platelet-sized elements were indeed generated by MK exposure to shear and did not simply arise in suspensions of mature MKs before their passage through the flow chamber.
Distribution of α-granule proteins and tubulin in shear-induced proplatelets and platelets. Confocal microscope analysis of immunofluorescence showing labeling (left panels) with anti–P-selectin, anti-tubulin antibodies, and overlay of (from top to bottom) an MK extending a thick proplatelet, a long and thin proplatelet, a dumbbell-shaped proplatelet with its central narrowing, and 2 platelets. P-selectin staining is seen in organelles in forming proplatelets and in platelets in different spots, whereas tubulin stains the entire microtubule shaft of the proplatelet and the periphery of platelets. Right panels: labeling with phalloidin, anti-tubulin antibodies, and overlay of (from top to bottom) an MK extending proplatelet, a long and thin proplatelet with its shaft as well as its tip, labeled for tubulin, a dumbbell-shaped proplatelet with its central narrowing, and a platelet exhibiting a circular labeling pattern. No colocalization between actin and tubulin is visible. The bars represent 10 μm.
Distribution of α-granule proteins and tubulin in shear-induced proplatelets and platelets. Confocal microscope analysis of immunofluorescence showing labeling (left panels) with anti–P-selectin, anti-tubulin antibodies, and overlay of (from top to bottom) an MK extending a thick proplatelet, a long and thin proplatelet, a dumbbell-shaped proplatelet with its central narrowing, and 2 platelets. P-selectin staining is seen in organelles in forming proplatelets and in platelets in different spots, whereas tubulin stains the entire microtubule shaft of the proplatelet and the periphery of platelets. Right panels: labeling with phalloidin, anti-tubulin antibodies, and overlay of (from top to bottom) an MK extending proplatelet, a long and thin proplatelet with its shaft as well as its tip, labeled for tubulin, a dumbbell-shaped proplatelet with its central narrowing, and a platelet exhibiting a circular labeling pattern. No colocalization between actin and tubulin is visible. The bars represent 10 μm.
Reversal of proplatelet elongation by nocodazole addition during proplatelet elongation. Frames from experiment shown in supplemental Video 3 were selected from (top to bottom) before (13 minutes) and during elongation (21 minutes), after addition of nocodazole (at 21 minutes and 20 seconds), during effect of nocodazole (25 minutes), and at the start (28 minutes), during the progress (31 minutes), and at the completion (38 minutes) of elongation reversal. Notice the modifications between the cell body and its anchorage point (white arrow) during different steps. Confocal microscope analysis of indirect immunofluorescence labeling with anti-tubulin antibody of an MK extending proplatelet before and after nocodazole treatment. MKs exhibit diffuse staining after action of nocodazole, indicating tubulin depolymerization. Bars represent 10 μm.
Reversal of proplatelet elongation by nocodazole addition during proplatelet elongation. Frames from experiment shown in supplemental Video 3 were selected from (top to bottom) before (13 minutes) and during elongation (21 minutes), after addition of nocodazole (at 21 minutes and 20 seconds), during effect of nocodazole (25 minutes), and at the start (28 minutes), during the progress (31 minutes), and at the completion (38 minutes) of elongation reversal. Notice the modifications between the cell body and its anchorage point (white arrow) during different steps. Confocal microscope analysis of indirect immunofluorescence labeling with anti-tubulin antibody of an MK extending proplatelet before and after nocodazole treatment. MKs exhibit diffuse staining after action of nocodazole, indicating tubulin depolymerization. Bars represent 10 μm.
Structure of shear-exposed MKs in the process of proplatelet and platelet release
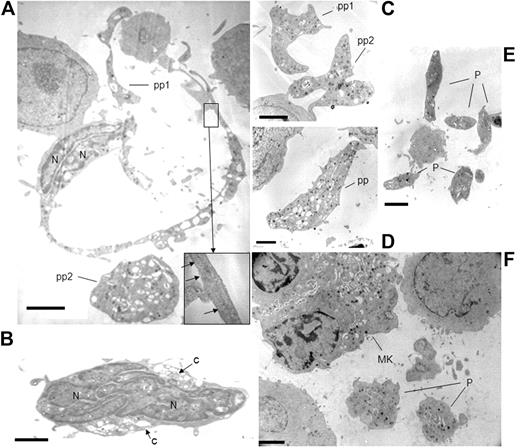
Having shown that proplatelets and platelets were truly formed in real time by MK exposure to a high shear rate, effluents were compared before and after perfusion in the flow chamber. Nonadherent flow-through of cells exposed to shear contained naked nuclei, whereas nonadherent cells in static conditions were devoid of them (data not shown). Electron microscopy showed long cytoplasmic shafts extending from the cell core, containing parallel bundles of microtubules and were sometimes swollen with cytoplasmic organelles (Figure 5A). Nuclear lobes with dense heterochromatin were located at one pole of the cell and elongated naked nuclei were occasionally seen (Figure 5B), in contrast to static cultures where they are virtually never observed. Large cytoplasmic fragments, devoid of nuclei, were roughly spherical, dumbbell-shaped, or elongated with slender extremities (Figure 5C-D). Platelet-like fragments were also visible (Figure 5E). In addition, ultrastructural data were obtained by sectioning the monolayer of the cells present on the VWF-coated coverslips removed from the chamber after they had been exposed to shear. It shows platelet-sized fragments located close to adhering MKs, displaying ultrastructural organization characteristic of platelets, and whose distribution pattern resembles that of the putative mother cell (Figure 5F).
Ultrastructure of MKs exposed to high shear rates leading to platelet formation. Effluent cell suspensions (A-E) or alternatively cells present on the coverslips (F) were processed for electron microscopy as described in “Electron microscopy.” Mature MKs were elongated, extending an often unique long cytoplasmic filopod enclosing parallel longitudinal microtubules (inset). These proplatelets exhibited regular swellings containing cytoplasmic organelles. A large spherical cytoplasmic fragment, probably a detached proplatelet (pp) was located nearby (A). The nuclear lobes (N) containing dense chromatin were elongated and located at one pole of the cell. Naked nuclei with an oval shape and compact nuclear lobes containing dense chromatin, which are normally absent from MK cultures, were retrieved in the effluents (B). Proplatelets (pp) filled with cytoplasmic organelles appeared as large cytoplasmic fragments, devoid of nuclei, roughly spherical, dumbbell-shaped, or elongated with slender extremities (C-D). Several isolated platelet-sized fragments (P) were observed (E). Panel F shows the section of the cell monolayer present on a VWF-coated coverslip removed from the chamber after exposition to shear. It shows 2 platelet-sized fragments (P) located close to an adhering MK, displaying α granules and surface-connected canalicular system characteristic of platelets, and whose distribution pattern resembles that of the putative mother cell (MK). Scale bar represents 2 μm.
Ultrastructure of MKs exposed to high shear rates leading to platelet formation. Effluent cell suspensions (A-E) or alternatively cells present on the coverslips (F) were processed for electron microscopy as described in “Electron microscopy.” Mature MKs were elongated, extending an often unique long cytoplasmic filopod enclosing parallel longitudinal microtubules (inset). These proplatelets exhibited regular swellings containing cytoplasmic organelles. A large spherical cytoplasmic fragment, probably a detached proplatelet (pp) was located nearby (A). The nuclear lobes (N) containing dense chromatin were elongated and located at one pole of the cell. Naked nuclei with an oval shape and compact nuclear lobes containing dense chromatin, which are normally absent from MK cultures, were retrieved in the effluents (B). Proplatelets (pp) filled with cytoplasmic organelles appeared as large cytoplasmic fragments, devoid of nuclei, roughly spherical, dumbbell-shaped, or elongated with slender extremities (C-D). Several isolated platelet-sized fragments (P) were observed (E). Panel F shows the section of the cell monolayer present on a VWF-coated coverslip removed from the chamber after exposition to shear. It shows 2 platelet-sized fragments (P) located close to an adhering MK, displaying α granules and surface-connected canalicular system characteristic of platelets, and whose distribution pattern resembles that of the putative mother cell (MK). Scale bar represents 2 μm.
Platelets generated by MK shear exposure are functional
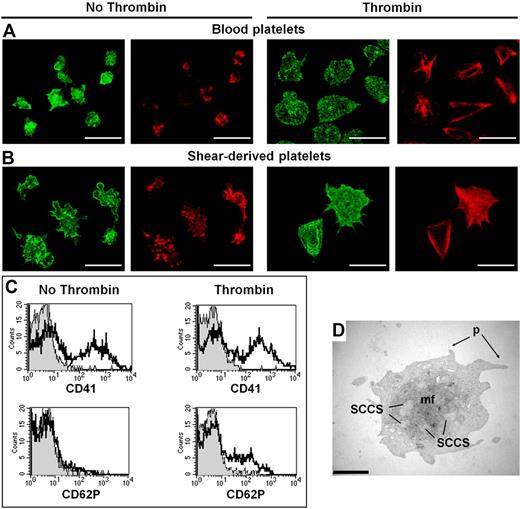
Platelet adhesion to fibrinogen mediated by αIIbβ3 integrin occurs in the absence of activation and is reinforced upon thrombin activation of this receptor. To confirm that platelets generated by MK shear exposure were functionally similar to blood platelets, cells collected in the flow-through were compared with isolated blood platelets, in a static adhesion assay to fibrinogen.26 Washed blood platelets prepared in a separate assay without exposure to shear were firmly adherent to fibrinogen in the absence of thrombin, demonstrating that αIIbβ3 was functional (Figure 6A). Similar findings were seen on shear-induced platelets and proplatelets in the absence of thrombin, as the cell elements positive for αIIbβ3 displayed diffuse actin staining (Figure 6B). After thrombin activation, actin filaments were reorganized in nucleated (not shown) and anucleated elements, showing lamellipod and filopod formation, and integrin membrane staining (Figure 6B). These MK-derived platelets generated by shear exposure displayed a similar cytoskeletal organization to the one of washed blood platelets (Figure 6A). Flow cytometry analysis of shear-exposed cells showed 3 populations of CD41+ cells, according to their size. The smaller sized population overlapped with that of blood platelets and responded to thrombin stimulation, as seen by up-regulation of P-selectin (CD62P) on the surface of shear-derived platelets (11.8% ± 1.8% compared with 6.2% ± 0.9% for nonactivated platelets, P < .001). Similarity with isolated blood platelets was confirmed by ultrastructural data: after thrombin activation, these cells displayed morphologic changes typical of activated platelets, a spherical shape, surface pseudopods, no cytoplasmic granulation but dense material within centralized and dilated cisternae of surface-connected canalicular system (SCCS), and a central bundle of microfilaments (Figure 6D).
Platelets obtained from MK exposure to high shear rates can be activated by thrombin. Cell effluents in the flow-through of MKs exposed to high shear rates were analyzed in a fibrinogen adhesion assay in static conditions, followed by confocal microscopy, with cell staining with phalloidin–Alexa 546 to visualize actin and Alexa 488 to visualize αIIbβ3. Washed blood platelets (A) and MK-derived platelets generated by shear exposure (B) adhered to fibrinogen in the absence (left panels) or in the presence (right panels) of thrombin. Nonactivated cells display diffuse actin staining and αIIbβ3 membrane localization. After thrombin activation, actin filaments are organized as stress fibers. They display a similar cytoskeletal organization to washed blood platelets. The bars represent 10 μm. Flow cytometry assay (C): Samples were labeled with anti–CD62P-FITC (FL1, P-selectin) and anti–CD41-PE (FL2, αIIb). Settings of FSC-SSC profiles of washed blood platelets were used to analyze flow-through cells. Histogram plot of activated platelets in the flow-through, in the absence or presence of thrombin, after labeling with nonimmune IgG (thin line, gray background), anti-CD62P, or anti-CD41a (thick line). Electron microscopy: (D) in the presence of thrombin, the platelet-sized fragment displayed morphologic changes characteristic of activated platelets, namely a spherical shape, surface pseudopods (p), dense material within dilated cisternae of SCCS, no granulation in the cytoplasm, and a central bundle of microfilaments, reminiscent of activated blood platelets. The bar represents 2 μm.
Platelets obtained from MK exposure to high shear rates can be activated by thrombin. Cell effluents in the flow-through of MKs exposed to high shear rates were analyzed in a fibrinogen adhesion assay in static conditions, followed by confocal microscopy, with cell staining with phalloidin–Alexa 546 to visualize actin and Alexa 488 to visualize αIIbβ3. Washed blood platelets (A) and MK-derived platelets generated by shear exposure (B) adhered to fibrinogen in the absence (left panels) or in the presence (right panels) of thrombin. Nonactivated cells display diffuse actin staining and αIIbβ3 membrane localization. After thrombin activation, actin filaments are organized as stress fibers. They display a similar cytoskeletal organization to washed blood platelets. The bars represent 10 μm. Flow cytometry assay (C): Samples were labeled with anti–CD62P-FITC (FL1, P-selectin) and anti–CD41-PE (FL2, αIIb). Settings of FSC-SSC profiles of washed blood platelets were used to analyze flow-through cells. Histogram plot of activated platelets in the flow-through, in the absence or presence of thrombin, after labeling with nonimmune IgG (thin line, gray background), anti-CD62P, or anti-CD41a (thick line). Electron microscopy: (D) in the presence of thrombin, the platelet-sized fragment displayed morphologic changes characteristic of activated platelets, namely a spherical shape, surface pseudopods (p), dense material within dilated cisternae of SCCS, no granulation in the cytoplasm, and a central bundle of microfilaments, reminiscent of activated blood platelets. The bar represents 2 μm.
Involvement of MK receptors and of matrix proteins in platelet formation
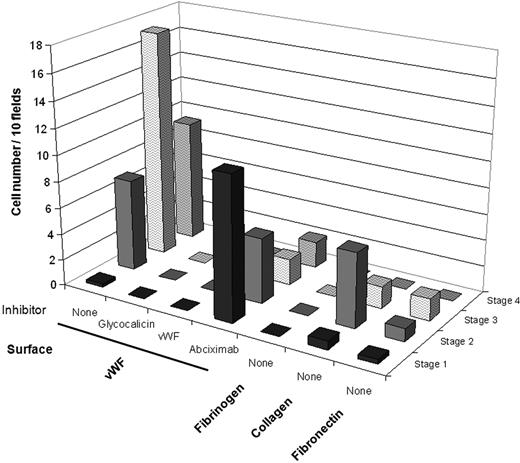
MK translocation and subsequent steps including proplatelet formation and platelet release were completely abolished in the presence of an antibody directed against glycocalicin or the GPIb-binding domain of VWF (Figure 7), demonstrating the crucial importance of the VWF-GPIb interaction. In the presence of the αIIbβ3 inhibitor abciximab, the proportion of cells without proplatelet (undeforming and early deforming MKs) remained constant in time, whereas proplatelet elongation was strongly reduced and platelet formation was almost completely abolished (Figure 7). As shown in supplemental Video 4, this inefficient proplatelet formation was due to loose contact with the VWF surface. Finally, to further establish the specificity of adhesive surface in the regulation of proplatelet formation, we examined the extent of proplatelet formation by MKs perfused over fibrinogen, collagen, or fibronectin at a high shear rate. No proplatelet was formed on these surfaces; the only change was a mild early MK deformation on collagen (Figure 7). In addition, we studied proplatelet formation from MKs perfused on a surface coated with a mutated type 2B-rVWF. We selected the V1316M substitution, a well-defined mutation, characterized in patients by enhanced VWF binding to platelet GPIb with mild thrombocytopenia and giant platelets.15,27 We found that the mean velocity of MK translocation on type 2B-rVWF (4.6 ± 0.3 μm/second) was much lower than on wild-type (wt)–rVWF (33.6 ± 2.5 μm/second, P < .001). All steps of proplatelet formation by MKs exposed to high shear rates were slower on 2B-rVWF than wt-rVWF, leading to decreased platelet production and increased proplatelet accumulation (supplemental Video 5).
MK deformations at high shear rate on different surfaces coated with matrix proteins and effect of VWF inhibitors. Cord blood MKs suspended in IMDM were perfused on VWF (in the absence or presence of inhibitors), fibrinogen, collagen, or fibronectin at 1800 s−1 for 10 minutes; then IMDM alone was perfused for 10 minutes. Cell numbers were counted in 10 fields at the end of perfusion (20 minutes) and classified according to the 4 stages defined in Figure 1. Intense cell deformation and proplatelet formation occur on VWF. Cell perfusion on VWF in the presence of GPIb-VWF interaction inhibitors, either a blocking antibody to glycocalicin or an anti-VWF MoAb directed against VWF binding site to GPIb, indicated a major inhibition of MK adhesion to VWF and of subsequent steps, thus abolishing proplatelet formation. Abciximab, blocking the interaction of αIIbβ3 with VWF, prevented proplatelet and platelet formation. MK deformations and proplatelet formation on non-VWF surfaces were minimal in shear conditions as shown for fibrinogen, fibronectin, and collagen.
MK deformations at high shear rate on different surfaces coated with matrix proteins and effect of VWF inhibitors. Cord blood MKs suspended in IMDM were perfused on VWF (in the absence or presence of inhibitors), fibrinogen, collagen, or fibronectin at 1800 s−1 for 10 minutes; then IMDM alone was perfused for 10 minutes. Cell numbers were counted in 10 fields at the end of perfusion (20 minutes) and classified according to the 4 stages defined in Figure 1. Intense cell deformation and proplatelet formation occur on VWF. Cell perfusion on VWF in the presence of GPIb-VWF interaction inhibitors, either a blocking antibody to glycocalicin or an anti-VWF MoAb directed against VWF binding site to GPIb, indicated a major inhibition of MK adhesion to VWF and of subsequent steps, thus abolishing proplatelet formation. Abciximab, blocking the interaction of αIIbβ3 with VWF, prevented proplatelet and platelet formation. MK deformations and proplatelet formation on non-VWF surfaces were minimal in shear conditions as shown for fibrinogen, fibronectin, and collagen.
Discussion
Mechanical forces play an important role in cell remodeling and cytoskeletal reorganization. Here, we studied platelet formation from human MKs perfused in a flow chamber reproducing high shear rate conditions of the microcirculation. A major finding was that during exposure to high shear rates (>1000 s−1), cytoplasmic MK deformation produced proplatelet extensions organized along the flow and rapidly leading to platelet formation. The involvement of shear forces on platelet production by mature MKs is supported by several observations. First, circulating differentiated MKs are frequently encountered.5,28 Second, direct images of MK fragmentation, platelet release, and naked nuclei are not seen in normal bone marrow hematopoietic islands.29 Third, although in vitro MK maturation has been greatly improved by the use of TPO, the yield of platelet shedding in culture has remained minimal.13 MKs respond to chemotactic stimuli and are able to cross the sinusoid barrier and enter the bloodstream.4 Recently, intravital microscopy of mouse skull microvessels indicated that MKs can extend protrusions or even penetrate inside the lumen of bone marrow capillaries, giving rise to large proplatelets connected to the MK cell body.8 In that in vivo model, the authors observed the detachment of large cytoplasmic fragments into circulating blood and suggested that high shear rates appeared to free them from the parent cells and might contribute to platelet formation. Our results are in agreement with this model and suggest an effect of MK exposure to high shear rates encountered in small vessels that contribute to the generation of single, mature platelets. We used a shear rate of 1800 s−1, which is encountered in small arterioles and capillaries of human organs30 and is higher than the ones existing in murine bone marrow sinusoids (50-165 s−1).8,31
The latter work was performed with murine species and, of note, many differences have been emphasized in megakaryocyte localization and ultrastructure of proplatelet formation, between mice and humans.32 The present study performed on human MKs sheds additional light on how these large protrusions can be induced to fragment into small platelets by shear forces in flowing blood. This is also in keeping with evidence that the pulmonary circulation could be an important site of platelet production, as the lung capillaries would be the first to be encountered by cells leaving the bone marrow.28 Indeed, the large cytoplasmic fragments and the isolated platelet-sized fragments that we observed in real time to form on the coverslip during the flow assay resembled those seen downstream of the pulmonary circulation in vivo.33 In our conditions, high shear rates were essential to proplatelet and platelet formation during an exposure time of 20 minutes. In contrast, no proplatelet or platelet was generated in static conditions during this short period. The 2-dimensional distribution of proplatelets generated in static conditions during several hours was multipolar,24 whereas it was bipolar or polar in our flow conditions. We demonstrated that this process was driven by microtubule polymerization to form proplatelets, as platelet formation was completely blocked when microtubule assembly was inhibited by nocodazole. This also indicated that the process we observed was indeed platelet production and not an artifact of cell death.3 Elongation after MK deformation shows similarities with the recently reported platelet cytoskeleton remodeling and shear-specific morphologic changes during surface translocation on VWF.34 The organization of shear-induced MK elongations resembled that previously reported in mice,1,3,23,24 as shown by the distribution of P-selectin, an α-granule membrane protein and by the characteristic tubulin staining. In addition, the reversal of proplatelet elongation and platelet shedding that we obtained by addition of nocodazole after the elongation had started demonstrates that proplatelet and platelet processes induced by shear are completely different from the previously reported membrane tethers. These tethers are dynamic structures extending from small, localized adhesion contacts under the influence of flow, induced by VWF-GPIb interaction, but that do not display any tubulin staining.25 Thus, not only platelets, but also MKs, may show a shear-sensitive functional response. Platelets generated in vitro by MK exposure to high shear rates were similar to normal human blood platelets as they responded to thrombin stimulation by up-regulating surface P-selectin expression, confirming a previous report in static conditions.35 The functional nature of shear-derived platelets was confirmed by their thrombin-induced cytoskeletal reorganization, indistinguishable from blood platelets, in a fibrinogen adhesion assay and by their ultrastructural aspect.
At high shear rates, human MKs rolled and attached to a VWF matrix in a fashion close to that reported with blood platelets. The latter adhere in a 2-step process consisting of a reversible initial contact (GPIb-dependent translocation) followed by an irreversible attachment mediated by integrin αIIbβ3 activation.36 Indeed, MK adhesion and platelet generation from MKs exposed to high shear rates were completely abrogated by antibodies inhibiting the VWF-GPIb interaction. In vitro proplatelet formation was previously reported to be inhibited by an anti-GPIb monoclonal antibody in static conditions.20,37 Our results thus suggest that the macrothrombocytopenia observed in BSS patients, characterized by a genetic defect of the GPIb-IX-V complex,12 may be in part related to impaired shear-induced thrombopoiesis, the giant platelets thus corresponding to incompletely fragmented proplatelets. Moreover, in a BSS mouse model, reconstitution of the GPIbα–null mouse with the GPIb intracellular domain fused to the extracellular domain of human IL4-R did not completely correct the macrothrombocytopenia, confirming the critical role of the GPIbα extracytoplasmic domain in platelet formation.38 Intriguingly, the platelet count and size are normal in severe hereditary VWF deficiency, the type 3 von Willebrand disease (VWD), but it is conceivable that other matrix proteins such as fibronectin or thrombospondin might replace VWF for GPIb binding in shear conditions in vivo.39,40 Recently, membrane-bound VWF was detected on proplatelet forming MKs, suggesting an autocrine stimulation of VWF bound to GPIb.20 Moreover, our findings obtained with a mutated type 2B-rVWF support a role for VWF interaction with GPIb in proplatelet formation under shear conditions. Indeed, in type 2B VWD patients, mutated VWF displays increased binding to GPIb, and a low dissociation rate from GPIb.27 This gain-of-function mutation accounts for the transient thrombocytopenia leading to platelet aggregates that are cleared from the circulation.41 Interestingly, macrothrombocytopenia has also been reported in type 2B VWD.42,43 The decreased rolling velocity of MKs on a 2B-rVWF matrix, compared with wt-rVWF that we describe, suggests a slower and less efficient process of platelet formation at high shear rates, responsible for an increased proportion of giant platelets in these patients.
We have observed proplatelet formation on human umbilical vein endothelial cell monolayers stimulated by known effectors of VWF release. Weibel-Palade bodies of endothelial cells release VWF by a tightly regulated mechanism that allows a rapid response to changes within the vascular microenvironment.21 Both Ca2+-raising agents and cAMP-raising agonists display specific patterns of cytoskeletal remodeling that lead to opposite effects on endothelial cell barrier function.21 Thus MK fragmentation leading to platelets may occur intravascularly and be finely regulated depending on the release of VWF from Weibel-Palade bodies, as well as on the VWF content of endothelial cells that is known to be heterogeneous along the vascular bed, and particularly high in the pulmonary artery.44,45 Indeed, there is evidence that platelet counts are higher in pulmonary venous samples than in pulmonary arterial blood,28,33 suggesting that circulating MK fragments and proplatelets may be processed into platelets when passing through the lung capillaries, where they are exposed to high shear rates. Shear rates conditions used in this study appear relevant to the pulmonary microcirculation, which has been suggested to be a site of platelet production. In humans, the diameter of pulmonary capillaries is 5 to 8 μm,46,47 thus smaller than that of bone marrow sinusoids (22-60 μm).48
VWF mediates platelet interactions with the vessel wall at the high shear rates encountered in capillaries and small arteries, through a tightly regulated interaction with GPIb, stabilized by signaling triggered by GPIb engagement that results in αIIbβ3 integrin activation.9 In our flow model, VWF interaction with αIIbβ3 was necessary for complete MK elongation and proplatelet formation. This is in agreement with the inhibiting effect of another αIIbβ3 antagonist, lotrafiban, on proplatelet formation in static conditions.49 In contrast to our shear conditions, the latter authors found that in static conditions, fibrinogen yielded a larger proportion of proplatelets than collagen or VWF. This suggests that shear forces may regulate the specificity of MK αIIbβ3 interactions with matrix proteins, as previously reported for platelets.50 However, it seems that, in vivo, the involvement of αIIbβ3 in platelet formation is more dispensable than GPIb, because αIIbβ3 congenital deficiency (Glanzmann thrombasthenia) does not lead to thrombocytopenia. Interestingly, the cytoplasmic part of the β3 subunit of αIIbβ3 was recently found to be involved in proplatelet formation, in a familial form of macrothrombocytopenia.51 A possible explanation is that αIIbβ3 is implicated only in MK anchorage to the surface, whereas GPIb is responsible mainly not only for MK arrest but also for several other downstream steps such as proplatelet elongation, swellings, and for the eventual detachment of platelets and proplatelets from the mother cell in a process similar to cytokinesis, through GPIb interaction with actin via filamin.52 In conclusion, our finding that mature MK interaction with VWF in flow conditions promotes human proplatelet formation represents a major breakthrough in understanding platelet formation, which may thus occur in capillaries or small arteries. The exploration of diseases with decreased platelet production would greatly benefit from a better understanding of platelet shedding from MKs. Finally, our results may also have important implications for research on platelet production for therapeutic purposes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Pierre Raynal for providing umbilical cord blood, Valerie Jalbert and Azzedine Yacia for technical assistance with MK culture, and Laurence Momeux for technical assistance with electron microscopy.
C.D.-L. was supported by a grant from Agence Nationale de la Recherche and Leducq International Network Against Thrombosis (LINAT).
Authorship
Contribution: C.D.-L. and D.B. conceived and designed the experiments; C.D.-L., D.B., and C.C. performed the experiments; C.D.-L., D.B., and E.C.-B. analyzed the data; D.B. and E.C.-B. designed the study and wrote the paper; S.F. and T.B. contributed reagents, materials, and analysis tools; and C.D.-L. prepared figures and videos.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominique Baruch, Inserm Unit 765, Faculté des Sciences Pharmaceutiques et Biologiques de Paris, 4 Avenue de l'Observatoire, 75270 Paris cedex 06, France; e-mail: dominique.baruch@univ-paris5.fr.
References
Author notes
*E.C.-B. and D.B. contributed equally to this work.