Infection of CD4+ chemokine coreceptor+ targets by HIV is aided and abetted by the proficiency of HIV in eliminating or neutralizing host cell–derived defensive molecules. Among these innate protective molecules, a family of intracellular apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like (APOBEC) cytidine deaminases, is constitutively expressed but inactivated by HIV viral infectivity factor. The ability of interferon-α (IFN-α) to augment cytidine deaminases offered the possibility that the balance between virus and target cell might be altered in favor of the host. Further characterization of transcriptional profiles induced by IFN-α using microarrays, with the intention to identify and dissociate retroviral countermaneuvers from associated toxicities, revealed multiple molecules with suspected antiviral activity, including IL-27. To establish whether IFN-α toxicity might be sidestepped through the use of downstream IL-27 against HIV, we examined whether IL-27 directly regulated cytidine deaminases. Although IL-27 inducesAPOBECs, it does so in a delayed fashion. Dissecting the underlying regulatory events uncovered an initial IL-27–dependent induction of IFN-α and/or IFN-β, which in turn, induces APOBEC3, inhibited by IFN-α/β receptor blockade. In addition to macrophages, the IL-27–IFN-α connection is operative in CD4+ T cells, consistent with an IFN-α–dependent pathway underlying host cell defense to HIV.
Introduction
Human immunodeficiency virus (HIV) ravages the immune system of those infected and continues to develop resistance to multiple therapeutic interventions. CD4+ T cells are primary viral targets, and as they are rapidly depleted during progression to acquired immunodeficiency syndrome (AIDS),1,2 the host becomes susceptible to opportunistic infections. In addition to CD4+ T lymphocytes, CD4+ chemokine coreceptor expressing monocyte-derived macrophages also serve as hosts for HIV. Macrophages often replicate virus in vacuoles to avoid immune surveillance, and being relatively resistant to viral cytopathicity may serve as long-term viral reservoirs.3,4 Because of rapid and persistent mutability of HIV and inherent difficulties in vaccine development, identifying host molecules that support or antagonize HIV as potential manipulative targets in defense against the virus remains an important goal. In this regard, considerable recent effort has focused on host cell molecules required for viral entry as a means to deflect the infection process.5,–7
Intracellular molecules commandeered by HIV to support its life cycle are also being considered as candidate targets to interfere with infection.8,–10 Among these, a family of intracellular cytidine deaminases, apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like (APOBEC) cytidine deaminases, particularly APOBEC3G, was identified in CD4+ T cells and macrophages with innate antiretroviral activity.11,–13 Although initially considered to be noninducible and overcome by its interaction with HIV viral infectivity factor (Vif), new evidence supports its unique regulation by interferon-α (IFN-α) in vitro,12,14,15 an incentive to explore the IFN-α impact on APOBEC relative to HIV in clinical trials16 (Peng G. et al, submitted). However, type I IFN generated by plasmacytoid dendritic cells and macrophages in response to HIV infection17,18 or delivered as a treatment modality16 is also associated with negative aspects, including induction of CD4+ T-cell apoptosis and/or HIV disease progression.18
In consideration of alternative mechanisms to enhance expression of the innate antiviral system of APOBEC family members, while minimizing potential cytopathicity, we explored IFN-α–stimulated gene (ISG) transcription in human macrophages.14 In addition to APOBEC, IFN-α modulated the expression of multiple genes with potential antiviral activity, including IL-27, recently linked to HIV suppression,19 and potentially allying these 2 cytokines in combating HIV. IL-27 is the most recently identified member of the IL-12 family, and its biologically active form consists of heterodimeric IL-27 p28 and an IL-12p40–like subunit, Epstein-Barr virus–induced 3 (EBI3).20,21 Characterized as a macrophage, dendritic cell (DC), and endothelial cell–derived proinflammatory cytokine for inducing early differentiation of the Th1 subset and IFN-γ production, IL-27 also demonstrates suppressive effects on the immune system.20,22,–24 Although emphasis has been on T lymphocytes that express the glycoprotein 130 (gp130) and WSX-1 receptor complex,25 further investigation of the role of IL-27 on innate cells and regulation of putative antiviral functions will enable an appreciation of its potential as a mediator of microbial defense. In this study, we demonstrate by microarray that IFN-α–induced macrophage antiviral molecules include IL-27 and that IL-27 can trigger synthesis of APOBEC cytidine deaminases to blunt HIV infection. However, IL-27 does not do this directly, but rather, induces an intermediary molecule, identified as type I IFN, which in turn, coordinates the antiretroviral activities attributed to IL-27. Revelation of IFN-α–orchestrated host defense mechanisms may circumvent the need for IFN-α and its related toxicities as potential interceptors of HIV infection.
Methods
Peripheral blood monocyte isolation and differentiation to macrophages
Human peripheral blood mononuclear cells (PBMCs), obtained by leukapheresis of normal volunteers (Department of Transfusion Medicine, National Institutes of Health), were diluted in endotoxin-free phosphate- buffered saline without Ca2+ and Mg2+ (BioWhittaker) and, for some experiments, separated by density centrifugation on lymphocyte sedimentation medium (Organon Teknika Corp) before elutriation to obtain monocytes and T lymphocytes.26 For differentiated macrophages, elutriated monocytes were adhered in serum-free Dulbecco modification of Eagle medium (DMEM) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich) at 37°C and 5% CO2 in 6-well (6 × 106 cells/well) plates (Corning) for 2 to 4 hours, 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) added and the cells cultured for 6 to 7 days.26
Cytokine treatment
Cell cultures were left untreated (control), stimulated with IFN-α (10 ng/mL; NCI-FCRDC; R&D Systems) or lipopolysaccharide (LPS) (Escherichia coli 055:B5, Sigma-Aldrich, 100 ng/mL) for 1 to 4 hours to evaluate transcriptional and functional responses. IL-27 (R&D Systems) was added at 5 to 200 ng/mL for 0.5 to 24 hours before RNA was isolated. Neutralizing type 1 IFN receptor (IFNAR) chain 2 antibody (CD118, R&D Systems, 10 μg/mL) was added in some experiments 20 minutes before cytokines or a Janus kinase (JAK) inhibitor (0-10 nM, Calbiochem) added 1 hour before cytokines.
Oligonucleotide microarrays and data analysis
Monocyte-derived macrophages from 3 independent donors were harvested, total RNA isolated, and further purified using RNeasy Mini kit (QIAGEN), as described.14,27 Preparation of biotin-labeled cRNA, hybridization, and scanning were performed per protocol (Affymetrix). Briefly, 10 μg total RNA was used to generate double-stranded cDNA using One-Cycle cDNA Synthesis Kit and oligo (dT)24 primer containing a 3′ T7 RNA polymerase promoter site. Biotin-labeled cRNA probes were produced from cDNA using an in vitro transcription (IVT) labeling kit. The probes were purified, fragmented, and hybridized to Affymetrix Plus 2.0 Microarrays that display greater than 47 000 unique probe sets and expressed sequence tags (ESTs) for 16 hours, washed, stained using Affymetrix Fluidics Station 450 and fluorescence measured using an Affymetrix GeneChip scanner. Affymetrix MAS5 signal and present call values were stored in the NIHLIMS, a database for storage and retrieval of chip data. The signal values for the 9 chips were subjected to S10 quantile-normalizing transformation. Principal components analysis on the S10 transformed data was also performed to permit the visualization of the samples in bivariate plots of the low-order principal components to detect possible outliers and to provide a global view of the study results. Data were statistically analyzed using the MSCL Analyst's Toolbox (P. J. Munson, J. J. Barb, 2004; http://abs.cit.nih.gov/MSCLtoolbox/) and the JMP statistical software package (SAS Inc; http://www.jmp.com).14 A 3-level, one-way blocked ANOVA compared baseline macrophages with macrophages treated with IFN-α or LPS. A posthoc test was used to identify probe sets that manifest significant false detection rate (FDR) less than 5% substantial (> 4-fold) differences among the 3 groups and were called present. Fold change calculations were made for IFN-α and LPS compared with control using the group means (IFN-α, LPS, control). The microarray data were deposited into the Gene Expression Omnibus public database under accession number GSE16755.
Infection with HIV-1
Macrophages were incubated with HIV-1BaL (HIV) at 103/TCID50/mL (ABI) in DMEM containing 10% fetal calf serum (FCS) for 2 hours at 37°C. After infection, cells were washed and cultured in DMEM containing 10% FCS for up to 14 days and half the supernatant collected and replaced with fresh medium every 2 to 3 days.26 Three-day phytohemagglutinin-blasted T lymphocytes were incubated with HIVIIIB at 103 TCID50/mL (ABI) for 2 hours, washed, and cultured for 7 to 9 days with supernatant collection every 2 to 3 days. IFN-α, IFN-β, and IL-27 were added at indicated times and concentrations. In indicated experiments, anti-CD118 (10 μg/mL) was added once to the macrophage cultures after infection and again when fresh medium was added. To measure viral infectivity, supernatant p24 levels were determined with HIV p24 ELISA kits (Perkin Elmer Life Sciences).
RNA isolation and polymerase chain reaction
Total cellular RNA was isolated and DNase digested using Qiagen RNeasy Mini Kit and RNase-Free DNase Set. For real-time (RT) polymerase chain reaction (PCR) and conventional PCR,12 2 μg total RNA was reverse-transcribed using oligodeoxythymidylic acid primer (Invitrogen). Amplification of the resulting cDNA for RT-PCR was performed with TaqMan expression assays for IL-27 p28 (Hs00377366_m1), IL-27 EBI3 (Hs00194957_m1 and Hs01057148_m1), IL-27Rα (Hs00175472_m1), IFN1α (Hs00353738_s1), IFN-β (Hs01077958_s1), APOBEC3A (Hs00377444_m1), APOBEC3B (Hs00358981_m1), APOBEC3C (Hs00819353_m1), APOBEC3F (Hs00736570_m1), APOBEC3H (Hs00419665_m1), and APOBEC3G (Hs00222415_m1; Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1) was used for normalization, data were analyzed using the 2−ddCT method, and results were reported as fold change.14,27
Western blots
Macrophages were treated or not with IFN-α at 10, 1, or 0.1 ng/mL and IL-27 at 100 and 5 ng/mL for 30 minutes. Cells were pelleted, lysed in lysis buffer,14 and lysates incubated on ice for 20 minutes and centrifuged (14 000 rpm, 20 minutes, 4°C) before supernatant proteins were quantified by Bio-Rad DC Protein Assay. Lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis in Tris-glycine gels. For Western blot, the proteins were transferred to nitrocellulose membranes. After blocking with 5% bovine serum albumin in Tris-buffered saline with 0.05%Triton X-100 (TBS-T), membranes were probed with mouse anti–STAT1 or 3 or rabbit anti–phospho-STAT1 antibody (Cell Signaling Technology) at 4°C overnight. Membranes were washed with TBS-T 3 times for 10 minutes, analyzed by adding Alexa Fluor 680 goat anti–rabbit or Alexa Fluor 750 goat anti–mouse antibodies (LI-COR), and the infrared fluorescence was detected with Odyssey infrared imaging system (LI-COR).
IFN-α ELISA
Supernatants from treated and untreated macrophages were collected at 7 to 18 hours and assayed by enzyme-linked immunosorbent assay (ELISA; PBL InterferonSource). The human-IFN-α multi-subtype assay detects 14 of 15 identified human IFN-α subtypes: IFN-αA, IFN-α2, IFN-αD, IFN-αB2, IFN-αC, IFN-αG, IFN-αH, IFN-αI, IFN-αJ1, IFN-αK, IFN-α1, IFN-α4a, IFN-α4b, and IFN-αWA.
Results
IFN-α–induced macrophage transcriptional profile by microarray analysis
Based on our recent observations that IFN-α induces macrophage HIV resistance, at least in part, through its ability to up-regulate APOBEC3 family cytidine deaminases,12,14 and the known regulatory role of IFN-α on other antiviral molecules,28 we treated human monocyte-derived macrophages with IFN-α to expose transcriptional expression of potential additional antiviral genes through oligonucleotide microarrays. To enhance selectivity, we evaluated only those inducible genes with a minimal false detection rate of 5% and expressing at least a 4-fold expression change. Based on this high stringency, 308 IFN-α–inducible genes were significantly enhanced in all donors within 4 hours after IFN-α exposure (see supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The 40 most affected gene symbols are represented in Table 1. Several probe sets were up-regulated more than 200-fold by IFN-α, including chemokine (C-X-C motif) ligand (CXCL10), tumor necrosis factor α–induced protein 6 (TNFAIP6), chemokine (C-C motif) ligand CCL20, and IFN-induced protein with tetratricopeptide repeats 2 (IFIT2). Additional classical IFN-responsive genes (IRG), including IFIT1 and IFIT3, IFN-regulatory factor (IRF)1, IFN-stimulated gene (ISG)20, IFN-α–inducible protein (clone IFI-15K), suppressor of cytokine signaling 3(SOCS3), guanylate binding protein (GBP)1 and GBP5, IFN-induced transmembrane protein 1 (IFITM1), and STAT4, increased greater than 10-fold, whereas eukaryotic translation initiation factor 2 alpha kinase 2 (PKR) increased only 3.35-fold, below our cutoff of 4-fold for this study. IFN-α triggered multiple chemokines (CCL5, CXCL11, CXCL9, CXCL1, CCL4, CCL8, CCL19, IL-8), reflective of its orchestration of innate immunity, but proinflammatory molecules may also contribute to IFN-α–related pathology. Moreover, cytokine and cytokine-related genes were also early macrophage IFN-α targets: pentraxin-related gene (PTX3), TNF (ligand) superfamily member 10 (TRAIL), IL-7R, TNF superfamily member 2 (TNF-α), TNFAIP2, TNFAIP3 interacting protein 3, TNF receptor-associated factor 1 (TRAF1), and IL-6 (IFN-β2), Epstein-Barr virus–induced 3 (EBI3), IL-12B(p40), IL-1B, and IL-15 (> 10-fold), along with other proinflammatory and/or potentially toxic molecules, including indoleamine(2,3)-dioxygenase (IDO) and GTP cyclohydrolase I (GCHI), are involved in neopterin production.29 Only a limited number of probe sets27 were down-regulated in macrophages upon exposure to IFN-α (see supplemental Table 1).
IFN-α–regulated macrophage genes
| Gene symbol . | Gene title . | FC, IFN-α/C* . |
|---|---|---|
| TNFAIP6 | Tumor necrosis factor, alpha-induced protein 6 | 404.21 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | 390.45 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 253.64 |
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 | 244.85 |
| RSAD2 | Radical S-adenosyl methionine domain containing 2 | 134.3 |
| INDO | Indoleamine-pyrrole 2,3 dioxygenase | 84.39 |
| ISG20 | Interferon stimulated exonuclease gene 20 kDa | 78.24 |
| CXCL11 | Chemokine (C-X-C motif) ligand 11 | 71 |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 70.45 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 70.2 |
| LOC730249 | Similar to immune-responsive protein 1 | 60.63 |
| IFIT3 | Interferon-induced protein with tetratricopeptide repeats 3 | 59.49 |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 | 58.59 |
| PTX3 | Pentraxin-related gene, rapidly induced by IL-1 beta | 56.11 |
| LAMP3 | Lysosomal-associated membrane protein 3 | 52.54 |
| TNIP3 | TNFAIP3 interacting protein 3 | 49.16 |
| IL7R | Interleukin-7 receptor | 48.11 |
| GCH1 | GTP cyclohydrolase 1 (dopa-responsive dystonia) | 44.27 |
| IL6 | Interleukin-6 (interferon, beta 2) | 43.62 |
| ISG15 | ISG15 ubiquitin-like modifier | 41.89 |
| EBI3 | Epstein-Barr virus induced gene 3 | 41.76 |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) | 34.96 |
| LRRC50 | Leucine rich repeat containing 50 | 34.81 |
| LOC440896 | Hypothetical gene supported by AK127288; AY343901 | 34.79 |
| OASL | 2′-5′-oligoadenylate synthetase-like | 33.49 |
| SOCS3 | Suppressor of cytokine signaling 3 | 32.8 |
| GBP1 | Guanylate binding protein 1, interferon-inducible, 67 kDa | 30.39 |
| IL12B | Interleukin 12B (natural killer cell stimulatory factor 2, p40) | 29.32 |
| CCL4 | Chemokine (C-C motif) ligand 4 | 27.97 |
| DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 26.81 |
| LOC129607 | Hypothetical protein LOC129607 | 26.46 |
| CCL8 | Chemokine (C-C motif) ligand 8 | 24.34 |
| IL8 | Interleukin 8 | 24.04 |
| ITGB8 | Integrin, beta 8 | 23.94 |
| CCR7 | Chemokine (C-C motif) receptor 7 | 23.68 |
| SOD2 | Superoxide dismutase 2, mitochondrial | 20.98 |
| ELOVL7 | ELOVL family member 7, elongation of long chain fatty acids (yeast) | 20.84 |
| MCOLN2 | Mucolipin 2 | 19.52 |
| SLAMF1 | Signaling lymphocytic activation molecule family member 1 | 17.26 |
| EGR1 | Early growth response 1 | 0.04 |
| Gene symbol . | Gene title . | FC, IFN-α/C* . |
|---|---|---|
| TNFAIP6 | Tumor necrosis factor, alpha-induced protein 6 | 404.21 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | 390.45 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 253.64 |
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 | 244.85 |
| RSAD2 | Radical S-adenosyl methionine domain containing 2 | 134.3 |
| INDO | Indoleamine-pyrrole 2,3 dioxygenase | 84.39 |
| ISG20 | Interferon stimulated exonuclease gene 20 kDa | 78.24 |
| CXCL11 | Chemokine (C-X-C motif) ligand 11 | 71 |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 70.45 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 70.2 |
| LOC730249 | Similar to immune-responsive protein 1 | 60.63 |
| IFIT3 | Interferon-induced protein with tetratricopeptide repeats 3 | 59.49 |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 | 58.59 |
| PTX3 | Pentraxin-related gene, rapidly induced by IL-1 beta | 56.11 |
| LAMP3 | Lysosomal-associated membrane protein 3 | 52.54 |
| TNIP3 | TNFAIP3 interacting protein 3 | 49.16 |
| IL7R | Interleukin-7 receptor | 48.11 |
| GCH1 | GTP cyclohydrolase 1 (dopa-responsive dystonia) | 44.27 |
| IL6 | Interleukin-6 (interferon, beta 2) | 43.62 |
| ISG15 | ISG15 ubiquitin-like modifier | 41.89 |
| EBI3 | Epstein-Barr virus induced gene 3 | 41.76 |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) | 34.96 |
| LRRC50 | Leucine rich repeat containing 50 | 34.81 |
| LOC440896 | Hypothetical gene supported by AK127288; AY343901 | 34.79 |
| OASL | 2′-5′-oligoadenylate synthetase-like | 33.49 |
| SOCS3 | Suppressor of cytokine signaling 3 | 32.8 |
| GBP1 | Guanylate binding protein 1, interferon-inducible, 67 kDa | 30.39 |
| IL12B | Interleukin 12B (natural killer cell stimulatory factor 2, p40) | 29.32 |
| CCL4 | Chemokine (C-C motif) ligand 4 | 27.97 |
| DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 26.81 |
| LOC129607 | Hypothetical protein LOC129607 | 26.46 |
| CCL8 | Chemokine (C-C motif) ligand 8 | 24.34 |
| IL8 | Interleukin 8 | 24.04 |
| ITGB8 | Integrin, beta 8 | 23.94 |
| CCR7 | Chemokine (C-C motif) receptor 7 | 23.68 |
| SOD2 | Superoxide dismutase 2, mitochondrial | 20.98 |
| ELOVL7 | ELOVL family member 7, elongation of long chain fatty acids (yeast) | 20.84 |
| MCOLN2 | Mucolipin 2 | 19.52 |
| SLAMF1 | Signaling lymphocytic activation molecule family member 1 | 17.26 |
| EGR1 | Early growth response 1 | 0.04 |
FC indicates fold change.
The 40 gene symbols with the largest IFN-α/control ratios, less than 5% false detection rate (FDR) on a posthoc test and called “present” (see “Methods”).
Dissociation of activation-induced genes from IFN-α–specific genes
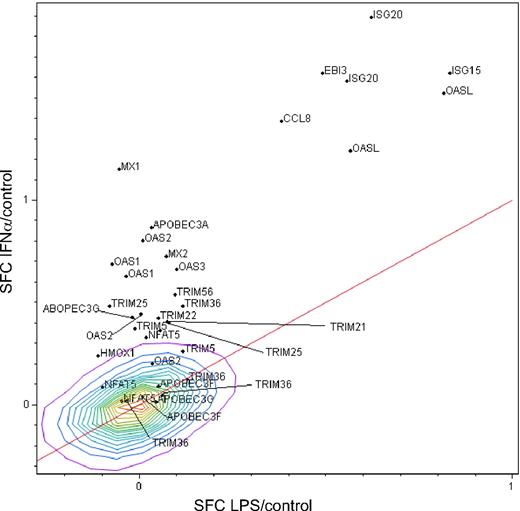
To distinguish potential antiviral genes uniquely expressed by IFN-α relative to activation-induced genes, we compared macrophage responses to IFN-α with those triggered by activation through a toll-like receptor (TLR)–mediated signal, LPS, in parallel with mock-treated cells. Macrophages exposed to a TLR4 stimulus (LPS) up-regulated a multitude of genes commonly associated with inflammation and host defense27 but not genes classically associated with antiviral activity seen in the high stringency IFN-α–induced transcriptional profile, represented by myxovirus (influenza virus) resistance 1 (MX1; >10-fold) and 2′-5′-oligoadenylate synthetase-like (OAS; >30-fold). This discrimination between LPS and IFN-α in inducing OAS and MX1 provided a basis for differentiating macrophage activation from IFN-α induction of potential antiviral genes. As summarized in Figure 1, IFN up-regulated genes (IFN-α/Control) are represented on the y-axis and LPS/Control on the x-axis, both in log scale. The red line represents the line of identity. Based on this analysis, IFN-α–induced genes above the red line and implicated in defense against HIV are listed in Table 2. Although LPS did not substantially up-regulate antiviral genes such as OAS or MX1, it did stimulate IFN-β1 greater than 100-fold and other molecules, which may directly or indirectly influence viral infection27 (see supplemental Table 1). Both IFN-α and LPS dramatically up-regulated chemokines, including CCL5, which may influence HIV entry by competing for coreceptor binding, but in this study, we did not focus on the plethora of shared genes found within the lines of identity or unique to LPS.27 This approach enabled dissociation of a cohort of genes selectively regulated by IFN-α, and because the majority of genes known or suspected to possess antiviral activity (Table 2) were found in this window, we focused our attention on these IFN-specific genes.
IFN-α and TLR-related antiviral molecules by microarray. Monocyte-derived macrophages (n = 3) were stimulated with IFN-α (10 ng/mL, 4 hours) in parallel with mock-treated cultures (see supplemental Table 1) and with LPS-stimulated cultures (100 ng/mL)27 and RNA extracted and processed for microarray analysis using the Affymetrix system. Macrophage gene expression after treatment with IFN-α as ratio to control (IFN-α/c) compared with LPS/Control ratio, in common logartithmic scale. Only probe sets of suspected antiviral functions are shown. The line of identity is shown to highlight the points that are similar in both measures. Density contours are shown for the entire set of 54 675 probe sets on the Affymetrix HG_U133 Plus2 chip. Ratios shown in common logarithmic scale. SFC = S10 transform fold change.
IFN-α and TLR-related antiviral molecules by microarray. Monocyte-derived macrophages (n = 3) were stimulated with IFN-α (10 ng/mL, 4 hours) in parallel with mock-treated cultures (see supplemental Table 1) and with LPS-stimulated cultures (100 ng/mL)27 and RNA extracted and processed for microarray analysis using the Affymetrix system. Macrophage gene expression after treatment with IFN-α as ratio to control (IFN-α/c) compared with LPS/Control ratio, in common logartithmic scale. Only probe sets of suspected antiviral functions are shown. The line of identity is shown to highlight the points that are similar in both measures. Density contours are shown for the entire set of 54 675 probe sets on the Affymetrix HG_U133 Plus2 chip. Ratios shown in common logarithmic scale. SFC = S10 transform fold change.
Genes differentiatially regulated by IFN-α and LPS with putative antiviral activity
| Probe set ID . | Gene title . | Gene symbol . | FC, IFN-α/C* . | FC, LPS/C . |
|---|---|---|---|---|
| 204698_at | Interferon-stimulated exonuclease gene 20 kDa | ISG20 | 78.24 | 4.18 |
| 205483_s_at | ISG15 ubiquitin-like modifier | ISG15 | 41.89 | 6.79 |
| 219424_at | Epstein-Barr virus induced gene 3 | EBI3 | 41.76 | 3.09 |
| 33304_at | Interferon-stimulated exonuclease gene 20 kDa | ISG20 | 38.35 | 3.6 |
| 205660_at | 2′-5′-oligoadenylate synthetase-like | OASL | 33.49 | 6.54 |
| 214038_at | Chemokine (C-C motif) ligand 8 | CCL8 | 24.34 | 2.4 |
| 210797_s_at | 2′-5′-oligoadenylate synthetase-like | OASL | 17.41 | 3.68 |
| 202086_at | Myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) | MX1 | 14.27 | 0.88 |
| 210873_x_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3A | APOBEC3A | 7.38 | 1.07 |
| 204972_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 6.38 | 1.02 |
| 204994_at | Myxovirus (influenza virus) resistance 2 (mouse) | MX2 | 5.33 | 1.18 |
| 202869_at | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | OAS1 | 4.88 | 0.84 |
| 218400_at | 2′-5′-oligoadenylate synthetase 3, 100 kDa | OAS3 | 4.63 | 1.25 |
| 205552_s_at | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | OAS1 | 4.28 | 0.92 |
| 231876_at | Tripartite motif-containing 56 | TRIM56 | 3.46 | 1.24 |
| 219736_at | Tripartite motif-containing 36 | TRIM36 | 3.06 | 1.3 |
| 224806_at | Tripartite motif-containing 25 | TRIM25 | 3.05 | 0.83 |
| 206553_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 2.8 | 1.01 |
| 204205_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G | APOBEC3G | 2.68 | 0.96 |
| 213293_s_at | Tripartite motif-containing 22 | TRIM22 | 2.66 | 1.12 |
| 204804_at | Tripartite motif-containing 21 | TRIM21 | 2.56 | 1.18 |
| 206911_at | Tripartite motif-containing 25 | TRIM25 | 2.53 | 1.2 |
| 210705_s_at | Tripartite motif-containing 5 | TRIM5 | 2.37 | 0.97 |
| 232666_at | 2′-5′-oligoadenylate synthetase 3, 100 kDa | OAS3 | 2.32 | 1.14 |
| 224984_at | Nuclear factor of activated T cells 5, tonicity-responsive | NFAT5 | 2.14 | 1.04 |
| 223830_s_at | Tripartite motif-containing 5 | TRIM5 | 1.83 | 1.31 |
| 203665_at | Heme oxygenase (decycling) 1 | HMOX1 | 1.75 | 0.77 |
| 228607_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 1.6 | 1.08 |
| 1565814_at | Tripartite motif-containing 36 | TRIM36 | 1.34 | 1.34 |
| 214994_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3F | APOBEC3F | 1.24 | 1.12 |
| 208003_s_at | Nuclear factor of activated T cells 5, tonicity-responsive | NFAT5 | 1.23 | 0.8 |
| 1565812_at | Tripartite motif-containing 36 | TRIM36 | 1.13 | 1.16 |
| 243912_x_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3F | APOBEC3F | 1.1 | 1.02 |
| 215092_s_at | Nuclear factor of activated T-cells 5, tonicity-responsive | NFAT5 | 1.05 | 0.89 |
| 231123_at | Tripartite motif-containing 36 | TRIM36 | 1.05 | 0.93 |
| 215579_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G | APOBEC3G | 1.04 | 1.1 |
| Probe set ID . | Gene title . | Gene symbol . | FC, IFN-α/C* . | FC, LPS/C . |
|---|---|---|---|---|
| 204698_at | Interferon-stimulated exonuclease gene 20 kDa | ISG20 | 78.24 | 4.18 |
| 205483_s_at | ISG15 ubiquitin-like modifier | ISG15 | 41.89 | 6.79 |
| 219424_at | Epstein-Barr virus induced gene 3 | EBI3 | 41.76 | 3.09 |
| 33304_at | Interferon-stimulated exonuclease gene 20 kDa | ISG20 | 38.35 | 3.6 |
| 205660_at | 2′-5′-oligoadenylate synthetase-like | OASL | 33.49 | 6.54 |
| 214038_at | Chemokine (C-C motif) ligand 8 | CCL8 | 24.34 | 2.4 |
| 210797_s_at | 2′-5′-oligoadenylate synthetase-like | OASL | 17.41 | 3.68 |
| 202086_at | Myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) | MX1 | 14.27 | 0.88 |
| 210873_x_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3A | APOBEC3A | 7.38 | 1.07 |
| 204972_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 6.38 | 1.02 |
| 204994_at | Myxovirus (influenza virus) resistance 2 (mouse) | MX2 | 5.33 | 1.18 |
| 202869_at | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | OAS1 | 4.88 | 0.84 |
| 218400_at | 2′-5′-oligoadenylate synthetase 3, 100 kDa | OAS3 | 4.63 | 1.25 |
| 205552_s_at | 2′,5′-oligoadenylate synthetase 1, 40/46 kDa | OAS1 | 4.28 | 0.92 |
| 231876_at | Tripartite motif-containing 56 | TRIM56 | 3.46 | 1.24 |
| 219736_at | Tripartite motif-containing 36 | TRIM36 | 3.06 | 1.3 |
| 224806_at | Tripartite motif-containing 25 | TRIM25 | 3.05 | 0.83 |
| 206553_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 2.8 | 1.01 |
| 204205_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G | APOBEC3G | 2.68 | 0.96 |
| 213293_s_at | Tripartite motif-containing 22 | TRIM22 | 2.66 | 1.12 |
| 204804_at | Tripartite motif-containing 21 | TRIM21 | 2.56 | 1.18 |
| 206911_at | Tripartite motif-containing 25 | TRIM25 | 2.53 | 1.2 |
| 210705_s_at | Tripartite motif-containing 5 | TRIM5 | 2.37 | 0.97 |
| 232666_at | 2′-5′-oligoadenylate synthetase 3, 100 kDa | OAS3 | 2.32 | 1.14 |
| 224984_at | Nuclear factor of activated T cells 5, tonicity-responsive | NFAT5 | 2.14 | 1.04 |
| 223830_s_at | Tripartite motif-containing 5 | TRIM5 | 1.83 | 1.31 |
| 203665_at | Heme oxygenase (decycling) 1 | HMOX1 | 1.75 | 0.77 |
| 228607_at | 2′-5′-oligoadenylate synthetase 2, 69/71 kDa | OAS2 | 1.6 | 1.08 |
| 1565814_at | Tripartite motif-containing 36 | TRIM36 | 1.34 | 1.34 |
| 214994_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3F | APOBEC3F | 1.24 | 1.12 |
| 208003_s_at | Nuclear factor of activated T cells 5, tonicity-responsive | NFAT5 | 1.23 | 0.8 |
| 1565812_at | Tripartite motif-containing 36 | TRIM36 | 1.13 | 1.16 |
| 243912_x_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3F | APOBEC3F | 1.1 | 1.02 |
| 215092_s_at | Nuclear factor of activated T-cells 5, tonicity-responsive | NFAT5 | 1.05 | 0.89 |
| 231123_at | Tripartite motif-containing 36 | TRIM36 | 1.05 | 0.93 |
| 215579_at | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G | APOBEC3G | 1.04 | 1.1 |
The 35 gene symbols were selected based on putative antiviral activities that are differentially regulated by IFN-α through IFNAR and LPS through toll-like receptors as fold change (FC; see “Methods”).
IFN-α–specific gene regulation
Genes and/or their products linked to antiviral functions represent a small subset of the IFN-α–induced transcriptional profile in macrophages (Figure 1 and Table 2), such as HO-1,30 multiple tripartite motif-containing (TRIM) superfamily members, several of which have been shown to inhibit retroviruses,31,32 CCL8, ISG15, an IFN-induced ubiquitin homolog that may mediate IFN-α inhibition of HIV release by blocking HIV-1 assembly through disrupting interactions between Gag and Tsg101,33 ISG20,34 CD4035 SOD,36 IFN-α–inducible protein (clone IFI-15K) G1P2,33 and members of the APOBEC cytidine deaminase family (APOBEC3A/F/G).12,14 Increased adenosine A2a receptor (ADORA2A) activation can induce cross-desensitization of CCR5 to limit susceptibility of monocytes to infection by an R5 strain of HIV37 and may also be neuroprotective.38 Notable by their absence were genes with recognized IFN inducibility and antiretroviral activity, including TETHERIN/CD137/BST239,40 up-regulated less than 2-fold. Collectively, enhanced expression of multiple HIV resistance molecules represents a substantial arsenal by which macrophages, under the influence of IFN-α, may fend off HIV. Additional genes expressed in a pattern similar to those with known or putative antiviral activity include genes, which are up-regulated by IFN-α more than 3-fold above TLR activation (see supplemental Table 1). Continuing studies of this cluster of genes may reveal new molecules and functions relevant to inhibition of HIV and/or other viruses.
IFN-α–inducible IL-27 and IL-27Rα
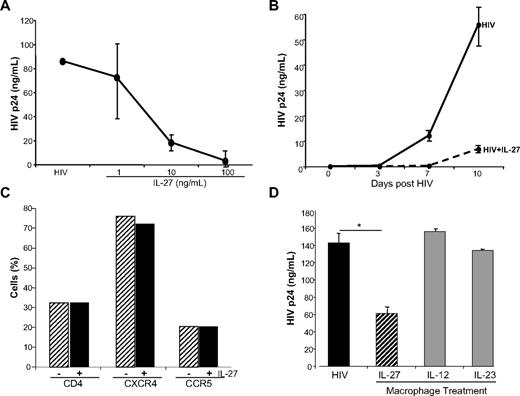
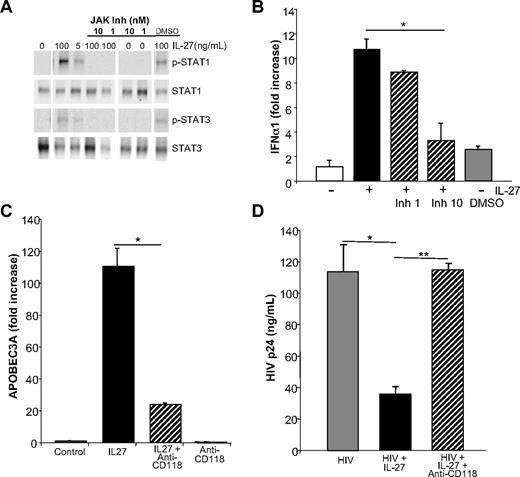
Among the IFN-α–inducible cytokine gene cluster dissociable from TLR activation by gene profiling, and recently linked to HIV,19 is the cytokine IL-27. Both the p28 and the EBI3 components of the heterodimer were up-regulated by IFN-α (Figures 1–2A), and confirmed by conventional RT-PCR (p28) and real-time PCR (p28 and EBI3; Figure 2B). The induction was dependent on JAK-STAT (signal transducer and activator of transcription) activation, because an inhibitor of JAK blocked the response (Figure 2C). Moreover, IFN-α modestly up-regulated expression of the IL-27Rα chain (WSX-1 subunit) in macrophage populations (Figure 2D), potentially enhancing responsivity to this cytokine. Exogenous IL-27 inhibited HIV replication in macrophages in a dose-dependent manner (Figure 3A), confirming an antiretroviral function19 and was effective early and sustained during infection (Figure 3B). Diminished HIV replication as monitored by supernatant p24 levels could not be attributed to IL-27–mediated alterations in viability, as viability in IL-27–treated macrophage cultures differed less than 2% to 5% from untreated cultures. IL-27–mediated antiviral activity appeared independent of altered expression of entry receptors, CD4, CXCR4, and CCR5 (Figure 3C), compatible with evidence that IL-27 was effective when added after the cells had been exposed to the virus. Inhibition of HIV appears selective to this member of the IL-12 family, in that other family members, IL-12 and IL-23, both up-regulated by IFN-α in parallel to IL-27 (see supplemental Table 1; p40 is 29-fold and IL-23p19 is ∼7-fold), did not suppress HIV replication when added to macrophage cultures (Figure 3D).
IFN-α up-regulates IL-27 and IL-27 receptor mRNA expression. (A) Monocyte-derived macrophages (n = 3) were stimulated with IFN-α (10 ng/mL, 4 hours) in parallel with mock-treated cultures, and RNA was extracted and processed for microarray analysis using the Affymetrix system. Expression of IL-27 p28 and EBI3 was up-regulated by IFN-α. (B) 6 × 106 monocyte-derived macrophages were treated with IFN-α (10 ng/mL) for 4 hours in DMEM with 10% FCS. Cells were lysed and processed for RNA following Qiagen's RNeasy protocol for conventional RT-PCR (primers were: 5′-TTCCCTTGCTCCTGGTTCAAG-3′ [forward], 5′-TGGAGATGAAGCAGAGACGCTC-3′ [reverse]; representative data) or real-time PCR (n = 5). (C) Inhibition of IFN-α–induced IL-27 transcription by JAK inhibitor (JAK Inh 1 and 10 nM) added 1 hour before IFN-α(10 ng/mL) and macrophage RNA processed for RT-PCR 4 hours after addition of IFN-α (mean ± SEM; *P < .01; representative experiment, n = 2). (D) Control or IFN-α treated macrophage cultures (n = 3) were processed for real-time PCR for IL-27R-α after 24 hours, and GAPDH was used for normalization.
IFN-α up-regulates IL-27 and IL-27 receptor mRNA expression. (A) Monocyte-derived macrophages (n = 3) were stimulated with IFN-α (10 ng/mL, 4 hours) in parallel with mock-treated cultures, and RNA was extracted and processed for microarray analysis using the Affymetrix system. Expression of IL-27 p28 and EBI3 was up-regulated by IFN-α. (B) 6 × 106 monocyte-derived macrophages were treated with IFN-α (10 ng/mL) for 4 hours in DMEM with 10% FCS. Cells were lysed and processed for RNA following Qiagen's RNeasy protocol for conventional RT-PCR (primers were: 5′-TTCCCTTGCTCCTGGTTCAAG-3′ [forward], 5′-TGGAGATGAAGCAGAGACGCTC-3′ [reverse]; representative data) or real-time PCR (n = 5). (C) Inhibition of IFN-α–induced IL-27 transcription by JAK inhibitor (JAK Inh 1 and 10 nM) added 1 hour before IFN-α(10 ng/mL) and macrophage RNA processed for RT-PCR 4 hours after addition of IFN-α (mean ± SEM; *P < .01; representative experiment, n = 2). (D) Control or IFN-α treated macrophage cultures (n = 3) were processed for real-time PCR for IL-27R-α after 24 hours, and GAPDH was used for normalization.
IL-27 inhibits HIV infection in macrophages. (A) Monocyte-derived macrophages were infected with 103 TCID50/mL BaL for 2 hours. Cells were washed, 10% DMEM added, and IL-27 at 0, 1, 10, and 100 ng/mL added only once after infection. Cells were fed every 3 to 4 days with 10% DMEM. Virus replication was measured by p24 ELISA at day 10 postinfection (n = 3). (B) Macrophages were infected with HIV for 2 hours, washed, 10% DMEM added, and IL-27 at 100 ng/mL added only once after the 2-hour infection period. Supernatant aliquots were obtained every 3 to 4 days and HIV measured by p24 ELISA (n = 3). (C) Macrophages (n = 3) were treated overnight with IL-27 (100 ng/mL) and the cells analyzed by flow cytometry after staining with antibodies specific for CD4, CXCR4, and CCR5. (D) In parallel to IL-27, IL-12 and IL-23 were added once at 100 ng/mL after HIVBaL was washed out. Cells were fed every 3 to 4 days with 10% DMEM, and virus replication measured in day 10 supernatants by p24 ELISA (*P < .02; n = 3, representative data shown).
IL-27 inhibits HIV infection in macrophages. (A) Monocyte-derived macrophages were infected with 103 TCID50/mL BaL for 2 hours. Cells were washed, 10% DMEM added, and IL-27 at 0, 1, 10, and 100 ng/mL added only once after infection. Cells were fed every 3 to 4 days with 10% DMEM. Virus replication was measured by p24 ELISA at day 10 postinfection (n = 3). (B) Macrophages were infected with HIV for 2 hours, washed, 10% DMEM added, and IL-27 at 100 ng/mL added only once after the 2-hour infection period. Supernatant aliquots were obtained every 3 to 4 days and HIV measured by p24 ELISA (n = 3). (C) Macrophages (n = 3) were treated overnight with IL-27 (100 ng/mL) and the cells analyzed by flow cytometry after staining with antibodies specific for CD4, CXCR4, and CCR5. (D) In parallel to IL-27, IL-12 and IL-23 were added once at 100 ng/mL after HIVBaL was washed out. Cells were fed every 3 to 4 days with 10% DMEM, and virus replication measured in day 10 supernatants by p24 ELISA (*P < .02; n = 3, representative data shown).
IL-27 induction of APOBEC
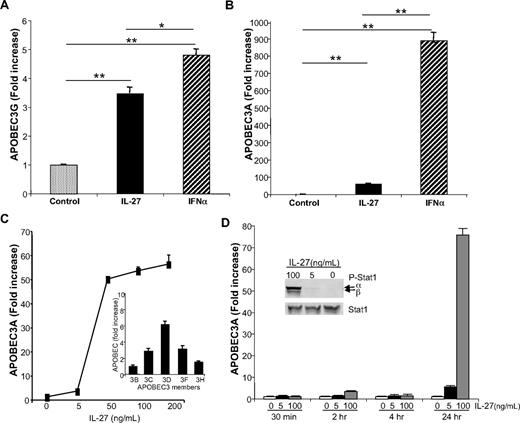
To explore the mechanism(s) by which IL-27 exerts activity against HIV, we examined its potential ability to directly influence members of the innate intracellular cytidine deaminase APOBEC family. Although IL-27 did up-regulate expression of APOBEC3G (> 3-fold), and even more substantively APOBEC3A (60-fold) after 24 hours (Figure 4A-B), the response was clearly less effective than with IFN-α (Figure 4A-B, APOBEC3A, P < .002; APOBEC3G, P = .03). To further define the IL-27 induction of APOBEC3A/G as its potential antiretroviral pathway, dose-response analyses revealed optimal effects at 50 ng/mL and above (Figure 4C), but kinetic studies revealed little or no evidence of APOBEC induction within the first 4 hours after exposure to IL-27. Rather, APOBEC3, as represented by APOBEC3A induction, was delayed and most prominent 24 hours after exposure of the cells to IL-27 (Figure 4D), compared with the evidence of much earlier induction of APOBEC by IFN-α (<4 hours).12 The delay in IL-27–triggered expression of APOBEC occurred despite evidence of rapid IL-27–induced signal transduction as monitored by STAT1 phosphorylation within minutes after addition to macrophage cultures (Figure 4D inset), as reported.41,42 The pattern of delayed and dose-dependent up-regulation of APOBEC3A (Figure 4C-D) was also seen for other members of the APOBEC family (Figure 4C inset), albeit to a lesser extent. Thus, the evidence supporting early IL-27 signaling of STAT1 phosphorylation appears inconsistent with the delayed induction of APOBECs and was suggestive of an intermediate molecule involved in driving APOBEC generation.
IL-27 enhances macrophage APOBEC RNA expression. Monocyte-derived macrophages were treated 18 to 24 hours with IL-27 at 100 ng/mL or IFN-α at 10 ng/mL. Cells were lysed and processed for RNA following QIAGEN's RNeasy protocol, and gene transcription was analyzed by real-time PCR for (A) APOBEC3G (n = 3) and (B) APOBEC3A (n = 5). GAPDH was used as normalization control. Data were analyzed using the 2−ΔΔct method and results reported as fold increase (*P < .05; **P < .005). (C) IL-27 (5-200 ng/mL) was added to macrophage cultures for 24 hours and RNA processed for real-time PCR with APOBEC3A primers (n = 4). Inset: IL-27 (50 ng/mL) was added to macrophage cultures for 24 hours and indicated members of APOBEC family expression profiles determined by RT-PCR (n = 2). (D) Kinetics (30 minutes to 24 hours) of IL-27 (0-100 ng/mL) induction of APOBEC3A expression as determined by real time PCR (n = 3). Inset: IL-27 at indicated concentrations was added to macrophage cultures for 30 minutes, and phospho-STAT1 and total STAT were monitored by Western blot.
IL-27 enhances macrophage APOBEC RNA expression. Monocyte-derived macrophages were treated 18 to 24 hours with IL-27 at 100 ng/mL or IFN-α at 10 ng/mL. Cells were lysed and processed for RNA following QIAGEN's RNeasy protocol, and gene transcription was analyzed by real-time PCR for (A) APOBEC3G (n = 3) and (B) APOBEC3A (n = 5). GAPDH was used as normalization control. Data were analyzed using the 2−ΔΔct method and results reported as fold increase (*P < .05; **P < .005). (C) IL-27 (5-200 ng/mL) was added to macrophage cultures for 24 hours and RNA processed for real-time PCR with APOBEC3A primers (n = 4). Inset: IL-27 (50 ng/mL) was added to macrophage cultures for 24 hours and indicated members of APOBEC family expression profiles determined by RT-PCR (n = 2). (D) Kinetics (30 minutes to 24 hours) of IL-27 (0-100 ng/mL) induction of APOBEC3A expression as determined by real time PCR (n = 3). Inset: IL-27 at indicated concentrations was added to macrophage cultures for 30 minutes, and phospho-STAT1 and total STAT were monitored by Western blot.
IL-27 induction of APOBEC involves type I IFN
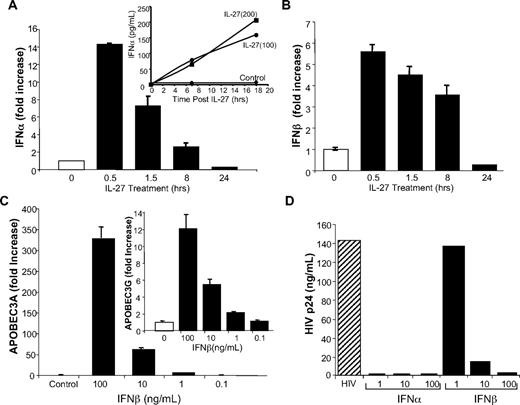
Because the delayed response suggested that IL-27 was possibly inducing an intermediate molecule, which, in turn, was responsible for regulating APOBEC expression, we considered that IFN-α was a possible candidate. It became apparent that within the first 30 minutes to 4 hours after exposure to IL-27, well before evidence of APOBEC augmentation, that there was a rapid up-regulation of IFN-α by RT-PCR, whether examined in PBMC, monocytes, or macrophages (Figure 5A, PBMC shown), accompanied by increased supernatant protein detection (Figure 5A inset). Further examination revealed a parallel IL-27 enhancement of expression of IFN-β (Figure 5B). In additional experiments, IFN-β, not previously linked to APOBEC expression, was shown to induce both APOBEC3A and APOBEC3G in macrophages (Figure 5C), reflecting shared signaling pathways via IFNAR and inhibition of HIV infection (Figure 5D).
IL-27 induction of IFN-α and IFN-β supports APOBEC3A/G expression and inhibition of HIV. (A) PBMC were treated with IL-27 at 100 ng/mL for 30 minutes to 24 hours. RNA was reverse transcribed to cDNA, and amplification was performed using Taqman expression array for IFN-α1. Inset: Supernatants from IL-27–treated (100 and 200 ng/mL) macrophages at indicated times were tested for IFN-α by ELISA (representative data, n = 3). (B) RNA from PBMC treated with IL-27 at 100 ng/mL for 30 minutes to 24 hours was reverse transcribed and amplification performed for IFN-β in parallel with GAPDH. (C) Macrophages were treated with indicated concentrations of IFN-β for 4 hours, and induction of APOBEC3A and APOBEC3G (inset) determined by RT-PCR (n = 2). (D) Macrophage cultures were exposed to HIV for 2 hours, washed, replenished with DMEM containing 10% FCS, and treated with IFN-α (1-100 ng/mL) or IFN-β (1-100 ng/mL). Cells were refed every 3 to 4 days with 10% DMEM. Virus replication was measured by p24 ELISA at day 10 postinfection (n = 3).
IL-27 induction of IFN-α and IFN-β supports APOBEC3A/G expression and inhibition of HIV. (A) PBMC were treated with IL-27 at 100 ng/mL for 30 minutes to 24 hours. RNA was reverse transcribed to cDNA, and amplification was performed using Taqman expression array for IFN-α1. Inset: Supernatants from IL-27–treated (100 and 200 ng/mL) macrophages at indicated times were tested for IFN-α by ELISA (representative data, n = 3). (B) RNA from PBMC treated with IL-27 at 100 ng/mL for 30 minutes to 24 hours was reverse transcribed and amplification performed for IFN-β in parallel with GAPDH. (C) Macrophages were treated with indicated concentrations of IFN-β for 4 hours, and induction of APOBEC3A and APOBEC3G (inset) determined by RT-PCR (n = 2). (D) Macrophage cultures were exposed to HIV for 2 hours, washed, replenished with DMEM containing 10% FCS, and treated with IFN-α (1-100 ng/mL) or IFN-β (1-100 ng/mL). Cells were refed every 3 to 4 days with 10% DMEM. Virus replication was measured by p24 ELISA at day 10 postinfection (n = 3).
Effects of IFN-α and IL-27 on T-cell HIV infection
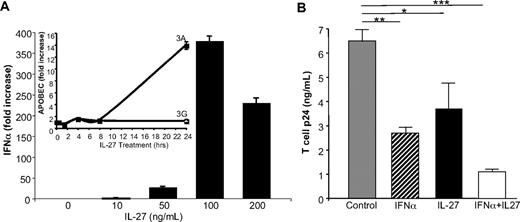
Whereas our evidence in this study and in our previous work12 indicates that IL-27 indirectly and IFN-α directly control cytidine deaminase expression in macrophages, similar studies are lacking in CD4+ T-cell HIV targets. Therefore, in parallel experiments, IFN-α and IL-27 were examined for their respective ability to suppress HIV infection in PBMC and in isolated T cells. As indicated in PBMC (Figure 5A-B), similar to monocytes and cultured monocyte-derived macrophages, IL-27 induced IFN-α and IFN-β within 30 minutes, preceding evidence of enhanced APOBEC3A and/or APOBEC3G. Moreover, when blasted CD4+ T-lymphocyte HIV targets were treated with IL-27, a dose-dependent increase in IFN-α occurred rapidly (Figure 6), whereas APOBEC3A was delayed (Figure 6A inset), without a parallel significant effect on T-cell APOBEC3G, as demonstrated (Gang Peng, W.J., T.G.W., Ke Jian Lei, Michael A. Polis, Richard Lempicki, Shyamasundaran Kottilil, S.M.W., manuscript in preparation). Thus, IL-27 increases expression of type I IFN in T cells, but not APOBEC3A, within the first 4 hours after stimulation, consistent with a stepwise induction of IFN, which in turn induces APOBEC3A and suppression of HIV replication (Figure 6). IFN-α directly suppressed T-cell virus replication, although not as significantly as in macrophages, and similarly, IL-27 blunted T-cell infection by HIV (Figure 6B). When both cytokines were added concurrently, enhanced suppression of HIV replication was seen (Figure 6B; P < .05), likely reflecting additional inducible antiviral mechanisms.
IL-27 inhibition of T-cell HIV includes an IFN intermediate. (A) IL-27 induction of IFN-α in T cells at 0.5 to 4 hours (4 hours shown; n = 2), without corresponding increase in APOBEC3A until 24 hours but no increase in APOBEC3G (inset; n = 3). (B) Isolated T cells were blasted with phytohemagglutinin (3 days) and treated with IFN-α, IL-27, or IFN-α plus IL-27 once at 2 hours after infection with HIVIIIB and HIV infection monitored by p24 ELISA after 7 days (*P = .04, **P < .01, ***P = .002 compared with HIV control; n = 2).
IL-27 inhibition of T-cell HIV includes an IFN intermediate. (A) IL-27 induction of IFN-α in T cells at 0.5 to 4 hours (4 hours shown; n = 2), without corresponding increase in APOBEC3A until 24 hours but no increase in APOBEC3G (inset; n = 3). (B) Isolated T cells were blasted with phytohemagglutinin (3 days) and treated with IFN-α, IL-27, or IFN-α plus IL-27 once at 2 hours after infection with HIVIIIB and HIV infection monitored by p24 ELISA after 7 days (*P = .04, **P < .01, ***P = .002 compared with HIV control; n = 2).
Disruption of IL-27–induced IFN and APOBEC blocks HIV suppression
To confirm that IL-27 bolsters APOBEC levels through a type I IFN intermediate, we first blocked IL-27–mediated signaling to determine whether this blunted IFN expression. IL-27 interaction with the IL-27 receptor triggers phosphorylation of STAT1, and to a lesser extent STAT3 (Figure 7A),25 presumably a requisite precursor step to IFN-α/β induction in human macrophages. Inhibition of this initial upstream IL-27 mediated JAK activation, by disrupting subsequent STAT phosphorylation, diminishes IL-27–induced type I IFN expression (Figure 7B).
Blocking IL-27 signaling reverses inhibition of HIV infection. (A) Inhibition of IL-27 signal transduction by blocking JAK activation interrupts STAT1 and STAT3 phosphorylation. Macrophages were treated with JAK inhibitor at 1 or 10 nM for 1 hour before addition of IL-27, and after 30 minutes, processed for Western blots using antibodies to STAT1, STAT3, p-STAT1, and p-STAT3 (representative data, n = 3). (B) Inhibition of JAK activation blocks IL-27 downstream transcription of IFN-α. Macrophages pretreated for 1 hour with JAK inhibitor (0-10 nM) before addition of IL-27 exhibited reduced induction of IFN-α after 30 minutes as determined by RT-PCR (mean ± SEM, *P = .01; n = 3). (C) Monocyte-derived macrophages were treated or not with IFN receptor antibody (CD118 at 10 μg/mL) 20 to 30 minutes before IL-27 (100 ng/mL) and APOBEC3A determined by RT-PCR after 24 hours (mean ± SEM, *P = .01; representative triplicate data, n = 8). (D) Monocyte-derived macrophages (n = 4) were infected with HIV, washed, and then treated or not with IFN receptor antibody (CD118 at 10 μg/mL), which was added 20 to 30 minutes before IL-27 (100 ng/mL). ELISA for p24 was used to monitor HIV replication in the treated and untreated cultures (mean ± SEM, *P = .01, **P = .001).
Blocking IL-27 signaling reverses inhibition of HIV infection. (A) Inhibition of IL-27 signal transduction by blocking JAK activation interrupts STAT1 and STAT3 phosphorylation. Macrophages were treated with JAK inhibitor at 1 or 10 nM for 1 hour before addition of IL-27, and after 30 minutes, processed for Western blots using antibodies to STAT1, STAT3, p-STAT1, and p-STAT3 (representative data, n = 3). (B) Inhibition of JAK activation blocks IL-27 downstream transcription of IFN-α. Macrophages pretreated for 1 hour with JAK inhibitor (0-10 nM) before addition of IL-27 exhibited reduced induction of IFN-α after 30 minutes as determined by RT-PCR (mean ± SEM, *P = .01; n = 3). (C) Monocyte-derived macrophages were treated or not with IFN receptor antibody (CD118 at 10 μg/mL) 20 to 30 minutes before IL-27 (100 ng/mL) and APOBEC3A determined by RT-PCR after 24 hours (mean ± SEM, *P = .01; representative triplicate data, n = 8). (D) Monocyte-derived macrophages (n = 4) were infected with HIV, washed, and then treated or not with IFN receptor antibody (CD118 at 10 μg/mL), which was added 20 to 30 minutes before IL-27 (100 ng/mL). ELISA for p24 was used to monitor HIV replication in the treated and untreated cultures (mean ± SEM, *P = .01, **P = .001).
To further link IL-27 and IFN, we treated macrophages with a neutralizing antibody to IFN-α/β receptor (IFNAR, CD118) before addition of IL-27 and found that the antibody blocks the ability of IL-27 to up-regulate APOBEC3A/G expression, consistent with the sequential effect of IL-27 on stimulation of IFN-α, which in turn induces APOBEC expression (Figure 7C). Furthermore, the IFNAR antibody partially reverses the ability of IL-27 to block HIV infection in macrophage cultures (Figure 7D, day 8 shown), in keeping with an IFN-dependent step in the induction of cytidine deaminases in defense against the retrovirus.
Collectively, our data support a circuitous connection between IFN and IL-27 in host defense against HIV. Type I IFNs or other stimuli induce IL-27 (p28 and EBI3), but IL-27, through rapid induction of IFN, drives enhanced expression of the intracellular antiretroviral cytidine deaminases. The outcome of this molecular cascade enables CD4+ T cells and macrophages to fend off advances by HIV.
Discussion
The conundrum of whether type I IFN is beneficial in HIV/AIDS or is a mediator of disease pathogenesis18 remains unresolved, and alternative regulators of innate antiretroviral defense pathways may alleviate treatment-related cytotoxicities. In these studies, we explored, through microarrays, potential IFN-α–induced molecules that may serve as downstream surrogates in battling HIV and focused on one such candidate, IFN-α–induced IL-27. Whereas IL-27 was shown to inhibit HIV and to up-regulate antiviral APOBEC3 cytidine deaminases,19 we provide evidence that IL-27 does not directly regulate APOBEC family members to counterattack HIV but rather induces IFN-α/β and in so doing augments macrophage defensive maneuvers against HIV. Based on our data, the recently reported parallel mechanisms of IL-27– and IFN-α–mediated inhibition of HIV43 are, in fact, sequential. The evidence in support of this conclusion is manifold. First, the ability of IL-27 to induce APOBEC3A/G is delayed, with minimal evidence of enhanced expression until after 4-hour stimulation with IL-27, optimal at 24 hours. Second, within the first 4 hours, IL-27 increased IFN-α, consistent with IFN-α–triggered enhancement of ISG. In previous studies, neither IL-27– nor IFN-α–mediated gene expression was monitored before 24 hours,43 negating detection of this intertwined connection. Third, stimulation of macrophages with IL-27 results in a very rapid JAK/STAT signaling response,25 despite belated increases in APOBEC. In T cells, STAT3 and STAT5 activation occurs in response to IL-27,41,42 and in fully activated CD4+ T cells, there is preferential activation of STAT3,22 which may underlie IL-27 induction of IFN and subsequent enhanced expression of antiretroviral cytidine deaminases. Fourth, the increase in APOBEC, particularly APOBEC3A, relevant to macrophage HIV resistance12,14 that occurs in response to IL-27 can be attenuated by a neutralizing antibody to the type I IFN receptor (CD118), consonant with dependence upon IFNAR signaling. Critically, the ability of IL-27 to enforce an HIV blockade is also impaired in the presence of the IFNAR antibody. IL-27 not only inhibits R5 HIV infection in macrophages but also suppresses X4 replication in CD4+ T cells, which is associated with elevations in IFN and downstream ISG. Finally, in a reciprocal arrangement, not only does IL-27 trigger IFN production, but IFN-α up-regulates IL-27 to establish a cyclic release of both cytokines with accentuation of APOBECs, TRIMs, and other ISG. Although not obviating the need for IFN-α, these data focus the search for additional modes of targeting the cytidine deaminases to thwart HIV, with the aim of minimizing IFN toxicity. These pathways appear to be distinct and/or complementary to the described ability of IL-27 to induce production of type II IFN (IFN-γ) in T cells and NK cells,24 important in microbe clearance.
Although most widely recognized for its pro- and anti-inflammatory properties and its regulation of Th-cell lineage differentiation and function,22,24,42 the evidence that IL-27 can influence activity against HIV provides an impetus to further explore mechanisms of its induction and function. Functionally active as a heterodimer composed of the receptor-like subunit, EBI3, and the p28 helical protein, IFN-α directly induces both components of IL-27 necessary for efficient secretion and also reportedly mediates TLR4-stimulated IL-27p28.44 Studies in human macrophages illustrate 2 potential IFN-stimulated response elements (ISRE1 and 2) in the p28 gene promoter, and IFN-α stimulation results in the binding of IRF-1 to the p28 ISRE site.44 Moreover, IFN-β stimulates p28, as does LPS differentiation of DC through a type I IFN and IRF1 and IRF3 pathway45,46 after activation of receptor-associated JAKs, STAT phosphorylation, and NFκB activation.47,48 The link between IFN and IL-27 regulation may be central in defense against HIV, particularly in human macrophages, which serve as a host and reservoir for HIV3,49,50 and can be resistant to antiviral strategies4 but also in CD4+ T cells.
IL-27 represents a novel member of the IL-12 family in many aspects and is apparently distinct in its ability to target HIV resistance via IFN-α. IL-27 does not obviate the need for IFN but, rather, requires IFN to achieve an antiviral state through induction of APOBECs, TRIMs, and other molecules, known and to be established, that disrupt the viral life cycle. Conceivably, as the induced IFN may function proximally in an autocrine or paracrine manner in lesser concentrations, this may sublimate IFN-related toxicities. Although IFN is believed to inhibit HIV by multiple mechanisms, including OAS-mediated RNase L and PKR, deletion of these genes does not appear to impair the ability of IFN to inhibit HIV,15 underscoring the role of other ISG, such as APOBEC family members as pivotal links in IFN′s defensive armor against HIV, as validated in IFN-treated patients (Gang Peng, W.J., T.G.W., Ke Jian Lei, Michael A. Polis, Richard Lempicki, Shyamasundaran Kottilil, S.M.W., manuscript in preparation). Further exploration of the pathways regulating these antiviral ISG, including the cytidine deaminases, may uncover key signaling components that can be selectively regulated to avoid negative aspects of IFN/IL-27 treatment. To this end, our findings provide further evidence that the targeting of innate intracellular pathways of host defense against HIV may provide an important inducible counterattack against this lethal virus.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr N. Moutsopoulos for flow cytometry and Dara Stoney for editorial assistance.
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Dental and Craniofacial Research.
National Institutes of Health
Authorship
Contribution: T.G.-W., N.V., and W.J. designed and performed research, collected data, analyzed and interpreted data, and participated in writing the manuscript; S.M.W. designed research, analyzed and interpreted data, and wrote the manuscript; and Z.R. and P.M. collected data, analyzed and interpreted data, performed statistical analysis, and participated in manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr S. M. Wahl, Bldg 30, Room 320, 30 Convent Dr, MSC 4352; NIDCR, National Institutes of Health, Bethesda, MD 20892-4352; e-mail: smwahl@dir.nidcr.nih.gov.

![Figure 2. IFN-α up-regulates IL-27 and IL-27 receptor mRNA expression. (A) Monocyte-derived macrophages (n = 3) were stimulated with IFN-α (10 ng/mL, 4 hours) in parallel with mock-treated cultures, and RNA was extracted and processed for microarray analysis using the Affymetrix system. Expression of IL-27 p28 and EBI3 was up-regulated by IFN-α. (B) 6 × 106 monocyte-derived macrophages were treated with IFN-α (10 ng/mL) for 4 hours in DMEM with 10% FCS. Cells were lysed and processed for RNA following Qiagen's RNeasy protocol for conventional RT-PCR (primers were: 5′-TTCCCTTGCTCCTGGTTCAAG-3′ [forward], 5′-TGGAGATGAAGCAGAGACGCTC-3′ [reverse]; representative data) or real-time PCR (n = 5). (C) Inhibition of IFN-α–induced IL-27 transcription by JAK inhibitor (JAK Inh 1 and 10 nM) added 1 hour before IFN-α(10 ng/mL) and macrophage RNA processed for RT-PCR 4 hours after addition of IFN-α (mean ± SEM; *P < .01; representative experiment, n = 2). (D) Control or IFN-α treated macrophage cultures (n = 3) were processed for real-time PCR for IL-27R-α after 24 hours, and GAPDH was used for normalization.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/9/10.1182_blood-2009-03-211540/5/m_zh89990940800002.jpeg?Expires=1769253337&Signature=uiuqxtDTGKlKlzLQD9tgp9KX~OmqI89-kRiwS00jPm9gju59TYhZ1YZ5TqB7KzEp-F82e0BG~s0SF3yvQX3rHrQe6qMK9Jb~1iqY8UmEo004QbbqepZV2NJPshyrvU-smYk~--ZxkLEXWiRSRv~YSbx-TXa7x2d0TYsGT9wWdYZcTKTUbPKFZNRIF9ytW~uHMlqRkeGgykYyXbNH7tnWFmDo2afKZvT9ZiO2M5kg6walKXaBc6Y~P5UFDbx7Koj3PmggH506Z9dS2Gt2FDYO9nz0hYEqF0H22tXBI2pxmQl6QZHLQdOPnzeqymC~3i5kHmIyWbbK3eDpDkTtkRCm5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)